Abstract
Microwave energy based chemical synthesis has several merits and is important from both scientific and engineering standpoints. Microwaves have been applied in numerous inorganic and organic chemical syntheses; perhaps, from the time their ability to work as heat source was discovered. Recent laboratory scale microwave applications in biodiesel production proved the potential of the technology to achieve superior results over conventional techniques. Short reaction time, cleaner reaction products, and reduced separation-purification times are the key observations reported by many researchers. Energy utilization and specific energy requirements for microwave based biodiesel synthesis are reportedly better than conventional techniques. Microwaves can be very well utilized in feedstock preparation, extraction and transesterification stages of the biodiesel production process. Although microwave technology has advanced in other food, pharmaceutical and polymer chemistry related research and industry, it has yet to prove its potential in the biodiesel industry at large scale applications. This paper reviews principles and practices of microwave energy technology as applied in biodiesel feedstock preparation and processing. Analysis of laboratory scale studies, potential design and operation challenges for developing large scale biodiesel production systems are discussed in detail.

Similar content being viewed by others
Introduction
Renewable energy research is receiving increased attention in recent years. Main reasons for this evolution are energy, economic and environmental security related concerns. It is reported that the present petroleum consumption is 105 times faster than the nature can create [1] and at this rate of consumption, the world’s fossil fuel reserves will be diminished by 2050 [2]. Apart from this, the fuel consumption is expected rise by 60% or so in the next 25 years [3]. To reduce dependency on the fossil fuel sources and imports from oil-rich countries and maintain environmental sustainability, many countries have committed to renewable energy production increases and/or greenhouse gas emission reductions at national and international levels [4]. Policy amendments and changes in energy management strategies have been considered as well.
Among many renewable energy sources solar thermal and photovoltaic collectors are still not mature and are cost-prohibitive. For instance, energy conversion efficiency of the photovoltaic modules available in the market is at the maximum of 15%. Photovoltaic cells are also referred to as solar energy harvesting factories with an input to output ratios of 1:7. The return energy production rate from the photovoltaic modules is slow over 20-25 years [5]. Wind and geothermal sources have limitations such as location, availability, and intensity. Since most of the transportation and industrial sectors need liquid fuels to drive the machinery and engines, more emphasis is needed on alternative fuel sources such as biodiesel [6]. Biodiesel is composed of methyl or ethyl esters produced from vegetable oil or animal oil and has fuel properties similar to diesel fuel which renders its use as biofuel. Biodiesel offers many benefits: (a) serves as alternative to petroleum-derived fuel, which implies a lower dependence on crude oil foreign imports; (b) provides favorable energy return on energy invested; (c) reduces greenhouse emissions in line with the Kyoto Protocol agreement; (d) lowers harmful gaseous emissions; (e) biodegradable and nontoxic fuel, being beneficial for reservoirs, lakes, marine life, and other environmentally sensitive areas [7–9]. It has been realized that local biodiesel production can address challenges related to energy independence, economic prosperity, and environmental sustainability in any nation. Towards this end, the United States (US) and Europe have encouraged large scale industrial biodiesel production. For example, biodiesel production in the US has increased from 75 million gallons in 2005 to 250 million gallons in 2006 and 450 million gallons in 2007, with an expected total capacity of well over 1 billion gallons in the next few years [10, 11]. Also, the federal government has passed the energy independence and security act (EISA) in 2007 which requires a gradual increase in the production of renewable fuels to reach 36 billion gallons per year by 2022. Furthermore, 28 states have passed their own mandatory renewable energy legislation. For example, Arizona and California will replace 15% and 20% of their electricity sales with renewable energy by 2020, respectively. Texas has a mandate for 5880 MW of renewable electricity capacity by 2015. Other states have mandates to reduce greenhouse gas (GHG) emissions. For instance, Minnesota’s strategic goal is to reduce GHG emissions by 80% between 2005 and 2050 [8, 9].
Local biodiesel production holds great promise to solve the above mentioned energy and environmental related concerns; however there are two major challenges that inhibit biodiesel production: 1) cost of the feedstock; and 2) conversion process of oils to biodiesel. While using low cost feedstock and recycling waste cooking oils and animal fats can be an alternative to reduce the feedstock costs; process improvements and optimization help reduce the biodiesel conversion process costs. Biodiesel production involves two main steps: 1) extraction of oils from the feedstock, and 2) conversion (transesterification) of oils (fatty acids) to biodiesel (alkyl esters). Without these steps biodiesel production is not possible, as such, these two steps play important role and need detailed attention. Common methods employed to demonstrate these two steps simultaneously or in series include conventional heating, high pressure and temperature reactions such as thermal liquefaction and pyrolysis. These methods are employed based on the feedstock type and quality [11]. These methods are not energy-efficient and are expensive and offer scope for further improvements. Several process modification and improvements were performed both at laboratory research and industrial levels [11–13]. In this category, the effect of radiofrequency and ultrasound waves has been tested [4, 7]. Ultrasonic production has shown improvements in extraction and transesterification processes; however, the technology may require longer reaction times and larger volumes of solvents possibly with excess energy consumption compared to microwave based process [12]. Recently, microwaves have received increased attention due to their ability to complete chemical reactions in very short times. Microwaves have revolutionized the way chemical reactions can be performed with unexplainable results. This amazed the entire scientific and industrial community and resulted in “curious chemists” who applied microwaves in different areas of chemistry to benefit from these results. Few advantages with microwave processing can be listed as: rapid heating and cooling; cost savings due to energy, time and work space savings; precise and controlled processing; selective heating; volumetric and uniform heating; reduced processing time; improved quality (“reportedly”) and properties; and effects not achievable by conventional means of heating [14–20]. Microwaves have been used by many researchers around the world in many organic and inorganic syntheses at exploratory levels [14–20]. Recently, many industries have successfully implemented microwave based processes, examples include: ceramic/ceramic matrix composite sintering and powder processing, polymers and polymer-matrix composites processing, microwave plasma processing of materials, and minerals processing [14]. Microwaves have the ability to induce reactions even in solvent-free conditions offering “Green Chemistry” solutions to many environmental problems related to hazardous and toxic contaminants [19]. Due to these advantages, microwaves provide for tremendous opportunities to improve biodiesel conversion processes from different feedstock and oils. The intention of this review is to provide the basics of microwave energy applications specific to biodiesel preparation and processing, preliminary understanding and explanation of microwave effect on the chemical reactions (extraction and transesterification), update on process utilization and improvements, and information related to different process configurations and reactor designs available for biodiesel production. This review paper provides basic information related to microwave based biodiesel processing for novice researchers and those actively practicing in the biodiesel industry.
Microwave characteristics
Microwave irradiation is the electromagnetic irradiation with frequency range of 0.3-300 GHz. They lie in the electromagnetic spectrum between infrared waves and radio waves with wavelengths between 0.01 and 1 m. Commercial microwave ovens approved for domestic applications operate at a frequency of 2.45 GHz to avoid interference with telecommunication and cellular phone frequencies. Typical bands approved for industrial applications are 915 and 2450 MHz. Most of the reported microwave chemistry experiments are conducted at 2450 MHz (the corresponding wavelength is 12.24 cm) since this frequency is approved worldwide and used in currently available commercial microwave chemistry equipment. One reason is that near to this frequency, the microwave energy absorption by liquid water is maximal. Interaction of dielectric materials with microwaves leads to what is generally described as dielectric heating due to a net polarization of the substance [21–24]. There are several mechanisms which are responsible for this, including electronic, ionic, molecular (dipole), and interfacial (space-charge) polarization which will be discussed further [25].
Microwave energy
Energy associated with microwaves is lower than the energy of Brownian motion which is not strong enough to even break chemical bonds as such microwaves cannot induce chemical reactions. The influence of microwave energy on chemical or biochemical reactions is both thermal and non-thermal. The microwave energy quantum is given by the well-known equation, W = hν. Within the frequency domain of microwaves and hyper-frequencies (300 MHz - 300 GHz), the corresponding energies are 1.24 × 10-6 -1.24 × 10-3 eV, respectively. These energies are much lower than ionization energies of biological compounds (13.6 eV), of covalent bond energies such as OH- (5 eV), hydrogen bonds (2 eV), van der Waals intermolecular interactions (lower than 2 eV) and even lower than the energy associated with Brownian motion at 37°C (2.7 10-3eV) [26–28]. Microwaves, as an energy source, produce heat by their interaction with the materials at molecular level without altering the molecular structure [29, 30]. Microwave heating offers several advantages over conventional heating such as non-contact heating (reduction of overheating of material surfaces), energy transfer instead of heat transfer (penetrative radiation), reduced thermal gradients, material selective and volumetric heating, fast start-up and stopping and reverse thermal effect, i.e. heat starts from the interior of material body. In terms of biodiesel production, the resultant value could include: more effective heating, fast heating of catalysts, reduced equipment size, faster response to process heating control, faster start-up, increased production, and elimination of process steps [28].
Microwave heat transfer mechanism
Microwave heating mechanism is complex. The microwave method of heating can be illustrated as shown in Figure 1. A comparison with conventional heating method would provide a base to compare the differences in heating mechanisms and further realize the advantages associated with microwave heating.
In conventional heating as well as supercritical methods, heat transferred to the sample volume is utilized to increase the temperature of the surface of the vessel followed by the internal materials. This is also called “wall heating”. Therefore, a large portion of energy supplied through conventional energy source is lost to the environment through conduction of materials and convection currents. Heating effect in the conventional method is heterogeneous and dependent on thermal conductivity of materials, specific heat, and density which result in higher surface temperatures causing heat transfer from the outer surface to the internal sample volume as seen in Figure 2. As a result, non-uniform sample temperatures and higher thermal gradients are observed [31, 32].
Figure 2a shows the temperature profiles for a 5 mL sample of ethanol boiled at 160°C in a single mode closed vessel microwave irradiation and open vessel oil bath heating conditions. The temperature profiles show that microwave heating method allows for rapid increase of solvent temperature and quick cooling as well, whereas in conventional heating (oil bath) rate of heating and cooling are very slow. Figure 2b shows thermal behavior of microwave versus oil bath heating. Temperature gradients shown in Figure 2b suggest that microwave irradiation rises the temperature of the whole volume evenly and simultaneously whereas in oil bath heating the reaction mixture in contact with the vessel wall is heated first. Inverted thermal gradient differences can be observed between the two heating methods [33–35]. The advantages of this enabling technology have more recently also been exploited in the context of multistep total synthesis and medicinal chemistry/ drug discovery and have additionally penetrated fields such as polymer synthesis,6 material sciences, nanotechnology, and biochemical processes [36].
Materials in general can be classified into three categories based on their interaction with microwaves: (1) materials that reflect microwaves, which are bulk metals and alloys, e.g. copper; (2) materials that are transparent to microwaves, such as fused quartz, glasses made of borosilicate, ceramics, Teflon, etc.; and (3) materials that absorb microwaves which constitute the most important class of materials for microwave synthesis, e.g. aqueous solutions, polar solvent, etc. Dissipation factor (often called the loss tangent, tan δ), a ratio of the dielectric loss (loss factor) to the dielectric constant, is used to predict material’s behavior in a microwave field. The microwave absorption ability of a material is directly proportional to its dissipation factor [34].
Microwaves transfer energy into materials by dipolar polarization, ionic conduction and interfacial polarization mechanisms to cause localized and rapid superheating of the reaction materials (Figure 3). If a molecule possesses a dipole moment, when it is exposed to microwave irradiation, the dipole tries to align with the applied electric field. Since the electric field is oscillating, the dipoles constantly try to realign to follow this movement. At 2.45 GHz, molecules have time to align with the electric field but not to follow the oscillating field exactly (Figure 4). This continual reorientation of the molecules results in friction and thus heat. If a molecule is charged, then the electric field component of the microwave irradiation moves the ions back and forth through the sample while also colliding them into each other. This movement again generates heat. In addition, because the energy is interacting with the molecules at a very fast rate, the molecules do not have time to relax and the heat generated can be, for short times, much greater than the overall recorded temperature of the bulk reaction mixture. In essence, there will be instantaneous localized superheating. Thus, the bulk temperature may not be an accurate measure of the temperature at which the actual reaction is taking place. The interfacial polarization method can be considered as a combination of the conduction and dipolar polarization mechanisms. It is important for heating systems that comprise a conducting material dispersed in a non-conducting material such as metal oxides in polar solvents [25, 28, 30, 32]. Figure 4 shows the range of microwave frequency and the variations of ionic conduction and dipolar polarization with the microwave frequency [233].
Microwave role in biodiesel production
Biodiesel production technologies
Currently, commercial biodiesel production processes are based on either conventional or supercritical heating methods. Commonly used methods are: 1) Pyrolysis, 2) Micro-emulsions, 3) Dilution, and 4) Transesterification of oils to esters [37–41]. Among these methods, transesterification has proven to be the simplest and the most economical route to produce biodiesel, with physical characteristics similar to fossil diesel and little or no deposit formation when used in diesel engines. Transesterification of oils from any feedstock is to simply reduce the viscosity of the oils derived from them. Transesterification is a process in which an alcohol (methanol or ethanol) in the presence of a catalyst (acid or alkali or enzyme) is used to chemically break the molecule of the vegetable oils or animal fats into methyl or ethyl esters of the renewable fuel.
