Abstract
A nanocomposite photocatalyst consisting of titanium dioxide (TiO2) supported on multiwalled carbon nanotubes (MWCNTs) has been successfully prepared and used for the treatment of wastewater contaminated with tetracycline (TC), a recalcitrant antibiotic pollutant. The TiO2/MCNT composites were prepared by a simple evaporation-drying method. The properties of MWCNT/TiO2 were optimized by dispersing different amounts of TiO2 onto MWCNT. The structural and optical characteristics of the nano-engineered photocatalyst composite were characterized using scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier-transform infrared spectroscopy (FTIR) techniques. Photocatalytic degradation of TC was conducted in a quartz glass reactor. Different kinetic models were used to demonstrate the governing mechanisms. The findings revealed that the TiO2/MWCNT composite had enhanced photocatalytic activity (95% TC removal) compared to TiO2 (86% removal). The photocatalyst nanocomposite exhibited overall pseudo-second-order reaction kinetics and favored the Langmuir adsorption isotherm. Although up to 95% degradation of TC was achieved, only 75% of it was mineralized as a result of the formation of stable refractory intermediates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is a critical component of environmental, social, and governance (ESG) and sustainability challenges faced globally. Finding solutions for wastewater management that are environmentally sustainable remain the main challenge given the increasing demands of the ever-growing world population (Boffo and Patalano 2020; Amdeha and Salem 2022). The presence of inorganic and organic pollutants from human, industrial, and agricultural activities makes the water supply challenges complex (Luo et al. 2023). Every day new recalcitrant chemicals such as pharmaceuticals and their metabolites are identified in the water environment posing a serious risk to aquatic ecosystems (Liu et al. 2018). Antibiotics are among the significant pharmaceutical components and have been widely used in veterinary and human medicine for treating and preventing bacterial infections (Xu et al. 2017). It has been reported that the concentration of antibiotics in hospital and pharmaceutical wastewater can be as high as 500 mg/L and between 100 ng/L and 6 μg/L in untreated domestic wastewater (Cao et al. 2018; Wang et al. 2016a, 2016b). Tetracycline (TC) is one of the most extensively produced, used, and effective antibiotics all over the world. Due to its high chemical stability, tetracycline poses serious harm to humans and the environment. Emerging recalcitrant contaminants such as TC possess low biodegradability and are not amenable to conventional biological treatment methods (Kumar et al. 2019).
Several treatment methods, such as adsorption, ion exchange, ozonation, membrane separation, Fenton, electrochemical oxidation, and chemical precipitation, have been used to treat antibiotic-contaminated wastewater with varying degrees of success and drawbacks (Ji et al. 2023; Abou-Hadid et al. 2022). Heterogenous photocatalysis, which is an advanced oxidation process (AOP), has attracted considerable interest as a unique green purification technology due to the degradation of pollutants adsorbed on the surface of the photocatalyst that can be simultaneously regenerated under light irradiation. Nanosized photocatalyst composites are promising due to their high performance and unique properties (Luo et al. 2019a, 2019b). Nano-photocatalyst composites exhibit not only remarkable photocatalytic properties but also strong adsorption capacity due to their extraordinarily high surface area-to-mass ratio. Since photocatalysis is a surface reaction that occurs mostly on/near the surface of the catalyst, contaminants are removed more effectively if they can easily diffuse to the photocatalyst’s surface. Thus, the adsorption process may affect the performances of catalysts, particularly nano-photocatalysts (Luo et al. 2019a, 2019b; Zhou et al. 2019).
Heterogeneous photocatalysis using TiO2 and UV light is a cost-effective and eco-friendly treatment technology for the removal of recalcitrant compounds and to increase the biodegradability of persistent pollutants (Zhou et al. 2019). TiO2 is the leading photocatalyst due to its high biological and chemical stability, relatively low cost, good photoactivity, and low toxicity. Several studies have investigated the heterogeneous UV/TiO2 photocatalysis of many emerging contaminants (Luo et al. 2019a, 2019b; Yu et al. 2021; Xue et al. 2011). It should be noted that the mass transfer limitation has to be minimized for an effective TiO2-based heterogeneous photocatalysis application in wastewater treatment. The main critical issue is to recover the TiO2 particles from the treated wastewater. In addition, the efficiency of TiO2 has been unsatisfactory given the rapid electron-hole pair recombination (Otieno et al. 2017; Brooms et al. 2018). To enhance the photocatalytic activity of TiO2, it is crucial to reduce the recombination rate of photogenerated electron and hole pairs (Tolosana-Moranchel et al. 2017; Manassero et al. 2017). TiO2 can be supported onto a conductive material with a large specific surface area to overcome these challenges.
Multiwalled carbon nanotubes (MWCNTs) present the advantages of being easy to synthesize, high essential electrical conductivity, suitable specific surface area, and improved chemical and mechanical stabilities (Liu et al. 2019; Dutta et al. 2018). Thus, MWCNTs are very promising materials that can be composited with TiO2. Furthermore, it is assumed that supporting TiO2 onto MWCNTs can promote dispersion, induce charge transfer, and thus improve the photocatalytic activity of TiO2 for degrading recalcitrant emerging pollutants (Sun et al. 2018). Many researchers have combined adsorptive and photocatalytic means for the removal of aqueous contaminants by using engineered nanomaterials, including adsorbents and photocatalysts with environmental applications (Mohamed et al. 2022; Dubey et al. 2022; Luo et al. 2023). However, few studies have focused on how photocatalytic activity and adsorption work together. In addition, the design and use of effective TiO2/carbon material systems depend on a complete understanding of this interaction (Meng et al. 2022).
