Abstract
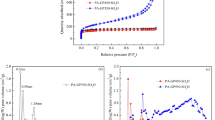
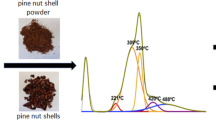
Biodiesel production from non-edible oils utilizing a highly efficient eco-friendly catalyst is a crucial necessity for replacing fossil fuels. In the present work, biochar has been applied for both energy and environmental purposes. The biochar was made by slow pyrolysis from a variety of biomass, primarily cassava peel, irul wood sawdust, and coconut shell. All biochars were used as adsorbents to remove an anionic dye (methyl orange) by conducting batch adsorption studies. The biochar made from cassava peels showed the highest dye adsorption, and it was characterized using elements analysis (CHNS), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), scanning electron microscopy (SEM), surface area analyzer (BET), total acid density, and sulfonic acid group density to successfully confirm the presence of weak (–OH) and strong (–COOH, –SO3H) acidic groups. Furthermore, for microwave-assisted biodiesel production from Millettia pinnata seed oil, the dye adsorbed biochar made from cassava peel was utilized as a Brønsted acid catalyst. The catalyst having a surface area of 4.89 m2/g, an average pore width of 108.77 nm, a total acid density of 3.2 mmol/g, and a sulfonic acid group density of 1.9 mmol/g exhibits distinctive mesoporous properties that contribute to a biodiesel yield of 91.25%. By utilizing the catalyst for three more cycles and getting a yield of more than 75%, the reusability of the catalyst was investigated.


Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428. https://doi.org/10.1016/j.biortech.2011.11.084
Chellappan S, Nair V, Sajith V, Aparna K (2018a) Synthesis, optimization and characterization of biochar based catalyst from sawdust for simultaneous esterification and transesterification. Chinese J Chem Eng 26(12):2654–2663. https://doi.org/10.1016/j.cjche.2018.02.034
Chellappan S, Nair V, Sajith V, Aparna K (2018b) Experimental validation of biochar based green Bronsted acid catalysts for simultaneous esterification and transesterification in biodiesel production. Bioresour Technol Reports 2:38–44. https://doi.org/10.1016/j.biteb.2018.04.002
Chellappan S, Aparna K, Chingakham C, Sajith V, Nair V (2019) Microwave assisted biodiesel production using a novel Brønsted acid catalyst based on nanomagnetic biocomposite. Fuel 246:268–276. https://doi.org/10.1016/j.fuel.2019.02.104
Corro G, Pal U, Tellez N (2013) Biodiesel production from Jatropha curcas crude oil using ZnO/SiO2 photocatalyst for free fatty acids esterification. Applied Catal B, Environ 129:39–47. https://doi.org/10.1016/j.apcatb.2012.09.004
Dawodu FA, Ayodele O, Xin J, Zhang S, Yan D (2014) Effective conversion of non-edible oil with high free fatty acid into biodiesel by sulphonated carbon catalyst. Appl Energy 114:819–826. https://doi.org/10.1016/j.apenergy.2013.10.004
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40. https://doi.org/10.1016/j.susmat.2016.06.002
Dehkhoda AM, Ellis N (2013) Biochar-based catalyst for simultaneous reactions of esterification and transesterification. Catalysis Today 207:86–92
Dehkhoda AM, West AH, Ellis N (2010) Biochar based solid acid catalyst for biodiesel production. Appl Catal A Gen 382:197–204. https://doi.org/10.1016/j.apcata.2010.04.051
Ergene A, Ada K, Tan S, Katircioǧlu H (2009) Removal of remazol brilliant blue R dye from aqueous solutions by adsorption onto immobilized Scenedesmus quadricauda: equilibrium and kinetic modeling studies. Desalination 249:1308–1314. https://doi.org/10.1016/j.desal.2009.06.027
Fadhil AB, Aziz AM, Al-Tamer MH (2016) Biodiesel production from Silybum marianum L. seed oil with high FFA content using sulfonated carbon catalyst for esterification and base catalyst for transesterification. Energy Convers Manag 108:255–265. https://doi.org/10.1016/j.enconman.2015.11.013
Gude V, Patil P, Martinez-Guerra E, Deng S, Nirmalakhandan N (2013) Microwave energy potential for biodiesel production. Sustain Chem Process 1:5. https://doi.org/10.1186/2043-7129-1-5
Igboke OJ, Odejobi OJ, Orimolade T, Prevatt GH, Krishnan S (2023) Composition and morphological characteristics of sulfonated coconut shell biochar and its use for corncob hydrolysis. Waste and Biomass Valorization., pp 1–17. https://doi.org/10.1007/s12649-023-02080-0
Iwuozor KO, Ighalo JO, Emenike EC, Ogunfowora LA, Igwegbe CA (2021) Adsorption of methyl orange: a review on adsorbent performance. Curr Res Green Sustain Chem 4:100179. https://doi.org/10.1016/j.crgsc.2021.100179
Konwar LJ, Boro J, Deka D (2014a) Review on latest developments in biodiesel production using carbon-based catalysts. Renew Sustain Energy Rev 29:546–564. https://doi.org/10.1016/j.rser.2013.09.003