The overall transesterification process is a sequence of three consecutive and reversible reactions, in which di and monoglycerides are formed as intermediates, yielding one ester molecule in each step. The stoichiometric reaction requires 1 mole of a triglyceride and 3 moles of the alcohol. However, excess amount of alcohol is used to increase the yields of the alkyl esters by shifting the equilibrium towards the formation of esters and to allow its phase separation from the glycerol formed as a by-product. The product of transesterification process is known as “biodiesel”.
While transesterification of oils to produce biodiesel is a well-established method, there exist conversion and energy utilization inefficiencies in the process which result in the high cost of biodiesel. These are mainly associated with the heating method employed in the process. Transesterification of organic feedstock to yield biodiesel can be performed by the following methods: 1) conventional heating with acid, base catalysts and co-solvents [42–51]; 2) sub- and super-critical methanol conditions with co-solvents and without catalyst [52–57]; 3) enzymatic method using lipases [58–63]; and 4) microwave irradiation with acid, base and heterogeneous catalysts [64–67]. Among these methods, conventional heating method requires longer reaction times with higher energy inputs and losses to the ambient [66]. Super and sub-critical methanol process operates in expensive reactors at high temperatures and pressures resulting in higher energy inputs and higher production costs [53, 67–69]. The enzymatic method, though operates at much lower temperatures, requires much longer reaction times [40]. Microwave-assisted transesterification, on the other hand, is energy-efficient and quick process to produce biodiesel from different feedstocks [65, 66].
The production methods include pyrolysis, thermochemical liquefaction, supercritical reactors, oil and sand baths, and jacket type heating. Ultrasound treatment was also favored in some processes. In recent years, many researchers have tested application of microwaves in biodiesel production and optimization studies with various feedstocks. Microwave energy, a non-conventional heating method is utilized in biodiesel production in two main stages: 1) oil extraction and 2) chemical transesterification reaction. It can be beneficial to combine the above two steps to perform a single-step extractive transesterification reaction as discussed later. Biodiesel production involves mixing of appropriate ratios of oil, methanol (solvent) and catalysts as shown in Figure 5. The mixture is then processed through a microwave reactor followed by separation of products to yield biodiesel and glycerin.
Thermodynamic justification
The advantage of microwave assisted reactions clearly reflects in short reaction times by rapid heating and cooling. Perhaps, the motivation for microwave reactions was derived from the desire to reduce reaction times and produce cleaner reaction products. A very high increase in (5-1000 times) reaction rates was reported by early researchers [53, 70–73]. It is also possible to observe different product composition under microwave and conventional heating. Probable explanation for this phenomenon is that microwave heating significantly increases the reaction temperature and it is possible that the reaction temperature (due to dielectric heating) could exceed the ignition temperature for an additional reaction, which is not possible at the lower temperatures achieved by conventional heating. Many theories attempt to elaborate on the special microwave effects of heating. Since reactions involve thermodynamics of materials, fundamental thermodynamic equation (the Arrhenius equation) for reactions can be taken as a basis to explain the special microwave heating effect [74]:
From the above equation, it can be noted that there are only two possible ways to increase the rate of reaction. First, by increasing the pre-exponential factor “A” which is the molecular mobility that depends on frequency of the vibrations of molecules at the reaction interface [75]. This relates to the microwave effects of dipolar polarization and ionic conduction mechanisms explained earlier. The pre-exponential factor “A” is expressed as :
where γ = number of neighbor jump sites, λ = jump distance, and Γ = jump frequency [76].
The other way is to decrease the activation energy, ΔG, which is given in terms of enthalpy and entropy (ΔG = ΔH – TΔS). In microwave assisted reactions, entropy generation is higher due to quick and random dipolar movement and molecular level microwave interactions which increases the value of second term in the equation. The expedited superheating can also contribute to reduction in activation energy [75]. Kappe mentioned that non-thermal effects essentially result from a direct interaction of the electric field with specific molecules in the reaction medium. It has been argued that the presence of an electric field leads to orientation effects of dipolar molecules and hence changes the pre-exponential factor A or the activation energy (entropy term) in the Arrhenius equation. A similar effect should be observed for polar reaction mechanisms, where the polarity is increased going from the ground state to the transition state, thus resulting in an enhancement of reactivity by lowering the activation energy [35, 77].
Microwave effects result from material-wave intractions and due to the dipolar polarization phenomenon, the greater the polarity of a molecule (such as the solvent) the more pronounced the microwave effect when the rise in temperature is considered [77]. In terms of reactivity and kinetics, the specific effect has therefore to be considered according to the reaction mechanism and particularly with regard to how the polarity of the system is altered during the progress of the reaction. When polairty is increased during the reaction from the ground state towards the transition state, specific microwave effects can be expected for the polar mechanism. The outcome is essentially dependent on the medium and the reaction mechanism. If stabilization of the transition state (TS) is more effective than that of the ground state (GS), this results in an enhancement of reactivity by a decrease in the activation energy (Figure 6a, 55). Alteration of esterification kinetics under microwave irradation was reported by Jermolovicius et al [78].
In biodesel transesterication reactions, the solvent used mostly is methanol. Methanol is known to have high microwave absorption capacity and is an organic solvent with high polarity. It can therefore be understood that oil-methanol-catalyst involved transesterification reaction can be enhanced by microwave interactions through dipolar polarization and ionic conduction. In water containing feedstock biodiesel reactions, microwave assisted supercritical reactions can turn the water as organic solvent because water molecules possess a dipole moment. A dipole is sensitive to external electrical fields and will attempt to align itself with the field by rotation to generate local superheating (Figure 6b, 74).
Microwave based biodiesel production
Microwave applications in biodiesel production can be separated based on different feedstock types. Many reports include research on microwave-enhanced transesterification of 1) edible oils, 2) non-edible oils, and 3) oils from algae and other cellulose based renewable feedstock.
-
i)
Edible oils (first generation)
Rudolph diesel has first tested the engine by using the peanut oil and discovered that the vegetable oils can serve as engine fuels after further treatment. Edible oils commonly used as feedstock for the biodiesel production have been soybean, canola, corn, coconut, palm tree, rapeseed, rice bran, sunflower, safflower, camelina and cottonseed oils to just name a few. Among these, soybean oil is the dominant feedstock and palm tree produces highest quantity of oils per cultivated area [79, 80]. Rapeseed and sunflower oils are predominant in the European Union. Although use of vegetable oils to prepare biodiesel was well received in the early stage, soon it turned out be a food versus fuel issue. This conflict arose due to increase in vegetable oil demands and prices.
-
ii)
Non-edible oils (second generation)
Among possible alternative biodiesel feedstocks are oils of non-edible crops such as jatropha, castor, neem, karanja, rubber seed, used frying oils (waste cooking oil), animal fats, beef and sheep tallow [81]. pongamia pinnata, maize, yellow grease, poultry fat, castor, and Chinese tallow tree. While these feedstock do not conflict with food interest, they conflict with other commercial products such as cosmetics and industrial products.
-
iii)
Algae and other feedstock (third generation)
Third generation biodiesel feedstock are those that do not conflict with any food, feed or cosmetic related human consumption interests. Macro and microalgae, cyanobacteria, wastewater treatment plant activated sludge, switch grass and other microbial communities belong to this type. Among these, algae seem to be a superior feedstock and offer several advantages as follows: 1) Algae can utilize non-arable land; 2) oil content in algae is orders of magnitude higher than from other feedstocks such as corn, sugar cane, jatropha, etc.; 3) Algae need CO2 to photosynthesize and can be used to sequester CO2 from industrial sources of flue and flaring gas; 4) Algae-based fuels are carbon-neutral or even more carbon-capturing than releasing; 5) Algae can be used to remediate high-nutrient water sources such as sewage treatment plant and agricultural runoff; 6) End-products include biodiesel and/or other higher value feed (protein), pharmaceutical, and health-related products. 7) Different species of algae can be grown in polluted, saline, brackish, and freshwater; 8) Co-location of algal ponds with industrial production plants for potential recycling of CO2 and impaired waters. Algal biofuels are thus renewable, sustainable, and environmentally-benign [82–85].
Microwave-assisted oil extraction
Microwaves can be used either as a thermal pretreatment or process enhancement technique for extraction of oils and lipids from biodiesel feedstock [86]. Microwave extraction is more efficient than other conventional extraction methods in many ways. Microwaves allow for rapid and selective extraction of organic compounds with low solvent and energy consumptions [87, 88]. In conventional extraction the extractability of different components depends mainly on the solubility of the compound in the solvent, mass transfer kinetics of the product and matrix interactions [89], whereas under microwave-assisted extraction localized superheating rate plays an important role in extraction efficiency. This heating rate is influenced by factors such as microwave power level, frequency, initial temperature and design of microwave applicator, and can be selected for a particular processing application. Microwaves have been successfully applied for the extraction of natural compounds from foodstuffs like flavonoids and polyphenols compounds from tea [90] and grape seeds [91], constituents from herbals [92], pigments from paprika [93], antioxidants from rice bran [94], isoflavones from soybeans [95, 96] and also for trace analysis of organic compounds in solid and liquid samples [97–99]. Microwaves may also allow for solvent free extraction of essential oils from plant materials [100].
Selection of solvent is another important consideration in microwave extraction. Microwaves are effective on materials that have high dielectric properties, an intrinsic property of the material that requires empirical measurement but is mostly influenced by the moisture liquid/solid mixture content and spatial distribution of the water and other polar/ionic compound in the matrix. The dielectric properties of materials are defined in terms of their relative complex permittivity. For a solvent/matrix to heat up rapidly under the microwave radiation, it has to have a high dielectric constant, associated with the potential for electrical energy storage in the material, and a high dielectric loss which is related to the electrical energy dissipation in the material [101]. The heating of a dielectric material in the presence of an electromagnetic field is based on intermolecular friction that arises via ionic conduction and dipolar rotation [102]. N-hexane is widely used as solvent for extraction with other commonly used solvents such as isopropanol, methanol, ethanol, acetone and water [89, 90, 103, 104].
Extraction of lipids and oils from plant leaves and seeds depend on the microwave penetration ability. Disruptions of the oilseed cells take place when temperature of water molecule inside the cells reach the boiling point leading to high pressure gradients and rupture of cell walls, causing migration of selected compounds from sample matrix into the extraction solvent [98]. This particularity makes the technology appealing for biodiesel, as biodiesel is produced from vegetable oil. The microwave thermal effects (localized microscopic super heating) naturally match the requirements for the disruption process of tissues and could be used to induce rupture of cells for efficient extraction of oils and other components from plants. The above mechanism of extraction applies to algal cells as well. In a recent study, lipid extraction from microalgae was tested by various methods including autoclaving, bead-beading, microwaves, sonication, and a 10% NaCl solution. Microwave based extraction proved to be the most simple, easy and effective method for disruptive extraction of lipids from Botryococcus sp., Chlorella vulgaris, and Scenedesmus sp. [105].
Extraction by microwaves can be fast and simple. Kanitkar conducted microwave assisted extraction of oils from soybeans, rice bran and Chinese tallow tree seeds. About 95% of recoverable oils were extracted from these seeds by microwave extraction process in just 20 minutes which would otherwise have taken hours of processing using other solvent and mechanical extraction methods. It was observed that the enhanced extraction was due to the specific interaction of the microwave field with the solvent-feedstock matrix, where higher temperature and pressure gradients develop at the microscopic level, leading to enhanced mass transfer coefficients [106].
Extraction kinetics can be explained using the Arrhenius equation. An explanation provided by Cooney is as follows. Solvent extraction of bio-oils from biomass is a process whereby the target analyte is transferred from one phase (e.g., a solid phase in the case of dried biomass and an aqueous liquid phase in the case of wet biomass) to a second immiscible phase (e.g., an alcohol such as methanol or an alkyl halide such as chloroform). In other words, the analyte (i.e., lipid) molecule must dissolve into the solvent and form a solution. The solubility of the analyte in the solvent is governed by the Gibbs free energy of the dissolution process, which is directly related to the equilibrium constant governing the concentration of the analyte in either phase.
As more of the analyte dissolves into the solvent phase, the natural logarithm of the quotient becomes positive and the Gibbs free energy for this reaction becomes negative, indicating that the reaction has proceeded more favorably in the direction of the analyte dissolving into the solvent. As the analyte fully dissolves into the solvent phase, the quotient approaches infinity and the equilibrium lies totally to the right, and the target analyte (i.e., lipid) is considered fully extracted into the solvent phase.