Previous studies on the integration of adsorption and photocatalysis have overlooked the role of adsorption kinetics when evaluating pollutant degradation by assuming the adsorption/desorption interaction is always at equilibrium (Luo et al. 2019a, 2019b; Xue et al. 2011; Martínez et al. 2011; Son et al. 2004; Ji et al. 2009). It is important to emphasize that the active species created by photo-induced carriers, which are crucial in the continuous degradation of the adsorbed reactant, hinder the adsorption/desorption interaction equilibrium from being attained in photocatalytic processes. According to Zhou et al. (2019), adsorption and photocatalytic degradation processes are indivisible and are widely used for pollutant degradation with practical environmental applications. To guide the development and improvement of nano-photocatalysts and advance the real-world applications of photocatalysis, a thorough understanding of the foundations of the modeling of the integrated adsorption and photocatalytic degradation of pollutants is required (Luo et al. 2019a, 2019b). The purpose of this research was to investigate the feasibility of using TiO2/MWCNT nanocomposite for the degradation of TC and to reveal the synergetic effect of surface adsorption and photocatalytic potential on the removal of a recalcitrant contaminant. Of special interest was the modeling of the photodegradation process and adsorption isotherms. Experiments were carried out in a systematic way to assess the removal of the TC contaminant by TiO2/MWCNTs under various conditions.
Materials and methods
Materials
TiO2 (P25, ca. 80% of anatase, and 20% of rutile) powder, supporting multiwalled carbon nanotubes (MWCNTs) with a specific surface area of 233 m2/g, an inner diameter of 3–5 nm and outer diameter of 5–8 nm, and recalcitrant emerging contaminant, tetracycline (TC, 95% purity), were purchased from Sigma-Aldrich, South Africa. All chemicals were of analytical grade and were used without further purification. Deionized water was used for the preparation of all aqueous solutions.
Preparation and characterization of MWCNTs-TiO2 nanocomposite
According to Malikov et al. (2014), KMnO4 is an effective oxidizing agent since elements become more electronegative as the oxidation states of atoms increase. Furthermore, since oxidation occurs under mild conditions (i.e., lower temperatures), it is less costly than oxidation with HNO3 (Malikov et al. 2014). Pure MWCNTs were oxidized for 3 h in a 0.1 M KMnO4 solution with magnetic stirring at 80 °C. The solution was filtered, and the resulting residue was oven-dried at 100 °C for 12 h after cooling to room temperature and being rinsed with distilled water until pH 7 was reached. TiO2/MWCNT composites with varying TiO2/MWCNTs ratios were prepared by transferring weighed amounts of MWCNTs and TiO2 in ethanol and ultrasonically dispersing them for 2 h. The mixtures were then stirred magnetically for 30 min at 100 °C to enhance components’ interaction. The solutions were finally calcined at 450 °C for 2 h to produce the TiO2/MWCNT nanocomposites.
Characterizations of the catalyst
Scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier-transform infrared (FTIR) spectroscopy were used to examine the morphology, crystal phase, and nature of the surface molecular groups of the materials, respectively. X-ray diffraction (XRD, Rigaku – Ultima IV X-ray diffraction, scan speed 4.00=min, scan range 3–90) was used to investigate crystallinity and was performed using monochromatic Cu K radiation and a positional sensitive detector. FTIR spectra were recorded using KBr transparent discs at 64 scans between 4000 and 400 cm−1 with a resolution of 4 cm−1. The 105 N vacuumed-dried samples were pressed into a transparent disk with a diameter of 14 mm by applying force. The absorbance of KBr-sample discs was measured.
Adsorption and photocatalytic experiments
To investigate the adsorption and photocatalytic activity of the prepared TiO2/MWCNTs, tetracycline (TC) was used as a model recalcitrant contaminant. Experiments for photolysis, adsorption in the dark, and photocatalytic degradation by TiO2/MWCNTs were carried out in a 250-mL cylindrical batch reactor (Fig. 1) under continuous stirring. Adsorption tests were conducted in the dark, and the minimum time required to ensure equilibrium was set at 60 min. After reaching adsorption-desorption equilibrium, photocatalytic degradation was carried out using a 12-W Xenon lamp and UV light (= 254 nm). Several parameters were investigated, including composite composition, substrate pH, and initial concentration. Samples were collected at various time intervals during the experiments and centrifuged at 3500 rpm for 2 min to separate the solution from the composite photocatalyst. A UV-Vis spectrophotometer was used to measure the concentration of TC at the maximum absorption wavelength of 357 nm (Wu et al. 2020).
The initial TC concentration was set in the range of 10–100 mg/L at a composite photocatalyst dose of 1 g/L for the adsorption isotherm study. The adsorption amount (qe) retained per gram of composite photocatalyst at reaction time t was calculated as:
where Ci and Ce (mg/L) are the TC concentration at initial and equilibrium time; V is the total solution volume (L); and m is the mass of TiO2/MWCNT added in the solution (g).