Konwar LJ, Das R, Thakur AJ, Salminen E, Maki-Arvela P, Kumar N, Mikkola J-P, Deka D (2014b) Biodiesel production from acid oils using sulfonated carbon catalyst derived from oil-cake waste. J Mol Catal A Chem 388:167–176. https://doi.org/10.1016/j.molcata.2013.09.031
Kumar R, Kumar GR, Chandrashekar N (2011) Microwave assisted alkali-catalyzed transesterification of Pongamia pinnata seed oil for biodiesel production. Bioresour Technol 102:6617–6620. https://doi.org/10.1016/j.biortech.2011.03.024
Liu W-J, Tian K, Jiang H, Yu H-Q (2013) Facile synthesis of highly efficient and recyclable magnetic solid acid from biomass waste. Sci Rep 3:2419. https://doi.org/10.1038/srep02419
Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70:1–15. https://doi.org/10.1016/S0960-8524(99)00025-5
Motasemi F, Ani FN (2012) A review on microwave-assisted production of biodiesel. Renew Sustain Energy Rev 16:4719–4733. https://doi.org/10.1016/j.rser.2012.03.069
Nair V, Vinu R (2016) Peroxide-assisted microwave activation of pyrolysis char for adsorption of dyes from wastewater. Bioresour Technol 216:511–519. https://doi.org/10.1016/j.biortech.2016.05.070
Noiroj K, Intarapong P, Luengnaruemitchai A, Jai-In S (2009) A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renew Energy. 34(4):1145–1150. https://doi.org/10.1016/j.renene.2008.06.015
Odeyemi SO, Iwuozor KO, Emenike EC, Odeyemi OT, Adeniyi AG (2023) Valorization of waste cassava peel into biochar: an alternative to electrically-powered process. Total Environ Res Themes 6:100029. https://doi.org/10.1016/j.totert.2023.100029
Ormsby R, Kastner JR, Miller J (2012) Hemicellulose hydrolysis using solid acid catalysts generated from biochar. Catal Today 190:89–97. https://doi.org/10.1016/j.cattod.2012.02.050
Sukartono UWH, Nugroho WH, Kusuma Z (2011) Simple biochar production generated from cattle dung and coconut shell. J Basic Appl Sci Res 1:1680–1685
Thushari I, Babel S (2018) Sustainable utilization of waste palm oil and sulfonated carbon catalyst derived from coconut meal residue for biodiesel production. Bioresour Technol 248:199–203. https://doi.org/10.1016/j.biortech.2017.06.106
Wang C, Du M, Feng H, Jin H (2022) Experimental investigation on biomass gasification mechanism in supercritical water for poly-generation of hydrogen-rich gas and biochar. Fuel 319:123809. https://doi.org/10.1016/j.fuel.2022.123809
Yuan H, Yang BL, Zhu GL (2009) Synthesis of biodiesel using microwave absorption catalysts. Energy & Fuels 23(1):548–552
Yusuff AS, Thompson-Yusuff KA, Porwal J (2022) Sulfonated biochar catalyst derived from eucalyptus tree shed bark: synthesis, characterization and its evaluation in oleic acid esterification. RSC Adv 12:10237–10248. https://doi.org/10.1039/d1ra09179d
Zeng D, Liu S, Gong W, Wang G, Qiu J, Chen H (2014) Synthesis, characterization and acid catalysis of solid acid from peanut shell. Appl Catal A Gen. 69:284–289. https://doi.org/10.1016/j.apcata.2013.09.038
Zhang H, Zhou Q, Chang F, Pan H, Liu X-F, Li H, Hu D-Y, Yang S (2015) Production and fuel properties of biodiesel from Firmiana platanifolia L.f. as a potential non-food oil source. Ind Crops Prod 76:768–771. https://doi.org/10.1016/j.indcrop.2015.08.002
Zhou Z, Yao D, Li S, Xu F, Liu Y, Liu R, Chen Z (2021) Sustainable production of value-added sulfonated biochar by sulfuric acid carbonization reduction of rice husks. Environ Technol Innov 24:102025. https://doi.org/10.1016/j.eti.2021.102025
Funding
We are grateful for the financial assistance given by TEQIP-II sanctioned under NITC/TEQIP-II/R&D/2015-16, National Institute of Technology Calicut.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Suchith Chellappan. The first draft of the manuscript was written by Suchith Chellappan, Aparna Kallingal, Sajith Vandana, Vaishakh Nair, and Chingakham Chinglenthoiba and all authors commented on previous versions of the manuscript. Final review and editing were performed by Suchith Chellappan and Chingakham Chinglenthoiba. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1:
Table S1 Kinetic parameter studies for MO adsorption on cassava peel biochar (BC-CP). Table S2 Characterization of cassava peel biochar (BC-CP) and dye adsorbed catalyst (D-CP-SO3H).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chellappan, S., Kallingal, A., Vandana, S. et al. Methyl orange dye adsorbed biochar as a potential Brønsted acid catalyst for microwave-assisted biodiesel production. Environ Sci Pollut Res 30, 125158–125164 (2023). https://doi.org/10.1007/s11356-023-28269-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-28269-3