The solubility of the target analyte in various solvents is governed by two independent parameters (which may, or may not, work together): the enthalpy of mixing (ΔH) and the entropy of mixing (ΔS). The solubilization of the analyte in the solvent is therefore favored when the dissolution process gives off energy (i.e., ΔH) and/or when the dissolution process increases entropy (ΔS). Since these two properties are interdependent, a favorable change in one may (or may not) offset an unfavorable change in the other. How the analyte molecule chemically interacts with the selected solvent will dictate whether the change in enthalpy is positive or negative, whether the change in entropy is positive or negative, and whether their combined sum yields a favorable Gibbs free energy of dissolution. The overall sum of these two terms is defined by the total relative contribution of all intermolecular forces that occur between the analyte and solvent molecules: Electrostatic, London forces, hydrogen bonds, and hydrophobic bonding. Consequently, the development of any solvent based extraction process must comprise a choice of solvent (or co-solvent mixture) that yields a set of chemical interactions between the analyte and solvent molecules that is more favorable than the chemical interactions between (i) the solvent molecules themselves (i.e., self association), and (ii) the analyte with the matrix it was already associated with. As a general rule analytes that strongly self associate dissolve best in strongly associated solvents, while analytes that weakly associate dissolve best in weakly associated solvents. In other words, polar solutes will dissolve in similarly polar solvents and non-polar solutes will dissolve better in similarly non-polar solvents [107].
An improved process of Soxhlet extraction assisted by microwave, called microwave-integrated Soxhlet (MIS) was tested for the extraction of oils and fats from different food matrixes such as oleaginous seeds, meat and bakery products. Results have shown that MIS parameters do not affect the composition of the extracts. For the generalization of the study with several food matrices, MIS extraction results obtained were then compared to conventional Soxhlet extraction in terms of crude extract and fatty acid composition and shown that the oils extracted by MIS were quantitatively and qualitatively similar to those obtained by conventional Soxhlet extraction. MIS labstation can be considered as a new and general alternative for the extraction of lipids by using microwave energy [108].
Microwave-enhanced transesterification
The chemical conversion of the oil to its corresponding fatty ester (biodiesel) is called transesterification. Transesterification is the process of using a monohydric alcohol in the presence of an alkali catalyst, such as sodium hydroxide (NaOH) or potassium hydroxide(KOH), to break chemically the molecule of the raw renewable oil into methyl or ethyl esters of the renewable oil with glycerol as a byproduct [109]. Microwave effect on the transesterification reaction can be two-fold: 1) enhancement of reaction by a thermal effect, and 2) evaporation of methanol due to the strong microwave interaction of the material [110, 111]. The microwave interaction with the reaction compounds (triglycerides and methanol) results in large reduction of activation energy due to increased dipolar polarization phenomenon [112]. This is achieved due to molecular level interaction of the microwaves in the reaction mixture resulting in dipolar rotation and ionic conduction [74, 96, 113]. The amount, by which the activation energy is reduced, is essentially dependent on the medium and reaction mechanism [112]. Methanol is a strong microwave absorption material and in general, the presence of an -OH group attached to a large molecule behaves as though it were anchored to an immobile raft and the more localized rotations result in localized superheating which assists the reaction to complete faster (Figure 6b) [114]. For this reason, methanol is preferred over ethanol for microwave-assisted transesterification process [115]. Comparison between three heating methods for biodiesel preparation through transesterification reaction is shown in Table 1. Supercritical conditions (high pressure and temperatures) eliminate the need for catalyst and provide for quick transesterification of oils and biomass lipids while the most commonly used conventional heating methods are slow and energy consuming.
Camelina Sativa oil as a feedstock was evaluated by Patil et al. [118, 119]. These studies included different methods of heating such as conventional, supercritical and microwave methods. Among which the microwave method proved to be superior due to inherent advantages of shorter reaction time and lower energy requirements. Microwave assisted reactions not only reduce the reaction time and increase the biodiesel yield but also reduce the product separation time significantly [66]. It was reported that the product separation in conventional heating method required 480 minutes which was around 30 minutes in microwave assisted heating method. Microwave irradiation resulted in reduction of the reaction time by about 97% and the separation time by about 94% [120]. Saifuddin and Chua [121] reported that the separation time was between 45-60 min for ethyl esters.
Continuous preparation of fatty acid ethyl esters (FAEE) from coconut, rice bran and used frying (palm) oils in a modified conventional microwave oven (800 Watts) were reported by Lertsathapornsuk et al. In a continuously mixed batch reactor system, rapid reaction rate and higher conversion yield of FAEE in the presence alkali catalyst of three vegetable oils was observed with excess amounts of alcohol. The reaction time was reduced to 30 - 60 seconds which was 30 - 60 times higher when compared with conventional and super critical methods [122, 123]. Refaat and Sheltawy reported that microwave irradiation also allows for use of high free fatty acid (FFA) containing feed stocks, including animal fats and used cooking oils, in existing transesterification processes by promoting the removal of the fatty acid. Radio frequency microwave energy further improves product recovery in the separation of the biodiesel product from alcohol and glycerin in the reaction mixture [124].
Mazzocchia et al. have shown that microwave irradiation is a fast and energy saving method compared to the conventional transesterification method for biodiesel production from different feedstocks. It was reported that microwave irradiation method prevented product degradation, when barium hydroxide was employed as a catalyst. The separation of the reaction products was quick and increased with Ba(OH)2 H2O when anhydrous and barium hydroxide is employed [125]. The total microwave irradiation power on the non-catalytic reaction indicated conversion up to 60% in 60 min of reaction in the esterification of oleic acid (C18). The effects of alcohol type (methanol or ethanol), temperature (150-225°C) and molar ratio of alcohol/fatty acid (3.5-20) on the ester yield were studied in detail [67]. To enhance the synthesis process for biodiesel from castor oil (fatty acid methyl ester, FAME), microwave absorption solid acid catalysts (H2SO4/C) were used for transesterification under microwave radiation. A maximum yield of 94% was obtained using 12:1 (MeOH to Oil ), 5 wt % catalyst , and 55 wt % H2SO4 loading amounts of catalyst at 338 K under microwave radiation after 60 min [111].
An efficient microwave-assisted transesterification (MAT) technique was developed by Zhang and co-workers to prepare biodiesel from yellow horn (Xanthoceras sorbifolia) oil with a heteropolyacid (HPA) catalyst namely Cs2.5H0.5PW12O40. A conversion yield higher than 96% was achieved by using a lower catalyst amount (1% w/w of oil) with a lower molar ratio of methanol/oil (12:1) in a relatively shorter reaction time (10 min) at 60°C [126]. The transesterification of high FFA jatropha curcas oil was carried out using microwave irradiation with homogenous catalyst. Biodiesel with 99% conversion can be achieved at 7 minutes reaction time [127]. It was studied that rapeseed oil can be converted to fatty acid butyl esters by means of microwave irradiation without using a catalyst or supercritical conditions of the alcohol [128]. The microwave assisted solvent extraction was studied effectively for Tallow tree. The major advantage of this implemented process was the reduced time of extraction required to obtain total recoverable lipids, with corresponding reduction in energy consumption costs per unit of lipid extracted [113].
Moseley and Woodman reported the energy efficiency of microwave- and conventionally heated reactors compared at meso scale for organic reactions. The results obtained from the study showed that at meso scale, microwave heating is generally more energy-efficient than conventional heating [129]. Barnard et al. developed a continuous-flow approach for the preparation of biodiesel using microwave heating. The methodology used for this process allows for the reaction to be run under atmospheric conditions and performed at flow rates of up to 7.2 L/min using a 4 L reaction vessel. This study assessed a range of different processing techniques for the scale-up of microwave-promoted reactions, taking them from the milligram to at least the multigram level for batch and continuous flow processing [130, 131]. Microwave assisted extraction and transesterification was performed using various types of feedstock ranging from edible oils to non-edible and waste frying oils. The experimental studies are summarized in Table 2[64, 106, 111, 118, 120–125, 127, 130, 132–139],[141–155].
Catalyst and alcohol-oil ratio
Among the most commonly used alkaline catalysts in the biodiesel industry are potassium hydroxide (KOH) and sodium hydroxide (NaOH) flakes which are inexpensive, easy to handle in transportation and storage, and are preferred by small producers. Alkyl oxide solutions of sodium methoxide or potassium methoxide in methanol, which are now commercially available, are the preferred catalysts for large continuous-flow production processes. However, both NaOH and KOH catalysts cause separation and purification a difficult process due to their high solubility in the both biodiesel and glycerin [109, 156, 157]. Biodiesel with the best properties was obtained using sodium hydroxide as catalyst in many studies. On the other hand, many other studies achieved best results using potassium hydroxide [120]. Refaat used 500 mL reactor at a reaction temperature of 65°C with a microwave power of 500 W controlled by microprocessor. A methanol/oil molar ratio of 6:1 was employed, and potassium hydroxide (1%) was used as a catalyst. Barium hydroxide was also used a homogeneous catalyst. the range of homogeneous catalysts applied was between 0.1 and 5% (Table 2) [132]. Slightly higher concentrations of KOH will be required compared to NaOH catalyst due to its higher molecular weight. For feedstock containing high free fatty acid content such as animal fats and used vegetable oils, KOH proved to be a better performer [120, 158]. Transesterification reaction depends on the type of oil and catalyst applied and the effects of catalysts vary with types of oils.
Although homogeneous catalysts are advantageous in terms of fast reaction rates, the drawback of this application is that the reaction products require longer separation and purification times. Use of heterogeneous catalysts can be advantageous in microwave-enhanced transesterification reactions since the catalyst can provide locations for hotspots for rapid heating. In addition, they are recyclable and reusable with acceptable performance. Patil et al. employed heterogeneous catalysts such as BaO, CaO, MgO, and SrO for transesterification of Camelina Sativa oil into biodiesel. They reported the kinetic rate constants for different catalysts. Two orders of magnitude of difference in the kinetic rate constants between the conventional heating method and microwave heating methods was reported in their study [159]. Sol gel type catalysts were also developed and tested by the same group of researchers. Heterogeneous catalysts also reportedly provide for cleaner products and easier separation of the end products. Variety of heterogeneous catalysts were tested. Few examples include: diphenylammonium salts - DPAMs (mesylates), DPABs (benzenesulfonate), DPATs (tosylate), sulfated zirconia, ZnO/ La2O2CO3, TiO2/SO4, heteropolyacids, aluminum oxides with sulfuric acid. whether reactions involving homogeneous or heterogeneous catalysts, when the reaction is carried out under microwaves, transesterification is efficiently activated, with short reaction times, and as a result, a drastic reduction in the quantity of by-products and a short separation time is obtained (> 90% reduction in separation time), and all with a reduced energy consumption [66, 136]. The rate acceleration in solid-state catalytic reactions, on exposure to microwave radiation, is attributed to high temperatures on the surface of the catalyst. The increase in the local surface temperature of the catalyst results in enhancement of the catalytic action, leading to an enhanced rate of reaction. It has been observed that when the catalyst is introduced in a solid granular form, the yield and rate of the heterogeneous oxidation, esterification and hydrolysis reactions increases with microwave heating, compared to conventional heating under the same conditions [160]. Solid base catalysts are more efficient than solid-acid catalysts. The advantage with the solid catalysts is that they are not sensitive to the presence of water in the reactants [25]. Breccia et al. reported on the use of a domestic microwave apparatus for the synthesis of biodiesel by reaction between methanol and commercial seed oils [161]. In this work, they found that the reaction was complete in less than 2 min under microwave irradiation. Activities of several catalysts such as sodium methylate, sodium hydroxide, sodium carbonate, sulfuric acid, benzensulfonic acid and boron carbide were also briefly discussed in their study.
The transesterification reaction is governed by the amount and type of alcohol participating in the reaction. Considering the type of the alcohol, the use of methanol is advantageous as it allows the simultaneous separation of glycerol. The same reaction using ethanol is more complicated as it requires a water-free alcohol, as well as an oil with a low water content, in order to obtain glycerol separation [162]. Methanol is the most commonly used reactant both in conventional and microwave assisted transesterification reactions. Ethanol is more sensitive to the presence of moisture content in the oil causing soap formation and has less dielectric constant compared to methanol. Ethanolysis proceeds at a slower rate than methanolysis because of the higher reactivity of the methoxide anion in comparison to ethoxide. As the length of the carbon chain of the alkoxide anion increases, a corresponding decrease in nucleophilicity occurs, resulting in a reduction in the reactivity of ethoxide in comparison to methoxide [163]. An example of this phenomenon is the transesterification (at 25°C) of canola oil with a 1:1 mixture of ethanol and methanol (to provide an overall molar ratio of alcohol to oil of 6:1) that results in 50% more methyl than ethyl esters [164, 165]. Therefore, for microwave assisted reactions, it is more favorable to use methanol as a solvent. On the other hand, ethanol has environmental acceptance due to its environmental friendly production from biomass. Since the transesterification reaction is an equilibrium reaction, excess amounts of alcohols need to be added to drive the reaction to completion within reasonable time. Alcohol-oil ratios of wide ranges (30:1) have been tested by many researchers with most common ratio being 9:1.
Direct extractive-transesterification of microalgae
In certain applications, it can be advantageous to perform extraction and transesterification reactions simultaneously. Biodiesel production from microalgae requires extraction of oils and lipids from the cellular mass prior to their transesterification. Microwaves can be used as efficient medium to perform these two tasks simultaneously. Algal biodiesel production essentially involves the following steps (Figure 7): 1) genetic development, 2) cultivation, 3) harvesting, 4) processing, and 5) separation of products [166–168]. Microwaves can be utilized in processing stage of the process i.e. for extraction and transesterification of oils.