Results and discussion
Characterization results
Figure 2 shows SEM images of TiO2 (P25), MWCNTs, and MWCNT/TiO2 composite photocatalysts (at various ratios). TiO2 and MWCNTs were found to have spherical and tubular structures, respectively. SEM images of MWCNT/TiO2 revealed similar morphologies for all ratios, demonstrating TiO2 interaction with MWCNTs. MWCNTs are seen to be deposited evenly and tightly around the surface of TiO2. Aggregation was observed in Fig. 2 at higher MWCNT loadings, and this could be caused by the high surface energy of the MWCNTs. The structural analysis revealed that MWCNTs were coated on the surface of TiO2 nanoparticles, despite the presence of agglomerates of TiO2, which was likely due to the relatively high TiO2 content (5%) in the nanocomposite. A crystalline material is distinguished by its orderly and continuously repetitive arrangements of atom planes in its crystal lattice (Ali et al. 2021).
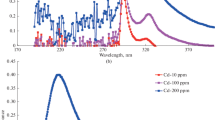
The XRD pattern of the o-MWCNTs revealed a high intense peak at approximately 30.95° and a low intense peak at 48.5°, as shown in Fig. 3. The peak at 30.95° in the XRD pattern for o-MWCNTs corresponds to the plane (002) of graphite hexagonal structure, and the next peak at around 48.5° corresponds to the plane (1.00) of graphite hexagonal structure (Natarajan et al. 2017; Caudillo-Flores et al. 2016). The XRD pattern for TiO2 nanoparticles showed several anatase phase peaks at 29.9°, 43.6.78°, 56.8°, 65.3°, and 74.7° (Meng et al. 2022). The presence of the rutile in TiO2 was also indicated by the peaks appearing at 42.6°, 52.04°, 64.42°, 78.21°, 89.51°, and 96.66° (Zhang et al. 2019). The MWCNT peak is not visible in the XRD pattern of TiO2/MWCNTs due to TiO2 being more abundant than MWCNTs. Furthermore, the crystalline extent of MWCNTs is much lower than that of TiO2, resulting in the shielding of MWCNTs peaks by TiO2 peaks (Shahnazi et al. 2020). These findings confirmed the successful synthesis of the TiO2/MWCNT nanocomposite.
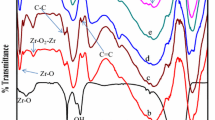
Figure 4 depicts the FTIR spectra of TiO2, MWCNTs, and TiO2/MWCNT composite particles. TiO2/MWCNT composite and TiO2 had nearly identical spectra. Fourier-transform infrared spectroscopy (FTIR) confirms the relationships and functions of various materials (Meng et al. 2022). The stretching and bending vibrations of the surface-OH group cause the adsorption peaks at 3480 cm−1 and 1600 cm−1 (Shahnazi et al. 2020). The peak due to Ti-O and O-TiO stretching and binding modes appears around 500 cm−1. In the MWCNT/TiO2 composite spectrum, the peak at 500 cm−1 is sharper than that of pure TiO2, which could be attributed to differences in crystallinity and size (Esfe et al. 2022).
Adsorption and photocatalytic degradation studies
Approximately 85% of TC was removed during the 90-min reaction time through adsorption and photodegradation, while 73% and 54% were removed in the photolysis and adsorption systems, respectively (Fig. 5a). The major challenges of TiO2 are aggregation and agglomeration, which affect light absorption and photoactivity. Furthermore, TiO2 photoactivity at low wavelengths is limited by the rapid recombination of photogenerated electron-hole pairs, as well as its large band-gap value of 3.2 eV. Carbon nanotubes used as support materials addressed these issues, preventing electron/hole pair recombination (Fig. 5b). This allowed more charge carriers to successfully diffuse into the surface, increasing TiO2 absorption affinity towards the target contaminants while also preventing TiO2 particle aggregation and agglomeration (Wang et al. 2015).
MWCNTs can also absorb photons, become excited, and inject electrons into the TiO2 conduction band, causing highly reactive hydroxyl and superoxide radicals to form (Czech and Tyszczuk-Rotko 2018). A decrease in photoactivity was observed at higher MWCNT percentages due to the TiO2 surface being completely covered with MWCNTs. Figure 5b shows the results of the TC removal obtained by varying the amounts of MWCNTs added to the TiO2 catalyst. The prepared 1% TiO2/MWCNTs composite photocatalyst achieved 95% efficiency in TC degradation. The removal of almost half of TC occurred within the first 15 min of reaction time for the 5% TiO2/MWCNTs composite photocatalyst, and this is attributable to more adsorption taking place than photodegradation.
Effect of catalyst loading on adsorption and photocatalytic degradation
The effect of optimal loading of TiO2/MWCNT nanocomposite on photocatalytic degradation of the recalcitrant compound was investigated at TC concentration of 20 mg/L, pH value of 6.5, and irradiation time of 120 min (Fig. 6). Figure 6 shows that TC photocatalytic degradation efficiency increased as photocatalyst loading increased, with the highest removal observed at 0.1 g/L after 90 min. The increase in the number of adsorption and photocatalytic sites available on the surface of the TiO2/MWCNT nanocomposite can be attributed to this result. In a similar reaction time, there was no further improvement for loadings greater than 0.1 g/L. High photocatalyst loading can cause light scattering and consequently reduce the specific activity of the photocatalyst. Furthermore, particle agglomeration at high loading can interfere with the homogeneous-like structure of the suspension, thereby reducing the number of active sites (Wang et al. 2009).
Effect of solution pH on adsorption and photocatalytic degradation
The TC degradation was least effective at alkaline pH and more effective at neutral and acidic pH conditions (Fig. 7). At the alkaline pH of 9, the adsorption and photocatalytic degradation of TC were hampered, leading to an overall removal of 51% (Fig. 7). At the acidic pH of 4, the adsorption and degradation efficiencies were greatest (93% TC removal). The initial pH of the solution is critical in the degradation and adsorption of the target organic compounds. The pH of the surface of the photocatalyst affects its electrical charge properties and determines the ionization state (Wang et al. 2009; Yuan et al. 2022). The observed adsorption and photocatalytic behavior can be attributed to a combination of TC species speciation, TiO2/MWCNT surface charge properties, and possible complexation between TC and TiO2/MWCNTs (Ahmadi et al. 2017). The increase in adsorption and photocatalytic degradation at acidic and neutral pH can be attributed to the point-of-zero charge (PZC) of the TiO2/MWCNT nanocomposite and TiO2, which have been reported to be approximately 6.6 (Saleh and Gupta 2012) and 6.5, respectively (Wang et al. 2009).
The decrease in adsorption and degradation efficiency at pH 9 can be attributed to either TC or TiO2 changing to an anionic form, in which case repulsive electrostatic forces prevented TC sorption onto TiO2 (Ahmadi et al. 2017). Furthermore, changes in solution pH affect the formation of hydroxyl radicals on TiO2, and the type of products and reactants via the corresponding pKa values (Yuan et al. 2016). TiO2 nanoparticles have a positively charged surface in acidic pH conditions and a negatively charged surface in the alkaline pH range (pH ZPC = 6.5). Tetracycline exists as neutral (H2TC) in pH values between 3.3 and 7.68, cationic (H3TC+) in pH values less than 3.3, and anionic (HTC− and TC−2) in pH values greater than 7.68 (Liu et al. 2015). As a result of the surface charges of TC and TiO2, at a pH range of 3.3 to 7.68 TC appears in molecular form favoring its sorption, thus improving the photocatalytic process.
Adsorption and photodegradation kinetics
To obtain the heterogeneous photodegradation rate constant, the first-order kinetic (Eq. 2), second-order (Eq. 3), and modified Elovich (Eq. 4) models were used to fit the experimental data:
where Ct and C0 are the TC concentrations (mg/L) at any time t and at time 0, respectively; k1, k2, (min−1), and ke (min−1) are the degradation rate constants for the first-order, second-order, and modified Elovich models, respectively. β is a constant inversely proportional to the removal capacity (Massai et al. 2020; Mouhamadou et al. 2023).
When the three models were compared (Table 1), the second-order model simulated the experimental kinetic data the best, with R2 values of 0.979 and 0.968 at pH 4 and pH 6.5, respectively. The findings confirm that adsorption plays an important role in photodegradation and thus speeds up TC degradation by TiO2/MWCNT. The experimental data are well fit by the Elovich model (Table 1), suggesting that it might be used to explain the TC removal kinetics by the composite TiO2/MWCNT photocatalyst. When composite photocatalysts like TiO2/MWCNTs are utilized to degrade pollutants under light irradiation, the adsorption and photodegradation processes are crucial to the kinetics (Qing et al. 2022). The results support and clarify the underlying mechanism of the synergy between adsorption and degradation (Wei et al. 2021).
Modeling adsorption isotherms
To investigate the adsorption equilibrium of TC, the Langmuir and Freundlich isotherms were used (Kinniburgh 1986; Luo et al. 2023). The Langmuir model predicts a homogeneous surface by monolayer adsorption in which all binding sites have the same adsorption affinity and no interactions between adsorbate and adsorbent are considered (Repo et al. 2010; Luo et al. 2023). The mathematical expression for the Langmuir adsorption isotherm is:
where Ce (mg/L) and qe (mg/g) represent the equilibrium concentration of TC and the adsorption capacity of TiO2/MWCNTs, respectively. qm (mg/g) is the maximum adsorption capacity of TiO2/MWCNTs, while KL (L/mg) is the energy of the adsorption.
The Freundlich model assumes adsorption on a heterogeneous surface without adsorbent binding site saturation (Luo et al. 2019a, 2019b). The Freundlich model is defined as:
where Kf (mg/g) is a unit capacity coefficient and nf is the Freundlich parameter associated with the degree of system heterogeneity. The larger the nf parameter (usually greater than unity), the more heterogeneous the system is (Luo et al. 2023). The Langmuir model correlated well with the experimental data, as shown in Fig. 8 and Table 2. The Langmuir model provided a more accurate estimate of the maximum adsorption capacity for TC without compromising the quality of the fit for the prepared composite photocatalyst. The Freundlich model demonstrated that the effect of surface heterogeneity results in a stronger adsorbate-adsorbent interaction.