High lipid yielding microalgae are cultivated and grown either in open or closed raceway ponds or in photobioreactors. Photobioreactors are designed to maximize the lipid yield and to minimize contamination and to improve the efficiency of the process. Algae are harvested by coagulation, flocculation, sedimentation and filtration methods followed by extraction and transesterification steps. The algal culture is usually concentrated to 15-20% by volume from its original concentration of 0.02-05% concentration in the cultivation ponds. One can notice that all of the above steps require large quantities of energy.
There are three well-known methods to extract the oil from algae: (1) mechanical expeller/press, (2) solvent extraction with hexane, and (3) supercritical fluid extraction. A simple process is to use a press to extract a large percentage (70–75%) of the oils out of algae. However, this method requires large volumes of samples. Algal oil can be extracted using chemicals. The most popular chemical for solvent extraction is hexane, which is relatively inexpensive. To be successful, any extracting solvent must be able to (1) penetrate through the matrix enclosing the lipid material, (2) physically contact the lipid material, and (3) solvate the lipid. As such the development of any extraction process must also account for the fact that the tissue structure and cell walls may present formidable barriers to solvent access. This generally requires that the native structure of the biomass must be disrupted prior to extraction [169]. Supercritical fluid extraction is far more efficient than traditional solvent separation methods. Supercritical fluids are selective, thus providing the high purity and product concentrations. This can extract almost 100% of the oils all by itself. In the supercritical fluid carbon dioxide (CO2) extraction, CO2 is liquefied under pressure and heated to the point that it has the properties of both a liquid and gas. This liquefied fluid then acts as the solvent in extracting the oil [170].
In general, two basic mechanisms by which extraction of a lipid can possibly occur: (1) diffusion of lipids across the cell wall, if the algal biomass is suspended in the solvent with higher selectivity and solubility (or large partition coefficient) for lipids and (2) disruption of the cell wall with release of cell contents in the solvent. The relative contribution of each of these mechanisms depends on the extraction technique. It could be easily perceived that diffusive mechanism will have less efficiency (in terms of long extraction time and smaller yield of lipid) due to the slow diffusion of lipid molecules across the cell wall. On the other hand, a disruptive mechanism is likely to cause faster extraction of lipids with high yields, as it involves the direct release of the lipid droplets in cytoplasm in to the bulk liquid with rupture of cell wall [171]. Diffusive mechanism is more predominant in extraction methods such as solvent extraction, soxhlet extraction and others. Disruptive mechanism refers to mechanical breakdown of the cell as in mechanical pressing and supercritical high pressure and high temperature treatment. However, it has been reported that mechanical pressing is inefficient method of extraction for algal biomass due to their rigid wall structure.
Even though dried plant material is used for extraction in most cases, but still plant cells contain minute microscopic traces of moisture that serves as the target for microwave heating. The moisture when heated up inside the plant cell due to microwave effect, evaporates and generates tremendous pressure on the cell wall due to swelling of the plant cell. The pressure pushes the cell wall from inside, stretching and ultimately rupturing it, which facilitates leaching out of the active constituents from the ruptures cells to the surrounding solvent thus improving the yield of phytoconstituents. This phenomenon can even be more intensified if the plant matrix is impregnated with solvents with higher heating efficiency under microwave (higher tan value) [172]. A microwave assisted straining method was developed to determine the amount of neutral lipids in microalgae. The microwave pretreatment and straining process only took 50 and 60 seconds respectively. Microwaves are suitable for this application since the conventional fluorescence method is unsuccessful in algae with thick, rigid cell walls [173].
Supercritical conditions can be applied in direct extractive-transesterification of vegetable oils and algal oils. Water at supercritical conditions can act as organic solvent and thus eliminating the need for solvent use. many studies have focused on this method to extract and transesterify bio-oils from different feedstock. The process operates at high temperatures and high pressures close to sub and supercritical conditions of water or solvent. In these studies, it was observed that higher temperatures favored extraction and transesterification process, however, at certain temperatures decomposition of biomass was inevitable [174–184]. Apart from it, safety of pressurized vessels is another concern. Advantages of this process are high quality extracts and end products which require easy separation [107].
Direct transesterification of freeze-dried microalgae in various solvents and using various catalysts was conducted by Cooney and co-workers under various experimental conditions. A 100% conversion of lipids (triglycerides) to FAMEs was observed. The same group has also executed this reaction in a novel ionic liquid based co-solvent that replaces the organic (i.e., chloroform) of the Bligh and Dyer co-solvent system with a hydrophilic ionic liquid (e.g., 1-ethyl-3-methylimidazolium methyl sulfate). It is proposed that the methanol facilitates the permeabilization of the cell wall and intracellular extraction of the lipids, while the ionic liquid facilitates the auto partitioning of the lipids to a separate immiscible phase [107, 167, 185]. Johnson and Wen have attempted extraction and transesterification of oils from Schizochytrium limacinum, heterotrophic microalga. They conducted their experiments by two methods: 1) oil extraction followed by transesterification (a two-stage method) or direction transesterification of algal biomass (a one-stage method). When freeze-dried biomass was used as feedstock, the two-stage method resulted in 57% of crude biodiesel yield (based on algal biomass) with a fatty acid methyl ester (FAME) content of 66.37%. The one-stage method (with chloroform, hexane, or petroleum ether used in transesterification) led to a high yield of crude biodiesel, whereas only chloroform-based transesterification led to a high FAME content. When wet biomass was used as feedstock, the one-stage method resulted in a much-lower biodiesel yield. The biodiesel prepared via the direct transesterification of dry biomass has met the ASTM standards. Different schemes using different solvents for one stage and two stage methods were also presented [186].
Aresta et al. conducted thermochemical liquefaction using wet algal biomass and supercritical CO2 extraction using dry algal biomass for direct transterification of bio-oils. Both of the processes seem to be energy intensive by the reaction conditions they reported (thermochemical liquefaction conditions: 250–395°C for 1 h and supercritical CO2 extraction conditions: 50°C, 2.60 MPa for 7 h). The two technologies resulted in different extraction capacities; the extraction with sc-CO2 allows to obtain a higher amount of long chain FA, while the liquefaction gives a higher amount of oily material. Also, the isolated yield of poly-unsaturated species (18.2, 20:4, 20:5) is higher with the sc-CO2 extraction compared to thermochemical liquefaction. Thermochemical liquefaction requires temperature around 350 and 395°C in order to have the optimal amount of extracted oil. However, as explained earlier, its composition depends on the working temperature and the content of long chain FA is higher at lower temperature as decomposition may occur at higher temperatures. Between these two technologies, the thermochemical liquefaction seems to be more efficient than the extraction with sc-CO2 from the quantitative point of view (as expected) but decomposition of the FA may occur under the operative conditions [187].
Prof. Deng’s research group has demonstrated simultaneous extraction and transesterification (in situ transesterification) of the wet algal biomass in supercritical methanol conditions [188]. In a microwave-assisted extraction and transesterification process, as it has been demonstrated in many organic and biodiesel synthesis studies, it is anticipated that the reaction can be conducted at atmospheric pressures and temperatures merely close to the boiling point of methanol with much shorter reaction time [64, 66, 118, 130, 136, 150]. The same group also performed direct extractive-transesterification of dry algal biomass and optimized process parameters using microwave heat source. Response surface methodology (RSM) was used as an optimization technique to analyze the influence of the process variables (dry algae to methanol (wt/vol) ratio, catalyst concentration, and reaction time) on the fatty acid methyl ester conversion. From experimental results and RSM analysis, they reported the optimal conditions as: dry algae to methanol (wt/vol) ratio of around 1:12, catalyst concentration about 2 wt.%, and reaction time of 4 min. The algal biodiesel samples were analyzed by GC–MS and thin layer chromatography (TLC) methods. Transmission electron microscopy (TEM) images of the algal biomass samples before and after the extraction/transesterification reaction were also presented which are shown in Figure 8[188–190].
Koberg and co-workers at Bar-Ilan University (Israel), together with their industrial research collaborators have demonstrated the direct production (extraction and transesterifcation) of biodiesel from Nannochloropsis. The marine algae was cultivated using carbon dioxide liberated from industrial flue gas emissions (coal burning power station). direct conversion of algal oil into biodiesel in a single step or by following two steps was conducted. two new innovative heating methods, namely, microwave irradiation and ultrasonication were used. These two techniques were compared to identify the most effective bio-diesel production method. Based on their studies, it was concluded that the microwave oven method was the most simple and efficient method for the one-stage direct transesterification of the as-harvested Nannochloropsis algae [191].
Microwave based biodiesel properties
The use of 100% pure vegetable or animal fats to power diesel engines is not permissible due to several drawbacks such as high fuel viscosity, low power output, thickening or gelling of the lubricating oil, oxidative stability, and low volatility resulting in carbon deposits by incomplete combustion. When biodiesel is used in its 100% purity, it is referred to as B100 or “neat” fuel. Blended biodiesel means pure biodiesel is blended with petrodiesel. Biodiesel blends are referred to as BXX. The XX indicates the amount of biodiesel in the blend (i.e., a B80 blend is 80% biodiesel and 20% petrodiesel) [44]. Commercially, these blends are named as B5, B20 or B100 to represent the volume percentage of biodiesel component in the blend with petro diesel as 5, 20 and 100 vol.%, respectively. Biodiesel obtained by microwave heating process very well compares with that obtained by other conventional methods of production. A summary of 1st generation, second generation biodiesel properties obtained by microwave processing are shown in Table 3[126, 140, 141, 144, 192–194]. Also shown in Table 3 are fuel properties of algal biodiesel from a conventional process for a comparison.
Energy needs
Energy scenario of biodiesel production
A viable alternative fuel as a substitute to fossil fuel (ex: biodiesel) will not only provide comparable or superior environmental performance but also will result in an energy gain in the overall process [195, 196]. For instance, among current food-based biofuels, biodiesel provides 93% more usable energy than the fossil energy needed for its production, reduces greenhouse gas emissions by 41% compared with diesel, reduces several major air pollutants, and has minimal impact on human and environmental health through N, P, and pesticide release. Sustainability of biodiesel production can be evaluated by a new concept called “Net energy balance” ratio. Net energy balance simply means the ratio of energy derived from the renewable feedstock (energy-out) to the energy invested (energy-in) in the process. The following expression can be used to represent the net energy balance (NEB) ratio [168]. The overall savings in energy and greenhouse gas emissions over the lifecycle of the biofuel may be less than anticipated; for example for biodiesel from oilseed rape and soya the input of energy required over the life-cycle is_50% of the energy contained in the fuel (Scott 2010).
Since the energy invested in the biodiesel production (energy required for farming, harvesting, processing, transport, etc) is derived from non-renewable energy sources such as fossil fuels, the net energy balance can also be written as follows [81]:
Sheehan reported that the fossil energy ratio of biodiesel is equal to 3.2. In other words, biodiesel yields 3.2 units of energy for every unit of fossil energy consumed over its life cycle. In comparison, it was found that petroleum diesel’s life cycle yielded only about 0.84 units of energy per unit of fossil energy consumed [197]. Few other studies have reported similar results as in [198, 199]. It may be more appealing and sustainably acceptable alternative if renewable energy sources can be utilized to produce biodiesel. this means the fossil energy input can be replaced by other renewable sources such as solar thermal, photovoltaic, geothermal and wind energy. the substitution can be in part or as a whole wherever applicable.
A life cycle analysis of microalgal biomass production was conducted between open raceway ponds and tubular photobioreactors [200]. The net energy ratio for the photobioreactor reactor proved to be a negative value considering energy requirements in its construction and material production. Net energy ratio depends on many factors such as the cultivation, harvesting, production and processing methods and can vary from each process [201]. For instance, the US DOE reported in the algal biodiesel production roadmap as follows: The energy content of most algae cells is of the order of 5 watt-hours/gram if the energy content of lipids, carbohydrates, and proteins and the typical percentage of each in algae are considered [202]. It is possible to estimate the energy requirements in watt-hours/gram of algae for harvesting, de-watering, and drying as a function of the volume percentage of algae in harvested biomass. The energy requirements for flocculation and sedimentation and the belt filter press are expected to be minimal. However, based on the latent heat of vaporization of water at 0.54 watt-hours/gram, energy balance can become an issue in systems that propose to take algal biomass and concentrate / dry it to enable downstream processing and extraction because of the high volumes of water that must be evaporated away. In spite of gaps in data precluding more detailed analyses, algal biofuel production schemes at scale will likely need to implement innovative technologies and integrated systems in order to overcome this challenge. Possible approaches may include developing strains of algae with much higher energy content than available today, along with innovative solutions to lower the energy intensity of harvesting and drying algae [168, 203, 204].
Microwave energy efficiency and requirements
Energy generation efficiency of microwaves from electrical energy is in the range of 50-65%. This means 35-50% of electrical energy is not converted into microwave energy. Again, in chemical reactions, it is an assumption that all of the microwave energy has been absorbed by the materials participating in reaction. Although microwaves have shown to increase reaction rates by 1000 times in particular chemical synthesis, the downside of it is that the energy generation process is not competitive with conventional steam based production plants with energy conversion efficiencies in the range of 65% - 90% (Electricity to steam conversion - 90%; fossil fuel to steam - 65%) [129].