Mineralization and theoretical model mechanism of adsorption and photocatalytic degradation of TC
The mineralization of TC was investigated by analyzing the results of total organic carbon (TOC) and TC removal at optimal conditions as:
where TOC0 and TOCt are initial and final TOC concentrations (mg/L), respectively. It was observed that the adsorption and photocatalytic process nearly removed all the TC, but with only 75% TOC removal (Fig. 9). The differences in TC and TOC removal can be attributed to TC compounds breaking down into stable intermediates (Nezamzadeh-Ejhieh and Shirzadi 2014; Zhou et al. 2020). The intermediates are refractory to further attack by the radicals resulting in incomplete mineralization and leading to the incomplete reduction in TOC (Wang et al. 2018).
The rate-limiting processes are defined with a theoretical model in Fig. 10 to demonstrate the fundamental stages of TC removal by TiO2/MWCNTs and the relationship between the adsorption and heterogeneous degradation processes. Semiconductor photocatalysts such as TiO2 can absorb photons with ultra-bandgap energy, resulting in electron-hole pairs. According to the findings, the mechanism for the synergistic adsorption-photocatalytic degradation of TC by 1%TiO2/MWCNTs can be proposed. After UV irradiation, the TiO2/MWCNT surface (with a rate constant, ksur) rapidly adsorbs TC molecules (kads), resulting in a rapid reaction between the tetracycline (with a rate constant, kbulk) and h+ dispersed on the composite photocatalyst surface. The separation of e− and h+ is prompted by the consumption of h+. Meanwhile, the electron-hole pairs (e− and h+) react with H2O and O2 leading to the formation of ●O2− and ●OH radicals. The radicals then combine with TC molecules via redox reactions achieving effective degradation.
Material stability and reusability
Due to the high cost of catalyst synthesis, the reusability of the photocatalyst is critical from a financial perspective. The photocatalytic degradation performance of the MWCNT/TiO2 during 5 cycles was tested to assess the stability and reusability of the composite materials. At the end of each cycle, the photocatalyst was collected and used in the next cycle experiment. As illustrated in Fig. 11, the degradation rate could still exceed 90% during the first two cycles, and then, it slightly decreased in the ensuing cycles. The high TC removal of 72% after the fifth cycle indicated that the catalyst was stable and with high reusability.
Conclusion
The MWCNT/TiO2 nano-engineered composite was successfully synthesized and demonstrated higher photocatalytic activity for the degradation of recalcitrant TC than pure TiO2. The resulting 1% TiO2/MWCNT composite photocatalyst achieved 98% removal efficiency in 90 min, with high reusability, exceeding 90% in the first two cycles and 72% after the fifth cycle. The composite performed best in weak acid and neutral solutions, according to pH studies. The second-order model best described the kinetics of the integrated adsorption-heterogeneous degradation of TC by TiO2/MWCNTs; however, the modified Elovich model also fairly described the process, confirming the importance of both adsorption and photodegradation. The Langmuir isotherm, with a maximum adsorption capacity of 39.7 mg/g, provided the best fit for the experimental data. The effect of surface heterogeneity was also seen, indicating that TC adsorption was intense and multilayered. The UV/TiO2/MWCNTs effectively removed TC, but TOC removal efficiency was only 75%. Differences in TC and TOC removal are due to TC molecules decomposing into stable intermediates. A theoretical model illustrating the basic steps of TC removal by TiO2/MWCNTs and the connection between the adsorption and heterogeneous degradation processes was used to describe the rate-limiting mechanisms. This study emphasized the strong synergistic effect of the adsorption properties of MWCNTs and the photocatalytic property of TiO2 (in the composite material) under UV irradiation. Adsorption was a critical process in controlling the kinetics of recalcitrant contaminant degradation, and as the surface adsorption of TC onto the substrate increased, so did the efficiency of TC degradation. As a result, the photodegradation process improved TC removal by MWCNT/TiO2 regenerating the adsorption sites. Due to the synergy between adsorption and photodegradation, the study shows that nano-engineered photocatalysts are a promising material for effective contaminant removal from aqueous solutions.
Data Availability
All the datasets used in developing the manuscript are available on request.
References
Abou-Hadid AF, El-Behairy UA, Elmalih MM et al (2022) Production of efficient carbon fiber from different solid waste residuals for adsorption of hazardous metals from wastewater samples. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-03097-6
Ahmadi M, Motlagh HR, Jaafarzadeh N, Mostoufi A, Saeedi R, Barzegar G, Jorfi S (2017) Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J Environ Manag 186:55–63. https://doi.org/10.1016/j.jenvman.2016.09.088
Ali A, Chiang YW, Santos RM (2021) X-ray diffraction techniques for mineral characterization: a review for engineers of the fundamentals, applications, and Research Directions. https://doi.org/10.20944/preprints202112.0438.v1
Amdeha E, Salem M (2022) Facile green synthesis of ZnO supported on exfoliated graphite for photocatalytic degradation of dye under UV and visible-light irradiation. Egypt J Chem 65(132):557–569. https://doi.org/10.21608/ejchem.2022.130532.5931
Boffo R, Patalano R (2020) ESG investing: practices, progress and challenges. OECD Paris, www.oecd.org/finance/ESG-Investing-Practices-Progress-and-Challenges.pdf. Accessed Jan 2023
Brooms TJ, Otieno B, Onyango MS, Ochieng A (2018) Photocatalytic degradation of P-Cresol using TiO2/ZnO hybrid surface capped with polyaniline. J Environ Sci Health A Tox Hazard Subst Environ Eng 53(2):99–107
Cao J, Yang Z, Xiong WP, Zhou YY, Peng YR, Li X, Zhou CY, Xu R, Zhang R, Y. (2018) One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: simultaneous adsorption and photocatalysis. Chem Eng J 353:126–137
Caudillo-Flores U, Lara-Romero L, Zárate-Medina J, Muñoz-Batista MJ, Huirache-Acuña R, Rivera-Muñoz EM, Cortés JA (2016) Enhanced photocatalytic activity of MWCNT/TiO2 heterojunction photocatalysts obtained by microwave assisted synthesis. Catal Today 266:102–109. https://doi.org/10.1016/j.cattod.2015.12.005
Czech B, Tyszczuk-Rotko K (2018) Visible-light-driven photocatalytic removal of acetaminophen from water using a novel MWCNT-TiO2-SiO2 photocatalysts. Sep Purif Technol 206:343–355
Dubey M, Wadhwa S, Mathur A, Kumar R (2022) Progress in mesoporous ceria: a review on synthesis strategies and catalytic applications. Appl Surf Sci Adv 12:100340
Dutta AK, Ghorai UK, Chattopadhyay KK, Banerjee D (2018) Removal of textile dyes by carbon nanotubes: a comparison between adsorption and UV assisted photocatalysis. Phys E Low Dimens Syst Nanostruct 99:6–15
Esfe MH, Toghraie D, Alidoust S, Esfandeh S, Ardeshiri EM (2022) Laboratory study and statistical analysis of MWCNT (40%)-TiO2 (60%)/10w40 nanoparticles as potential new hybrid nano-lubricant. Colloids Surf A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2022.129078
Ji P, Zhang J, Chen F, Anpo M (2009) Study of adsorption and degradation of acid orange 7 on the surface of CeO2 under visible light irradiation. Appl Catal B Environ 85(2009):148–154
Ji W, Wang X, Ding S, Chakir T, Xu Y, Huang X, Wang H (2023) Electrospinning preparation of NYLON-6@UIO-66-NH2 fiber membrane for selective adsorption enhanced photocatalysis reduction of cr(vi) in water’. Chem Eng J 451:138973. https://doi.org/10.1016/j.cej.2022.138973
Kinniburgh DG (1986) General purpose adsorption isotherms. Environ Sci Technol 20(9):895–904
Kumar M, Jaiswal S, Sodhi KK, Shree P, Singh DK, Agrawal PK, Shukla P (2019) Antibiotics bioremediation: perspectives on its ecotoxicity and resistance. Environ Int 124:448–461
Liu Q, Zheng Y, Zhong L, Cheng X (2015) Removal of tetracycline from aqueous solution by a Fe3O4 incorporated PAN electrospun nanofiber mat. J Environ Sci (China) 28:29–36
Liu W, Zhou J, Zhou J (2019) Facile fabrication of multi-walled carbon nanotubes (MWCNTs)/α-Bi2O3 nanosheets composite with enhanced photocatalytic activity for doxycycline degradation under visible light irradiation. J Mater Sci 54(4):3294–3308
Liu Y, Kong J, Yuan J, Zhao W, Zhu X, Sun C, Xie J (2018) Enhanced photocatalytic activity over flower-like sphere Ag/Ag2CO3/BiVO4 plasmonic heterojunction photocatalyst for tetracycline degradation. Chem Eng J 331:242–254
Luo Y, Wei X, Gao B, Zou W, Zheng Y, Yang Y, Zhang Y, Tong Q, Dong L (2019b) Synergistic adsorption-photocatalysis processes of graphitic carbon nitrate (g-C3N4) for contaminant removal: Kinetics, models, and mechanisms. Chem Eng J 375:122019. https://doi.org/10.1016/j.cej.2019.122019
Luo Y, Wei X, Gao B, Zou W, Zheng Y, Yang Y, Zhang Y, Tong Q, Dong L (2019a) Synergistic adsorption-photocatalysis processes of graphitic carbon nitrate (G-C3N4) for contaminant removal: Kinetics, models, and Mechanisms. Chem Eng J 375:122019. https://doi.org/10.1016/j.cej.2019.122019