The energy efficiency of a microwave assisted reaction can be calculated using the following equations. Eq. 1 represents the heat energy supplied by the microwaves which is given in terms of the power dissipation and the time of exposure. The power dissipation level of the microwave device is usually reported by the manufacturer. Eq. 4 quantifies the thermal effect caused by the microwave radiation in the sample volume (i = reactant; ex: oil, catalyst, and solvent) which is simply the product of the mass of the sample multiplied the specific heat of the material and the temperature gain during the reaction. Energy efficiency of the microwave energy is the ratio of the observed resultant temperature effect to the total energy supplied to the sample as in Eq. 3 [102].
The energy efficiency of the microwave assisted reactions depends on several factors such as the sample volume, nature of the medium (solvents), dissipation level of the microwave device and the penetration depth of the microwaves required in the reaction sample volume. Poor efficiencies can be observed when a high power microwave device is used for a very small sample volume. It is very important to consider the effective level of power dissipation in microwave assisted chemical synthesis to eliminate the energy losses to the surroundings. Patil et al found that transesterification of the Camelina Sativa oil was even successful at reduced microwave power levels using domestic microwave unit. This observation suggests that effective utilization of microwave power can lead to process energy savings [118].
Energy calculations for microwave based process need to consider the actual microwave power applied into the process. Leadbeater conducted batch and continuous flow microwave experiments using 4.6 L batch vessel and flow rates 2 L/min, 7.2 L/min. Energy consumption rates reported from this study are comparable to energy consumption by conventional method. Process energy requirements were calculated based on both actual power consumed and actual microwave power delivered (65% of the power setting) by the system. Overall conversion (oil to FAMEs) rates of 97.9 and 98.9% were reported for these tests. For instance, considering preliminary analysis for 2 L continuous flow conditions, the initial assumption was that the microwave unit would operate at an average of 66% of maximum power (1100 W microwave input; power consumed 2600 W) as observed when the reaction was performed. On the basis of this, energy consumption would be 60.3 kJ/L of biodiesel prepared. If the microwave was operating at full power (1600 W; power consumed 2600 W), energy consumption would be 92.3 kJ/L of biodiesel prepared. For a batch process, calculations were based on the process to heat a 4.6 L reaction mixture to the target temperature of 50°C which takes 3.5 min using a microwave power of 1300 W. With a hold time of 1 min at 50°C, a total reaction time of 4.5 min is given. Assuming that the microwave power remains constant at 1300 W throughout the process, the energy consumption would be 90.1 kJ/L of biodiesel prepared. In reality, the power drops once the target temperature is reached. Thus, this is an overestimation of energy consumption [110].
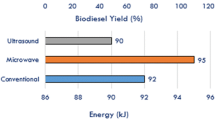
While few other studies attempted to report the energy efficiency and requirements for the microwave based biodiesel production, they are based on some rudimentary assumptions and calculations [133]. Some energy requirements are based on milliliter volumes without a measure of scale in laboratory studies [205]. This is one of the most serious drawbacks for the microwave based biodiesel process. A pilot scale demonstration study at a biodiesel production capacity of 1 ton/d may provide an estimate of actual energy requirements of the process. Results compiled from recent studies are shown in Table 4. Chand et al conducted biodiesel conversion process using ultrasonication method. They estimated an energy consumption of 91-100 kJ/L for the transesterification process with total energy requirements around 137.5 kJ/L. Their estimates at a large scale level are comparable to the conventional method [206].
Current status and potential for large scale industrial application
General microwave reactor concerns
One of the main limitations of the microwave technology reported by many experts is its inability to penetrate through large sample volumes. This limitation challenges the scalability of microwave applications from laboratory small-scale synthesis (millimolar level) to industrial multikilogram production (kmolar level). The replacement of conventional processes by microwave has several limitations. Measurement and control of temperature are difficult and temperature distribution is non-uniform in large batch reactors, it may indeed simulate thermal currents similar to conventional heating. Microwaves generally have a few centimeters depth of penetration capacity into the absorbing materials depending on their dielectric properties. As such, in large batch type reactors, the microwave power density varies greatly from outside surface to inside sample material. Therefore, materials in the center of the reaction vessel are heated only by convection and not by microwave dielectric heating. When trying to heat large quantities of materials, additional problems arise. As the volume of the mixture increases, the energy required for heating it also increases and higher radiation intensity is needed. Safety of the pressurized vessel with large quantities of batch operation needs to be considered as well.
The dissipation factor or penetration capacity of the microwave radiation depends on the factors such as ion concentration, the ions size, the dielectric constant and viscosity of the reacting medium and the microwave frequency. The dissipation factor of water and most organic solvents decreases with increasing temperature, i.e. the absorption of microwave radiation in water decreases at higher temperatures. In turn, the penetration depth of microwaves increases [102]. For some heterogeneous reactions, the microwaves may not be able to penetrate through large sample volumes. An important characteristic of microwave heating is the phenomenon of ‘hotspot’ formation, whereby regions of very high temperature form due to non-uniform heating [207]. This thermal instability arises because of the non-linear dependence of the electromagnetic and thermal properties of the material on temperature [208]. The formation of standing waves within the microwave cavity results in some regions being exposed to higher energy than others. This results in an increased rate of heating in these higher energy areas due to the non-linear dependence. Cavity design is an important factor in the control, or the utilization of this hotspot phenomenon. Considering high production flow rates, it is beneficial to design the reactor in a fashion that simulates the plug flow reactor. In this case, the sample volume exposed to microwave field can be sized to the power dissipation capacity of microwave heat source. Plug flow reactors or small quantities of batch reactions in a continuous chain type operation mode can be designed to enhance the utilization of microwave energy [209–211].
Microwave reactor design
Microwave ovens operating at 2450 MHz are common appliances in the households of USA and around the world. Hundreds of 2450 and 915 MHz systems between 10 to 200 kW heating capacities are used in the food industry for precooking bacons (e.g., used in Subways restaurants), tempering deep frozen meats when making meat patties, and precooking many other foods products. When evaluating an extraction process it is important to consider the various factors affecting it during scale up to commercial operations. In microwave processing this usually means a change in frequency from 2450 MHz to 915 MHz. Microwaves at 915 MHz (used industrially) have much higher penetration depths into the material as compared to the higher frequency of 2450 MHz commonly used in laboratory sized equipment. The higher penetration depths allow for much larger diameter tubes and processing flow rates, and microwave generators can be built for significantly higher power and efficiencies when compared to smaller generators.
Proper application of microwave energy may result in greater benefits in terms of energy efficiency and reaction product quality. Understanding the characteristics of the reactants and nature of the reactions desired is critical in many applications. In certain polymerization reactions where the reaction temperatures change with nature of the reactions (endothermic versus exothermic), a better control of the microwave power dissipation is desired. In these applications, pulsed type microwave heating rather than continuous heating might result in improved energy efficiencies without affecting the quality of the reaction products due to too high or too low reaction temperatures [31]. In some applications, the organic chemicals under study may not have the capacity to absorb the microwave energy. In such cases, it is beneficial to introduce materials that have strong microwave absorption capability. This helps initiate the desired chemical reactions using organic chemicals. Here, the materials introduced whether it is a solvent or metal particle acts both as a chemical catalyst as well as an energy converter. Also, by using a proper microwave pulse train, it is further possible to control the desired selectivity in the products formed.
Microwave reactors can be designed to function in two different modes: multimode and monomode (also referred to as single-mode) reactors. In multimode reactor instruments (which is similar to a domestic oven in concept), the microwaves that enter the cavity are reflected by the walls and the load over the typically large cavity. In most instruments a mode stirrer ensures that the field distribution is as homogeneous as possible. In the much smaller monomode cavities, the electromagnetic irradiation is directed through an accurately designed rectangular or circular wave guide onto the reaction vessel mounted at a fixed distance from the radiation source, thus creating a standing wave. The key difference between the two types of reactor systems is that in multimode cavities several reaction vessels can be irradiated simultaneously in multi vessel rotors (parallel synthesis), in monomode systems only one vessel can be irradiated at any time. In the latter case high throughput can be achieved by integrated robotics that move individual reaction vessels in and out of the microwave cavity [133].
Most instrument companies offer a variety of diverse reactor platforms with different degrees of sophistication with respect to automation, database capabilities, safety features, temperature and pressure monitoring, and vessel design. Importantly, single-mode reactors processing comparatively small volumes also have a built-in cooling feature that allows for rapid cooling of the reaction mixture with compressed air after completion of the irradiation period (see Figure 2). The dedicated single-mode instruments available today can process volumes ranging from 0.2 to about 50 mL under sealed vessel conditions (250°C, ca. 20 bar), and somewhat higher volumes (ca. 150 mL) under open-vessel reflux conditions. In the much larger multimode instruments several liters can be processed under both open- and closed-vessel conditions. Continuous-flow reactors are currently available for both single- and multimode cavities that allow the preparation of kilograms of materials by using microwave technology.
For extraction, two basic designs of microwave reactors are available. The first one is a scientific/industrial/ laboratory level multimode cavity which in principle is similar to the domestic microwave unit. In multimode, microwaves reflect of the walls and generate a standing wave pattern in which waves intersect at specific points in the cavity. The second mode use a focusing concentrated at one waveguide in which waves are reflected at specific location [212, 213]. In comparison, the design with a single mode applicator (as appeared to multimode commonly used in household microwaves) focuses the microwaves in the center of the applicator, where the material flows in a processing tube. This resonance mode allows for very high electric field values which increase the heating rate. This focusing creates an electrical field distribution with the highest values in the center of the applicator tube and decreasing as it nears the walls of the tube. Therefore, if the flow in the tube is laminar, the fluid with highest velocity in the center receives the highest amount of microwave energy. The fluid with the lowest velocity near the wall receives lower amounts of energy, therefore creating a more uniform temperature distribution when exiting the microwave applicator [214, 215]. While this difference in electric field distribution may not play a significant role in small diameter tubes, when scaling up to higher flow rates and consequently larger diameter tubes, temperature uniformity becomes more important. Continuous processes using a 5 kW, 915 MHz microwave have been successfully applied so far for beverage and vegetable purees sterilizations, for aseptic processing and for ballast water treatments [133, 212].
Batch operations are undoubtedly energy efficient when conducted at micromole or millimole level scale. Large volumes of batch reactors may not be energy efficient with microwaves and a continuous flow process is more favorable. In a continuous flow, the mixture is continuously pumped and heated in a microwave cavity. This process is more complex due to the addition of momentum transfer to the heat generation from microwave heat transfer in the solvent/solid matrix, mass transfer through the solid/solvent [98]. The drawbacks of a continuous-flow microwave apparatus are that it can be difficult to process solids, highly viscous liquids, or heterogeneous reaction mixtures. Also, adaptation of conditions from simple small scale reactions to the continuous-flow cell could end up being time-consuming [131]. Continuous flow systems will allow for large-scale production with reduced costs. A continuous flow system was tested by Groisman for tranesterification of canola and sunflower oils. A very high FAMEs yield of 92% and 89% were obtained for both the oils respectively. for comparison, a batch reaction with a volume of 500 mL of oil was conducted and this test resulted in 64% yield. Again, the batch test with a one-tenth volume (50 mL) of the oil resulted in 97% yield. This confirmed the inability of the microwaves to influence large scale reactions [32].
Microwave based continuous flow biodiesel production has not been developed to date. Many other industries, including food, rubber, ceramics, and mining, successfully use microwave heating on a large scale, often using different frequencies that can increase penetration depths through solvents and solids. The option to change the frequency may provide an alternative solution to the problem of microwave scale-up. However, these applications do not require the controlled heating of delicate organic molecules while suspended in relatively low boiling and often flammable solvents (the context for pharmaceutical chemistry) [129, 216].
Microwave reactors are manufactured and marketed by Anton Paar, Biotage, CEM, Milestone and few other vendors. Different types of reactors used in microwave based organic chemical synthesis are as follows: Anton Paar (synthos 3000, with XQ80); Biotage (Advancer); CEM (voyager, MARS-open, MARS-a/c); Milestone (flow SYNTH, microSYNTH-open, microSYNTH-a/c, autoclave). The functional, operational and process parametric data are provided in Tables 5 and 6. These tables were reproduced from the work presented by Moseley et al., and Bowman et al. For more information on these reactor specifications and performances, readers are referred to these references [131, 216]. Bowman and co-researchers have worked on different types of reactors in batch and continuous flow based configurations with the objective of identifying the concerns for scale-up needs as well as to compare their performances. For batch technologies, they found that open reactor vessels offer operational advantages while still giving good yields of desired products. In cases where volatile or toxic reagents are used, closed vessel reactors are better. For continuous flow processing, they suggest that homogeneity of the reaction mixture is key. When the mixture is homogeneous, it is possible to move from small scale sealed-vessel conditions to the continuous-flow apparatus without any modification of reaction conditions or loss in product yield. When either the starting materials or the product mixture contains particulate matter, continuous processing can prove a challenge, but reoptimization of reaction conditions as well as reduction of the concentration may allow these difficulties to be overcome [131, 216]. For biodiesel applications, assuming the reactants are well mixed and homogeneous, a focused continuous flow microwave reactor or serpentine plug-type reactor or helical reactor may be considered to monitor and control the reactants temperature and detention times as shown in Figure 9[133, 217, 218]. this type of reactor was used in solvent-free organic reactions and extraction of polycyclic aromatic hydrocarbons from sediment, soil, and air samples.