Luo Y, Zheng A, Li J, Han Y, Xue M, Zhang L, Yin Z, Xie C, Chen Z, Ji L, Hong Z, Xie X (2023) Integrated adsorption and photodegradation of tetracycline by bismuth oxycarbonate/biochar nanocomposites. Chem Eng J 457:141228. https://doi.org/10.1016/j.cej.2022.141228
Malikov E, Akperov O, Muradov M, Eyvazova G, Maharramov AL, Kukovecz A, Kónya Z (2014) Oxidation of multiwalled carbon nanotubes using different oxidation agents like nitric acid and potassium permanganate. News Baku Univ 4:49–59
Manassero A, Satuf ML, Alfano OM (2017) Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem Eng J 326:29–36
Martínez C, Canle ML, Fernández MI, Santaballa JA, Faria J (2011) Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walled carbon nanotubes–anatase composites. Appl Catal B Environ 102:563–571
Massai H, Raphael D, Sali M (2020) Adsorption of copper ions (Cu++) in aqueous solution using activated carbon and biosorbent from Indian jujube (Ziziphus mauritiana) seed hulls. Chem Sci Int J:13–24. https://doi.org/10.9734/csji/2020/v29i530177
Meng Y-J, Chen S-S, Luo C-B, Song Y-J, Xiong Z-W, Li J, Li D-Q (2022) Competitive coordination strategy for preparing TiO2/C nanocomposite with adsorption-photocatalytic synergistic effect. Appl Surf Sci 603:154395. https://doi.org/10.1016/j.apsusc.2022.154395
Mohamed WAA, Fahmy A, Helal A, Ahmed EAE, Elsayed BA, Kamoun EA, Gad EAM (2022) Degradation of local Brilliant Blue R dye in presence of polyvinylidene fluoride/MWCNTs/TiO2 as photocatalysts and plasma discharge. J Environ Chem Eng 10(1):106854
Mouhamadou S, Dalhatou S, Obada DO, Fryda L, Mahieu A, Bonnet P, Caperaa C, Kane A, Massai H, Zeghioud H (2023) Synthesis of PILIOSTIGMA reticulatum decorated TiO2 based composite and its application towards cr(vi) adsorption and bromophenol blue degradation: nonlinear kinetics, equilibrium modelling and optimisation photocatalytic parameters. J Environ Chem Eng 11(1):109273. https://doi.org/10.1016/j.jece.2023.109273
Natarajan TS, Lee JY, Bajaj HC, Jo W-K, Tayade RJ (2017) Synthesis of multiwall carbon nanotubes/TiO2 nanotube composites with enhanced photocatalytic decomposition efficiency. Catal Today 282:13–23. https://doi.org/10.1016/j.cattod.2016.03.018
Nezamzadeh-Ejhieh A, Shirzadi A (2014) Enhancement of the photocatalytic activity of ferrous oxide by doping onto the nano-clinoptilolite particles towards photodegradation of tetracycline. Chemosphere 107:136–144. https://doi.org/10.1016/j.chemosphere.2014.02.015
Otieno BO, Apollo SO, Naidoo BE, Ochieng A (2017) Photodecolorisation of melanoidins in vinasse with illuminated TiO2-ZnO/activated carbon composite. J Environ Sci Health A 52(7):1–8
Qing Y, Qing Y, Li Y, Hu D, Guo Z, Yang Y, Geng L, Li W (2022) Photocatalytic Bi2WO6/PG-C3N4-embedded in polyamide microfiltration membrane with enhanced performance in synergistic adsorption-photocatalysis of 17β-estradiol from water. J Environ Chem Eng 10(6):108648. https://doi.org/10.1016/j.jece.2022.108648
Repo E, Warchol JK, Kurniawan TA, Sillanpää MET (2010) Adsorption of Co(II) and Ni(II) by EDTA- and/or DTPA-modified chitosan: kinetic and equilibrium modeling. Chem Eng J 161(1–2):73–82
Saleh TA, Gupta VK (2012) Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide. J Colloid Interface Sci 371(1):101–106
Shahnazi A, Nabid MR, Sedghi R, Heidari B (2020) A thermosensitive molecularly imprinted poly-NIPAM coated mwcnts/TiO2 photocatalyst for the preferential removal of Pendimethalin pesticide from wastewater. J Photochem Photobiol A Chem 402:112802. https://doi.org/10.1016/j.jphotochem.2020.112802
Son HS, Lee SJ, Cho IHZ, K.D. (2004) Kinetics and mechanism of TNT degradation in TiO2 photocatalysis. Chemosphere 57:309–317
Sun M, Yan T, Tingting W, He Y, Shao Y, Wei D, Bin D (2018) Self-assembled hierarchical Sn3O4-multi-wall carbon nanotubes: facile fabrication, promoted charge separation, and enhanced photocatalytic performances. Mater Res Bull 103:104–113
Tolosana-Moranchel A, Casas JA, Carbajo J, Faraldos M, Bahamonde A (2017) Influence of TiO2 optical parameters in a slurry photocatalytic reactor: Kinetic modelling. Appl Catal B Environ 200:164–173
Wang F, Zhang YT, Xu Y, Wang X, Li S, Yang H, Liu X, Wei F (2016a) Enhanced photodegradation of rhodamine B by coupling direct solid-state Z-scheme N-K2TI4O9/G-C3N4 heterojunction with high adsorption capacity of UIO-66. J Environ Chem Eng 4(3):3364–3373. https://doi.org/10.1016/j.jece.2016.07.008
Wang H, Wang HL, Jiang WF (2009) Solar photocatalytic degradation of 2, 6-dinitro-p-cresol (DNPC) using multi-walled carbon nanotubes (MWCNTs)–TiO2 composite photocatalysts. Chemosphere 75(8):1105–1111