Microwaves for supercritical process
Microwave reactors that operate at supercritical conditions (high temperatures and pressures) are available for laboratory as well as commercial uses. In a microwave transparent pressurized vessel, batch heating of reactants can be extremely rapid. Usually these applicators involve pressures from 2–3 to 8–10MPa and temperatures from 150 to 250°C. They are used for hydrothermal and solvothermal preparation, staining, sterilization and so on. The combination of microwave and vapour pressure have been already applied successfully to polymers-composite reticulation in large (7500 l) multi-mode cavities [128, 219].
C18 fatty acids (Oleic acid) esterification at supercritical conditions (elevated pressures and temperatures) without catalyst was performed recently. The operating temperature was 150 -225°C and the pressure was 20 bar. Methanol and ethanol were used as solvents in this non-catalytic reaction. A microwave batch reactor (synthos 3000, Anton-Paar) equipped with two magnetrons, 1400 W of continuous microwave power at 2.45 GHz, a rotor system in which 8 quartz vessels with 80 mL of capacity can be inserted at one time, and a magnetic stirrer for agitation of the sample in each vessel (up to 600 rpm) was used. The equipment is projected to operate up to 300°C and 80 bar. The non-catalytic esterification of the oleic acid resulted in 60% conversion in 60 minutes which is similar to conventional heating [67]. A microwave based high pressure thermo-chemical conversion of sewage sludge as an alternative to incineration was performed by Bohlmann [128, 220]. A maximum oil yield of 30.7% with a heating value of 36.4 MJ/kg was achieved in this study. Microwave-assisted catalyst-free transesterification of triglycerides with 1-butanol under supercritical conditions was conducted by Geuens et al [128]. Microwave based pyrolysis of sewage sludge to recover bio-oils was also studied by many researchers in last few years [221–224].
Hybrid microwave/ultrasonic reactors
Combining the effects of the microwave and ultrasonic energy can be innovative and beneficial [219]. These two effects, in fact, complement each other, in that, dielectric heating and ionic conduction for selective heating by microwaves and acoustic cavitation with large amounts of concentrated energy by ultrasonics may result in far superior results. Constructing a single reactor vessel to operate the two mechanisms simultaneously may require some deep knowledge and understanding of the mechanisms. Currently, this hybrid technology is developed at a laboratory scale and is used in few green chemistry related experimental procedures. Large scale development of the technology is a challenge and if done successfully, can lead to breakthrough in operational performance. Hybrid reactors developed for different laboratory applications are shown in Figure 10 below. There are two types of reactor arrangements: 1) ultrasonic zone (horn) within microwave zone; 2) ultrasonic zone (horn) outside microwave zone. For reactors with ultrasonic zone within microwave zone, horn made by microwave transparent material is required. Possible extraction and transesterification mechanisms in a reactor with ultrasonic and microwave effects in series is illustrated in Figure 11. Possible extraction and transesterification mechanisms for ultrasonic and microwave induced algal biodiesel production are as follows (Figure 11): a) microalgal cells in water are exposed to ultrasonication; b) ultrasonics align the algal cells along the vibrations; c) ultrasonics coagulate to form floc and concentrate the algal cells; d) concentrated algal cells exposed to microwaves in the solvent medium; e) microwaves induce diffusive and disruptive mechanisms and create hotspots to extract the oils and lipids; f) algal lipids extracted and transesterified by the microwaves. Ultrasonics also induce the same effects that are produced by microwaves. Figure 11 shows the flocculation capability of the ultrasonics in process streams with dilute concentrations. For extraction reactions, ultrasonic effect can be explained as follows: 1) rapid movement of fluids caused by a variation of sonic pressure which causes solvent compression and rarefaction cycles; 2) cavitation, when large negative pressure gradient is applied to the liquid, the liquid will break down and cavities (cavitation bubbles) will be created. At high ultrasonic intensities, a small cavity may grow rapidly through inertial effects. So, bubbles grow and collapse violently. The formation and collapse of micro bubbles are responsible for most of the significant chemical effects and mass transfer increases by disrupting the interfacial boundary layers; and 3) acoustic streaming mixing [225]. A new technology including infrared radiation along with microwave and ultrasonic techniques is developed recently. This technology has yet to be tested for biodiesel production [226].
Possible extraction and transesterification mechanisms for ultrasonic and microwave induced reactors in series: a) microalgal cells in water are exposed to ultrasonication; b) ultrasonics align the algal cells along the vibrations; c) ultrasonics coagulate to form floc and concentrate the algal cells; d) concentrated algal cells exposed to microwaves in the solvent medium; e) microwaves induce diffusive and disruptive mechanisms and create hotspots to extract the oils and lipids; f) algal lipids extracted and transesterified by the microwaves.
Li and co-researchers have studied different types of extraction methods, namely, solvent extraction, microwave assisted extraction and ultrasonic assisted extraction. Microwaves were used as pretreatment technique before actual extraction of oils using extractant. different pretreatment times ((0, 0.5, 1, and 2 min), solvent (isopropanol, hexane, and 3:2 hexane − isopropanol mixture), and extraction time (0.5, 1, 1.5, 2, and 3 h) were considered.
Prior to actual extraction, the ground soybean was pretreated by heating in a microwave oven operating at 2450 MHz. A 600 W microwave oven with a 0.6 ft3 cavity and equipped with a turntable was used for the ground soybean pretreatment. For ultrasonic based extraction the intensity was changed between (0, 16.4, 20.9, and 47.6 W/cm2). Solvent extraction was accomplished by immersion of ground soybeans in a given volume of solvent at ambient temperature. Oil yields were found to increase with both intensity of the process assistance and extraction time under the different conditions, particularly with hexane and the mixed solvent.
With the longest microwave pretreatment (2 min), extracted oil yields with the mixed solvent increased from 5.08 to 6.10 g when extraction time was increased from 0.5 to 3 h. The highest yield result of 12.21 g from 100 g soybeans was obtained with the mixed solvent under 47.6 W/cm2 sonication [227].
Yet, another interesting study by Cravotto and group tested soybean germ and marine microalgae using ultrasound-assisted (US) and microwave-assisted (MW) extraction techniques to extract oils either separately or in combination of these two effects [228]. Ultrasound devices working at several frequencies (19, 25, 40 and 300 kHz), and a multimode microwave oven (operating with both open and closed vessels) were used for ultrasonic and microwave assisted extractions respectively. Combined treatments were also studied, such as simultaneous double sonication (at 19 and 25 kHz) and simultaneous US/MW irradiation, achieved by inserting a non-metallic horn in a MW oven. Extraction times and yields were compared with those from conventional procedures. With soybean germ the best yield was obtained with a cavitating tube prototype (19 kHz, 80 W), featuring a thin titanium cylinder instead of a conventional horn. Double sonication, carried out by inserting an immersion horn (25 kHz) in the same tube, improved the yield only slightly but halved the extraction time. Almost comparable yields were achieved by closed-vessel MAE and simultaneous US/MW irradiation. Compared with conventional methods, extraction times were reduced by up to 10-fold and yields increased by 50– 500%. In the case of marine microalgae, UAE worked best, as the disruption by US of the tough algal cell wall considerably improved the extraction yield from 4.8% in soxhlet to 25.9%. It was suggested that US and MW, either alone or combined, can greatly improve the extraction of bioactive substances, achieving higher efficiency and shorter reaction times at low or moderate costs, with minimal added toxicity. Compared with conventional methods much higher yields were also achieved with closed-vessel MW irradiation at 120°C and simultaneous US/MW irradiation. Results were even more striking in the case of seaweed extraction in another study, as the cell wall of the microalgae is very tough. In a pioneering study Chemat and associates showed that simultaneous MW/US irradiation enabled digestion and dissolution of solid and liquid samples to be carried out rapidly at atmospheric pressure, as exemplified in the determination of copper in olive oil and the dissolution of refractory oxides in ceramics [229].
The above examples clearly show that combined US/MW irradiation, being practically hazard-free, represents an emerging technological innovation that deserves widespread attention in fine-chemical and pharmaceutical research. Although the mechanisms of cavitation and microwave effects are not fully understood, processes requiring enhanced heat transfer and mass transport (especially heterogeneous reactions) will no doubt benefit from this green technique. Combinations of both energies may be simultaneous or sequential, and conditions can be tailored for the analytical and preparative modes. Besides saving energy, these green techniques promote faster and more selective transformations. As they are of a basically different nature (quantum and non-quantum fields), each must be fine-tuned by its specific parameters; a combined device will often be subject to additional hazard limitations. However, recent developments evidence that such a combination is certainly possible and safe, ranging from simple modifications to flow systems that are well suited for automation and scaling-up [220, 230–232]. Combining microwaves with radiofrequency waves may benefit the biodiesel process in that the radiofrequency waves have a higher wavelength than microwaves which allows for them to penetrate through larger objects and solid particles [231].
Concluding remarks
Microwave-enhanced organic/inorganic synthesis is considered as green chemistry and a preferred method due to several advantages such as lower energy consumption, substantial reduction in reaction times and solvent requirements, enhanced selectivity, and improved conversions with less by-product formation. Many reactions that do not occur under classical methods of heating can be carried out with high yields under microwave irradiation. Microwaves have the potential for large scale applications specifically in biodiesel production due to their ability to interact with a variety of reagents. Laboratory scale results in both batch and continuous conditions are encouraging and few pilot scale studies need to be developed to test their ability and efficiency for large scale adaptability. The reactor design, configurations, flow patterns, reactor safety and operational logistics are yet to be developed. Understanding the effect of microwaves on biomass extraction and transesterification reactions can be beneficial in the reactor design. Similarly, understanding microwave effect on different catalysts and solvents is crucial to develop safe reactors. Specific areas of challenges that need critical attention prior to large scale development are: controlled heating since biodiesel process is sensitive to temperature variations, efficient transfer of microwave energy into work area with fewer losses to the reactor walls and environment, compatibility of the process with rest of the process pipeline which includes biodiesel product separation and purification. Other important areas are better fundamental understanding and modeling of microwave-material interactions, better preparation of reaction mixtures and compositions tailored specific to microwave processing, better process controls, electronic tuning and automation (smart processing). Finally, availability of low-cost equipment, supporting technologies and other processing support hardware is to be considered. Combining the microwave effect with other innovative heating methods can be beneficial. Ultrasonics and radiofrequency waves can complement the microwave effect to improve the overall reaction performance in hybrid reactors; especially use of ultrasonic technology seems promising. Research in this area is in its infancy; however if successfully demonstrated, combined effect of these two innovative technologies can be enormous.
References
Satyanarayana KG, Mariano AB, Vargas JVC: A review on microalgae, a versatile source for sustainable energy and materials. Int J Energy Res. 2011, 35: 291-311. 10.1002/er.1695.
Demirbas A: Global renewable energy projections. Energy Sources, Part B. 2009, 4: 212-224. 10.1080/15567240701620499.
Rittmann RE: Opportunities for renewable bioenergy using microorganisms. Biotechnol Bioengr. 2008, 100: 203-212. 10.1002/bit.21875.
Fabbri D, Bevoni V, Notari M, Rivetti F: Properties of a potential biofuel obtained from soybean oil by transmethylation with dimethyl carbonate. Fuel. 2007, 86: 690-697. 10.1016/j.fuel.2006.09.003.
Pimentel D: Biofuels, solar and wind as renewable energy systems. 2008, New York: Springer Science + Business Media B.V
Energy independence and security act of 2007: summary of provisions. http://www.eia.gov/oiaf/aeo/otheranalysis/aeo_2008analysispapers/eisa.html,
Georgogianni KG, Kontominas MG, Tegou E, Avlonitis D, Gergis V: Biodiesel production: reaction and process parameters of alkali-catalyzed transesterification of waste frying oils. Energy & Fuels. 2007, 21: 3023-3027. 10.1021/ef070102b.
Yang J, Xu M, Zhang X, Hu Q, Sommerfeld M, Chen Y: Life-cycle analysis on biodiesel production from microalgae: water footprint and nutrients balance. Bioresour Technol. 2011, 102: 159-165. 10.1016/j.biortech.2010.07.017.
Hoekman SK: Biofuels in the U.S. - challenges and opportunities. Renewable Energy. 2009, 34: 14-22. 10.1016/j.renene.2008.04.030.
Kargbo DM: Biodiesel production from municipal sewage sludges. Energy Fuels. 2010, 24 (5): 2791-2794. 10.1021/ef1001106.
Haas MJ, McAloon AJ, Yee WC, Foglia TA: A process model to estimate biodiesel production costs. Bioresource Technology. 2006, 97: 671-678. 10.1016/j.biortech.2005.03.039.
Cintas P, Mantegna S, Gaudino EC, Cravotto G: A new pilot flow reactor for high-intensity ultrasound irradiation. Application to the synthesis of biodiesel. Ultrasonics Sonochemistry. 2010, 17: 985-989. 10.1016/j.ultsonch.2009.12.003.
Harvey AP, Mackley MR, Seliger T: Process intensification of biodiesel production using a continuous oscillatory flow reactor. J Chem Technol Biotechnol. 2003, 78: 338-341. 10.1002/jctb.782.
Clark DE, Sutton WH: Microwave processing of materials. Annual Review of Materials Science. 1996, 26: 299-331. 10.1146/annurev.ms.26.080196.001503.
Caddick S, Fitzmaurice R: Microwave enhanced synthesis. Tetrahedron. 2009, 65: 3325-3355. 10.1016/j.tet.2009.01.105.
Ku HS, Siores E, Taube A, Ball JAR: Productivity improvements through the use of industrial microwave technologies. Computers & Industrial Engineering. 2002, 42 (2–4): 281-290.
de la Hoz A, Diaz-Ortiz A, Moreno A: Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem Soc Rev. 2005, 34: 164-178. 10.1039/b411438h.
Roberts B, Strauss CR: Toward rapid, “green”, predictable microwave-assisted synthesis. Acc Chem Res. 2005, 38: 653-661. 10.1021/ar040278m.
Varma RS: Solvent- free organic syntheses using supported reagents and microwave irradiation. Green Chem. 1999, 1: 43-55. 10.1039/a808223e.
Giguere RJ, Bray TL, Duncan SM, Majetich G: Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett. 1986, 27: 4945-4948. 10.1016/S0040-4039(00)85103-5.
Stuerga D, Delmotte M: Microwaves in Organic Synthesis. Edited by: Loupy A. 2002, Wiley-VCH Weinheim, 1-34.
Mingos DMP: Microwave-assisted organic synthesis. Edited by: Lidstrom P, Tierney JP. 2004, Oxford: Blackwell, Chap. 1
Baghurst DR, Mingos DMP: Applications of microwave dielectric heating effects to the synthetic problems in chemistry. Chem Soc Rev. 1991, 20: 1-47. 10.1039/cs9912000001.
Gabriel C, Gabriel S, Grant EH, Halstead BS, Mingos DMP: Dielectric parameters relevant to microwave dielectric heating. Chem Soc Rev. 1998, 27: 213-223. 10.1039/a827213z.
Intellectual property report. Evaluserve analysis. Developments on microwave chemistry. 2005, http://www.rsc.org/images/evaluserve_tcm18-16758.pdf,
Metaxas AC, Meredith RJ: Industrial microwave heating. 1993, London: Peter Peregrinus Ltd
Peterson ER: Microwave chemical processing. Res Chem Intermed. 1994, 1: 93-96.
Chemat-Djenni Z, Hamada B, Chemat F: Atmospheric pressure microwave assisted heterogeneous catalytic reactions. Molecules. 2007, 12: 1399-1409. 10.3390/12071399.
Varma RS: Solvent-free accelerated organic syntheses using microwaves. Pure and Applied Chemistry. 2001, 73 (1): 193-198. 10.1351/pac200173010193.
Refaat AA: Different techniques for the production of biodiesel from waste vegetable oil. Int J Environ Sci Tech. 2010, 7 (1): 183-213.
Bogdal D: Microwave-assisted organic synthesis. 2005, Elsevier Ltd
Groisman Y, Gedanken A: Continuous flow, circulating microwave system and its application in Nanoparticle fabrication and biodiesel synthesis. J Phys Chem C. 2008, 112: 8802-8808. 10.1021/jp801409t.
Kappe CO: The impact of microwave synthesis on drug discovery. Nat Rev Drug Discov. 2006, 5: 51-63. 10.1038/nrd1926.
Li Y, Yang W: Microwave synthesis of zeolite membranes: a review. J Membrane Sci. 2008, 316: 3-17. 10.1016/j.memsci.2007.08.054.
Kappe CO: Controlled microwave heating in modern organic synthesis. Angew Chem. Int. Ed. 2004, 43: 6250-6284. 10.1002/anie.200400655.
Herrero MA, Kremsner JM, Kappe CO: Nonthermal microwave effects revisited: on the importance of internal temperature monitoring and agitation in microwave chemistry. J Org Chem. 2008, 73: 36-47. 10.1021/jo7022697.
Maher KD, Bressler DC: Pyrolysis of triglyceride materials for the production of renewable fuels and chemicals. Bioresour Technol. 2007, 98: 2351-2368. 10.1016/j.biortech.2006.10.025.
Hoekman SK: Biofuels in the U.S.-challenges and opportunities. Renewable Energy. 2009, 34: 14-22. 10.1016/j.renene.2008.04.030.
Sharma YC, Singh B, Upadhyay SN: Advancements in development and characterization of biodiesel: a review. Fuel. 2008, 87: 2355-2373. 10.1016/j.fuel.2008.01.014.
Akoh CC, Chang S, Lee G, Shaw JJ: Enzymatic approach to biodiesel production. J Agric Food Chem. 2007, 55: 8995-9005. 10.1021/jf071724y.
Kusdiana D, Saka S: Two-step preparation for catalyst-free biodiesel fuel production. Appl Biochem Biotechnol. 2004, 113–116: 781-791.
Freedman B, Pryde EH, Mounts TL: Variables affecting the yields of fatty esters from transesterified vegetable oils. J Am Oil Chem Soc. 1984, 61 (10): 1638-1643. 10.1007/BF02541649.
Srivastava A, Prasad R: Triglycerides-based diesel fuels. Renewable & Sustainable Energy Rev. 2000, 4 (2): 111-133. 10.1016/S1364-0321(99)00013-1.
Kaieda M, Samukawa T, Kondo A, Fukuda H: Effect of methanol and water contents on production of biodiesel fuel from plant oil catalyzed by various lipases in a solvent-free system. J Biosci and Bioengr. 2001, 91 (1): 12-15.
Komers K, Stloukal R, Machek J, Skopal F: Biodiesel from rapeseed oil, methanol and KOH 3: analysis of composition of actual reaction mixture. Eur J Lipid Scie Technol. 2001, 103 (6): 363-371. 10.1002/1438-9312(200106)103:6<363::AID-EJLT363>3.0.CO;2-3.
Zhang Y, Dube MA, Mclean DD, Kates M: Biodiesel production from waste cooking oil. 1. Process design and technological assessment. Bioresour Technol. 2003, 89 (1): 1-16. 10.1016/S0960-8524(03)00040-3.
Suppes GJ, Dasari MA, Doskocil EJ, Mankidy PJ, Goff MJ: Transesterification of soybean oil with zeolite and metal catalysts. Applied Catalysis A: General. 2004, 257 (2): 213-223. 10.1016/j.apcata.2003.07.010.
Haas MJ, McAloon AJ, Yee WC, Foglia TA: A process model to estimate biodiesel production costs. Bioresour Technol. 2006, 97: 671-678. 10.1016/j.biortech.2005.03.039.
Meher LC, Sagar DV, Naik SN: Technical aspects of biodiesel production by transesterification: a review. Renewable Sustainable Energy Reviews. 2006, 10 (3): 248-268. 10.1016/j.rser.2004.09.002.
Patil PD, Deng S: Optimization of biodiesel production from edible and non-edible vegetable oils. Fuel. 2009, 88: 1302-1306. 10.1016/j.fuel.2009.01.016.
Patil PD, Deng S, Rhodes I, Lammers P: Biodiesel production from waste cooking oil using ferric sulfate and supercritical methanol method. Fuel. 2010, 89: 360-364. 10.1016/j.fuel.2009.05.024.
King JW, Holliday RL, List GR: Hydrolysis of soybean oil in a subcritical water flow reactor. Green Chem. 1999, 1: 261-264. 10.1039/a908861j.
Demirbas A: Biodiesel from vegetable oils via transesterification in supercritical methanol. Energy Conversion Management. 2002, 43: 2349-2356. 10.1016/S0196-8904(01)00170-4.
Demirbas A: Biodiesel fuels from vegetable oils via catalytic and noncatalytic supercritical alcohol transesterifications and other methods: a survey. Energy Conversion Management. 2003, 44: 2093-2109. 10.1016/S0196-8904(02)00234-0.
Demirbas A: Biodiesel production from vegetable oils via catalytic and noncatalytic supercritical methanol transesterification methods. Progress in Energy and Combustion Science. 2005, 31: 466-487. 10.1016/j.pecs.2005.09.001.
Kusdiana D, Saka S: Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel. 2001, 80: 693-698. 10.1016/S0016-2361(00)00140-X.
Kusdiana D, Saka S: Effects of water on biodiesel fuel production by supercritical methanol treatment. Bioresour Technol. 2004, 91: 289-295. 10.1016/S0960-8524(03)00201-3.
Hernandez-Martin E, Otero C: Different enzyme requirements for the synthesis of biodiesel: Novozym1 435 and Lipozyme1 TL IM. Bioresour Technol. 2008, 99 (2): 277-286. 10.1016/j.biortech.2006.12.024.
Hsu AF, Jones KC, Foglia TA, Marmer WN: Continuous production of ethyl esters of grease using an immobilized lipase. J Am Oil Chem Soc. 2004, 81 (8): 749-752. 10.1007/s11746-004-0973-9.
Deng L, Xu XB, Haraldsson GG, Tan TW, Wang F: Enzymatic production of alkyl esters through alcoholysis: A critical evaluation of lipases and alcohols. J Am Oil Chem Soc. 2005, 82 (5): 341-347. 10.1007/s11746-005-1076-3.
Dossat V, Combes D, Marty A: Continuous enzymatic transesterification of high oleic sunflower oil in a packed bed reactor: Influence of the glycerol production. Enzyme Microb Tech. 1999, 25 (3–5): 194-200.
Kumari V, Shah S, Gupta MN: Preparation of biodiesel by lipasecatalyzed transesterification of high free fatty acid containing oil from Madhuca indica. Energy Fuels. 2007, 21 (1): 368-372. 10.1021/ef0602168.
Roy I, Gupta MN: Applications of microwaves in biological sciences. Curr Sci. 2003, 85: 1685-1693.
Azcan N, Danisman A: Alkali catalyzed transesterification of cottonseed oil by microwave irradiation. Fuel. 2007, 86: 2639-2644. 10.1016/j.fuel.2007.05.021.
Mazzocchia C, Kaddouri A, Modica G, Nannicini R: Advances in Microwave and Radio Frequency Processing. Fast synthesis of biodiesel from triglycerides in presence of microwaves. Edited by: Willert-Porada M. 2006, the Netherlands: Springer Berlin Heidelberg, 370-376. Part V
Refaat AA, El Sheltawy ST, Sadek KU: Optimum reaction time, performance and exhaust emissions of biodiesel produced by microwave irradiation. Int J Environ Sci Technol. 2008, 5: 315-322.
Melo-Junior CAR, Albuquerque CER, Fortuny M, Dariva C, Egues S, Santos AF, Ramos ALD: Use of microwaves irradiation in the non-catalytic esterification of C18 fatty acids. Energy & Fuels. 2009, 23: 580-585. 10.1021/ef800766x.
Yin J, Xiao M, Song J: Biodiesel from soybean oil in supercritical methanol with co-solvent. Energy Conversion Manage. 2008, 49: 908-912. 10.1016/j.enconman.2007.10.018.
Chen W, Wang C, Ying W, Wang W, Wu Y, Zhang J: Continuous production of biodiesel via supercritical methanol transesterification in a tubular reactor. Part 1: Thermophysical and transitive properties of supercritical methanol. Energy Fuels. 2009, 23 (1): 526-532. 10.1021/ef8005299.
Gedye RN, Smith FE, Westaway KC: The rapid synthesis of organic compounds in microwave ovens. Canad J Chem. 1988, 66 (1): 17-26. 10.1139/v88-003.
Gedye RN, Smith FE, Westaway KC: The rapid synthesis of organic compounds in microwave ovens –Part 2. Canad J Chem. 1991, 69: 706-10.1139/v91-106.
Wiesbrock F, Hoogenboom R, Schubert US: Microwave-assisted polymer synthesis: state-of-the-Art and future perspectives. Macromol Rapid Commun. 2004, 25: 1739-1764. 10.1002/marc.200400313.
Sinnwell S, Ritter H: Recent advances in microwave-assisted polymer synthesis. Aust J Chem. 2007, 60: 729-743. 10.1071/CH07219.
Lidstrom P, Tierney JP, Wathey B, Westman J: Microwave assisted organic synthesis – a review. Tetrahedron. 2001, 57: 9225-9283. 10.1016/S0040-4020(01)00906-1.
Perreux L, Loupy A: A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron. 2001, 57: 9199-9223. 10.1016/S0040-4020(01)00905-X.
Binner JGP, Hassine NA, Cross TE: The possible role of the pre-exponential factor in explaining the increased reaction rates observed during the microwave synthesis of titanium carbide. J Materials Sci. 1995, 30 (21): 5389-5393. 10.1007/BF00351548.
Kappe CO: Microwave dielectric heating in synthetic organic chemistry. Chem Sov Rev. 2008, 37: 1127-1139. 10.1039/b803001b.
Jermolovicius LA, Schneiderman B, Senise JT: Alteration of esterification kinetics under microwave irradiation. Advances in microwave and radio frequency processing. Edited by: Willert-Porada M. 2006, The Netherlands: Springer-Verlag Berlin Heidelberg, 377-385. Part V
Sim TS, Goh A, Becker EW: Comparison of centrifugation, dissolved air flotation and drum filtration techniques for garvesting sewage-grown algae. Biomass. 1988, 16 (1): 51-62. 10.1016/0144-4565(88)90015-7.