Wang H, Wu Y, Feng M, Tu W, Xiao T, Xiong T, Ang H, Yuan X, Chew JW (2018) Visible-light-driven removal of tetracycline antibiotics and reclamation of hydrogen energy from natural water matrices and wastewater by polymeric carbon nitride foam. Water Res 144:215–225
Wang H, Yuan X, Wu Y, Zeng G, Chen X, Leng L, Li H (2015) Synthesis and applications of novel graphitic carbon nitride/metal-organic frameworks mesoporous photocatalyst for dyes removal. Appl Catal B Environ 174–175:445–454
Wang H, Yuan X, Wu Y, Zeng G, Dong H, Chen X, Leng L, Wu Z, Peng L (2016b) In situ synthesis of In2S3 at MIL-125(Ti) core-shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl Catal B Environ 186:19–29
Wei X, Wang X, Pu Y, Liu A, Chen C, Zou W, Zheng Y, Huang J, Zhang Y, Yang Y, Naushad M, Gao B, Dong L (2021) Facile ball-milling synthesis of CEO2/G-C3N4 Z-scheme heterojunction for synergistic adsorption and photodegradation of methylene blue: Characteristics, kinetics, models, and Mechanisms. Chem Eng J 420:127719. https://doi.org/10.1016/j.cej.2020.127719
Wu Q, Yangab H, Kangab L, Gao Z, Ren F (2020) Fe-based metal-organic frameworks as Fenton-like catalysts for highly efficient degradation of tetracycline hydrochloride over a wide ph range: acceleration of Fe(II)/ Fe(III) cycle under visible light irradiation. Appl Catal B Environ 263:118282. https://doi.org/10.1016/j.apcatb.2019.118282
Xu H, Mi HY, Guan MM, Shan HY, Fei Q, Huan YF, Zhang ZQ, Feng GD (2017) Residue analysis of tetracyclines in milk by HPLC coupled with hollow fiber membranes-based dynamic liquid-liquid micro-extraction. Food Chem 232:198–202
Xue GL, Chen Q, Hills C, Tyrer M, Innocent F (2011) Synergy between surface adsorption and photocatalysis during degradation of humic acid on Tio2/activated carbon composites. J Hazard Mater 186(1):765–772. https://doi.org/10.1016/j.jhazmat.2010.11.063
Yu F, Tian F, Zou H, Ye Z, Peng C, Huang J, Zheng Y, Zhang Y, Yang Y, Wei X, Gao B (2021) ZnO/biochar nanocomposites via solvent free ball milling for enhanced adsorption and photocatalytic degradation of methylene blue. J Hazard Mater 415:125511. https://doi.org/10.1016/j.jhazmat.2021.125511
Yuan C, Hung CH, Li HW, Chang., W.H. (2016) Photodegradation of ibuprofen by TiO2 co-doping with urea and functionalized CNT irradiated with visible light – effect of doping content and pH. Chemosphere 155:471–478. https://doi.org/10.1016/j.chemosphere.2016.04.055
Yuan X, Yang J, Yao Y, Shen H, Meng Y, Xie B, Ni Z, Xia S (2022) Preparation, characterization and photodegradation mechanism of 0D/2D Cu2O/BiOCl S-scheme heterojunction for efficient photodegradation of tetracycline. Sep Purif Technol 291:120965
Zhang T, Li K, Pan JH (2019) Annealing temperature-dependent electrochemical properties of aeroxide p25 TiO2 nanoparticles as anode material for lithium storage. Prog Nat Sci Mater Int 29(6):679–684. https://doi.org/10.1016/j.pnsc.2019.11.008
Zhou J, Ma F, Guo H (2020) Adsorption behavior of tetracycline from aqueous solution on ferroferric oxide nanoparticles assisted powdered activated carbon. Chem Eng J 384:123290. https://doi.org/10.1016/j.cej.2019.123290
Zhou X, Zhou S, Ma F, Xu Y (2019) Synergistic effects and kinetics of rGO-modified TiO2 nanocomposite on adsorption and photocatalytic degradation of humic acid. J Environ Manag 235(1):293–302
Acknowledgements
The authors acknowledge the contribution of Mr Innocentia Shuro for SEM & XRD image analysis and their interpretations.
Funding
Open access funding provided by Vaal University of Technology. The research of this article was supported by the Water Research Commission (WRC, Project no. C2020/2021-00426) of South Africa and the German Academic Exchange Service (DAAD) within the framework of the climapAfrica programme with funds from the Federal Ministry of Education and Research.
Author information
Authors and Affiliations
Contributions
Kwena Yvonne Pete, John Kabuba, Benton Otieno, and Aoyi Ochieng contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by Kwena Yvonne Pete and Benton Otieno. The first draft of the manuscript was written by Kwena Yvonne Pete. John Kabuba and Aoyi Ochieng contributed to the subsequent revisions of the drafts and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
“Not applicable”; research does not report on or involve the use of any animal or human data or tissue.
Consent for publication
“Not applicable.”
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pete, K.Y., Kabuba, J., Otieno, B. et al. Modeling adsorption and photocatalytic treatment of recalcitrant contaminant on multi-walled carbon/TiO2 nanocomposite. Environ Sci Pollut Res 30, 94154–94165 (2023). https://doi.org/10.1007/s11356-023-28852-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28852-8