Patil PD, Deng S: Transesterification of camelina sativa oil using heterogeneous metal oxide catalysts. Energy & Fuels. 2009, 23: 4619-4624. 10.1021/ef900362y.
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B: Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1: 20-43. 10.1007/s12155-008-9008-8.
Sheehan J, Dunahay T, Benemann J, Roessler A: A look back at the US department of Energy’s aquatic species program-biodiesel from algae. 1998, National Renweable Energy Laboratory
Pienkos PT, Darzins A: The promise and challenges of microalgal-derived biofuels. Biofuels, Bioprod Bioref. 2009, 3: 431-440. 10.1002/bbb.159.
Gressel J: Transgenics are imperative for biofuel crops. Plant Sci. 2008, 174: 246-263. 10.1016/j.plantsci.2007.11.009.
Revellame E, Hernandez R, French W, Holmes W, Alley E: Biodiesel from activated sludge through in situ Transesterification. J Chem Technol Biotechnol. 2010, 85: 614-620. 10.1002/jctb.2317.
Giese J: Advances in microwave food-processing. Food Technology. 1992, 46 (9): 118-123.
Pare JRJ, Belanger JMR, Stafford SS: Microwave-assisted process (map(tm)) - a new tool for the analytical laboratory. Trac-Trends in Analytical Chem. 1994, 13 (4): 176-184. 10.1016/0165-9936(94)87033-0.
Letellier M, Budzinski H: Microwave assisted extraction of organic compounds. Analusis. 1999, 27 (3): 259-271. 10.1051/analusis:1999116.
Spigno G, de Faveri DM: Microwave-assisted extraction of tea phenols: a phenomenological study. J Food Eng. 2009, 93 (2): 210-217. 10.1016/j.jfoodeng.2009.01.006.
Pan XJ, Niu GG, Liu HZ: Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Process. 2003, 42 (2): 129-133. 10.1016/S0255-2701(02)00037-5.
Hong N, Yaylayan VA, Raghavan GSV, Pare JRJ, Belanger JMR: Microwave-assisted extraction of phenolic compounds from grape seed. Nat Prod Lett. 2001, 15 (3): 197-204. 10.1080/10575630108041280.
Guo ZK, Jin QH, Fan GQ, Duan YP, Qin C, Wen MJ: Microwave-assisted extraction of effective constituents from a Chinese herbal medicine Radix puerariae. Analytica Chimica Acta. 2001, 436 (1): 41-47. 10.1016/S0003-2670(01)00900-X.
Kiss GAC, Forgacs E, Cserhati T, Mota T, Morais H, Ramos A: Optimization of the microwave-assisted extraction of pigments from paprika (Capsicum annuum L.) powders. J Chromatogr A. 2000, 889: 41-49. 10.1016/S0021-9673(00)00440-4.
Zigoneanu IG, Wilhams L, Xu Z, Sabliov CM: Determination of antioxidant components in rice bran oil extracted by microwave-assisted method. Bioresour Technol. 2008, 99 (11): 4910-4918. 10.1016/j.biortech.2007.09.067.
Rostagno MA, Palma M, Barroso CG: Microwave assisted extraction of soy isoflavones. Analytica Chimica Acta. 2007, 588 (2): 274-282. 10.1016/j.aca.2007.02.010.
Terigar BG, Balasubramanian S, Boldor D, Xu Z, Lima M, Sabliov CM: Continuous microwave-assisted isoflavone extraction system-design and performance evaluation. Bioresour Technol. 2010, 101 (7): 2466-2471. 10.1016/j.biortech.2009.11.039.
Eskilsson CS, Bjorklund E: Analytical-scale microwave-assisted extraction. J Chromatogr A. 2000, 902 (1): 227-250. 10.1016/S0021-9673(00)00921-3.
Bhattacharya M, Basak T: On the analysis of microwave power and heating characteristics for food processing: Asymptotes and resonances. Food Res Int. 2006, 39 (10): 1046-1057. 10.1016/j.foodres.2006.09.012.
Hemwimon S, Pavasant P, Shotipruk A: Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep Purif Technol. 2007, 54: 44-50. 10.1016/j.seppur.2006.08.014.
Lucchesi ME, Chemat F, Smadja J: Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydrodistillation. J Chromatogr A. 2004, 1043: 323-327. 10.1016/j.chroma.2004.05.083.
Nelson SO: Measurement of microwave dielectric properties of particulate materials. J Food Eng. 1994, 21 (3): 365-384. 10.1016/0260-8774(94)90080-9.
Nuchter M, Ondruschka B, Bonrath W, Gum A: Microwave assisted synthesis – a critical technology overview. Green Chem. 2004, 6: 128-141. 10.1039/b310502d.
Virot M, Tomao V, Colnagui G, Visinoni F, Chemat F: New microwave-integrated Soxhlet extraction. An advantageous tool for the extraction of lipids from food products. J Chromatography A. 2007, 1174 (1–2): 138-144.
Duvernay WH, Assad JM, Sabliov CM, Lima M, Xu Z: Microwave extraction of antioxidant components from rice bran. Pharm Eng. 2005, 25 (4): 1-5.
Lee J, Yoo C, Jun S, Ahn C, Oh H: Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol. 2010, 101 (Suppl 1): S75-S77.
Kanitkar AV: Master’s Thesis. Parameterization of microwave assisted Oil extraction and its transesterification to biodiesel. 2010, Lousiana State University, Biological and Agricultural Engineering Department
Cooney M, Young G, Nagle N: Extraction of Bio-oils from microalgae. Separation & Purification Rev. 2009, 38: 291-325. 10.1080/15422110903327919.
Virot M, Tomao V, Ginies C, Visinoni F, Chemat F: Microwave-integrated extraction of total fats and oils. J Chromatogr A. 2008, 1196–1197: 57-64.
Demirbas A: Biodiesel from sunflower oil in supercritical methanol with calcium oxide. Energy Conversion and Management. 2007, 48: 937-941. 10.1016/j.enconman.2006.08.004.
Loupy A, Petit A, Ramdani M, Yvanaeff C: The synthesis of esters under microwave irradiation using dry-media conditions. Can J Chem. 1993, 71: 90-95. 10.1139/v93-013.
Yuan H, Yang BL, Zhu GL: Synthesis of biodiesel using microwave absorption catalysts. Energy & Fuels. 2009, 23: 548-552. 10.1021/ef800577j.
Perreux L, Loupy A: A tentative rationalization of microwave effects in organic synthesis according to the reaction medium and mechanistic considerations. Tetrahedron. 2001, 57: 9199-9223. 10.1016/S0040-4020(01)00905-X.
Boldor D, Kanitkar A, Terigar BG, Leonardi C, Lima M, Breintenbeck GA: Microwave assisted extraction of biodiesel feedstock from the seeds of the invasive Chinese tallow tree. Environ Sci Technol. 2010, 44: 4019-4025. 10.1021/es100143z.
Tierney JP, Lidstrom P: Microwave assisted organic synthesis. 2005, Oxford, UK: CRC Press
Yuan H, Yang BL, Zhu GL: Synthesis of biodiesel using microwave absorption catalysts. Energy & Fuels. 2009, 23: 548-552. 10.1021/ef800577j.
van Kasteren JMN, Nisworo AP: A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Resources Conservation and recycling. 2007, 50: 442-458. 10.1016/j.resconrec.2006.07.005.
Demirbas A: Biodiesel from vegetable oils via transesterification in supercritical methanol. J Sci Ind Res . 2005, 64: 854-865.
Patil PD, Gude VG, Lucy MC, Deng S: Microwave-assisted catalytic transesterification of camelina sativa Oil. Energy & Fuels. 2010, 24 (2): 1298-1304. 10.1021/ef9010065.
Patil PD, Gude VG, Deng S: Transesterification of camelina sativa oil using subcritical and supercritical methanol with Co-solvents. Energy & Fuels. 2010, 24 (2): 746-751. 10.1021/ef900854h.
Refaat A, Attia NK, Sibak HA, El Sheltawy ST, ElDiwani GI: Production optimization and quality assessment of biodiesel from waste vegetable oil. Int J Environ Sci Tech. 2008, 5 (1): 75-82.
Saifuddin N, Chua KH: Production of ethyl ester (biodiesel) from used frying Oil: optimization of transesterification process using microwave irradiation. Malays J Chem. 2004, 6 (1): 77-82.
Lertsathapornsuk V, Pairintra R, Krisnangkura K, Chindaruksa S: Direct conversion of used vegetable oil to biodiesel and its use as an alternative fuel for compression ignition engine. Proc First Int Conf Sustainable Energy and Green Architecture. 2003, 2003: SE091-SE096.
Lertsathapornsuk V, Ruangying P, Pairintra R, Krisnangkura K: Proceedings of the first international conference on energy network: 2005. Continuous transethylation of vegetable oils by microwave irradiation. 2005, Thailand, RE11-RE14.
Leadbeater NE, Barnard TM, Stencel LM: Batch and continuous-flow preparation of biodiesel derived from butanol and facilitated by microwave heating. Energy & Fuels. 2008, 22: 2005-2008. 10.1021/ef700748t.
Mazzocchia C, Modica G, Martini F, Nannicini R, Venegoni D: Proceedings of second international conference on microwave and its scientific applications: 2004. Biodiesel and FAME from triglycerides over acid and basic catalysts assisted by microwave vol 7. 2004, Ancona, Italy: CR Chimie, 601-
Zhang S, Zu Y-G, Fu Y-J, Luo M, Zhang D-Y, Efferth T: Rapid microwave-assisted transesterification of yellow horn oil to biodiesel using a heteropolyacid solid catalyst. Bioresource Technology. 2010, 101 (3): 931-936. 10.1016/j.biortech.2009.08.069.
Yaakob Z, Sukarman IS, Kamarudin SK, Abdullah SRS, Mohamed F: Proceedings of the 2nd WSEAS/IASME International Conference on renewable energy sources: October 26-28 2008. Production of biodiesel from jatropha curcas by microwave irradiation. 2008, Corfu, Greece, 235-239.
Geuens J, Kremsner JM, Nebel BA, Schober S, Dommisse RA, Mittelbach M, Tavernier S, Kappe CO, Maes BUW: Microwave-assisted catalyst-free transesterification of triglycerides with 1-butanol under supercritical conditions. Energy Fuels. 2008, 22: 643-645. 10.1021/ef700617q.
Moseley JD, Woodman EK: Energy efficiency of microwave- and conventionally heated reactors compared at meso scale for organic reactions. Energy Fuels. 2009, 23: 5438-5447. 10.1021/ef900598m.
Barnard TM, Leadbeater NE, Boucher MB, Stencel LM, Wilhite BA: Continuous-flow preparation of biodiesel using microwave heating. Energy & Fuels. 2007, 21: 1777-1781. 10.1021/ef0606207.
Bowman MD, Holcomb JL, Kormos CM, Leadbeater NE, Williams VA: Approaches for scale-Up of microwave-promoted reactions. Org Process Res Dev. 2008, 12: 41-57. 10.1021/op700187w.
Mazzocchia C, Modica G: Fatty acid methyl esters synthesis from triglycerides over heterogeneous catalysts in presence of microwaves. http://www.microwave-rf.org/MWRFApplKaddouri.pdf,
Terigar BG: Master’s Thesis. Advanced microwave technology for biodiesel feedstock processing. 2009, Louisiana State University, Biological and Agricultural Engineering Department
Terigar BG, Balasubramanian S, Boldor D: Effect of storage conditions on the Oil quality of Chinese tallow tree seeds. J Am Oil Chem Soc. 2010, 87: 573-582. 10.1007/s11746-009-1529-6.
Duz MZ, Saydut A, Öztürk G: Alkali catalyzed transesterification of safflower seed oil assisted by microwave irradiation. Fuel Process Technol. 2011, 92: 308-313. 10.1016/j.fuproc.2010.09.020.
Hernando J, Leton P, Matia MP, Novella JL, Alvarez-Builla J: Biodiesel and FAME synthesis assisted by microwaves. Homogeneous batch and flow processes. Fuel. 2007, 86 (10–11): 1641-1644.
Majewski MW, Pollack SA, Curtis-Palmer VA: Diphenylammonium salt catalysts for microwave assisted triglyceride transesterification of corn and soybean oil for biodiesel production. Tetrahedron Letters. 2009, 50: 5175-5177. 10.1016/j.tetlet.2009.06.135.
Rahmanlar I, Yucel S, Ozcimen D: The Production of methyl esters from waste frying oil by microwave method. Asia-Pacific Journal of Chemical Engineering. 2012, 7 (5): 697-704.
Nogueira BM, Carretoni C, Cruz R, Freitas S, Melo PA, Costa-Felix R, Pinto JC, Nele M: Microwave activation of enzymatic catalysts for biodiesel production. J Mol Catalysis B: Enzymatic. 2010, 67: 117-121. 10.1016/j.molcatb.2010.07.015.
Lertsathapornsuk V, Pairintra R, Aryusuk K, Krisnangkura K: Microwave assisted in continuous biodiesel production from waste frying palm oil and its performance in a 100 kW diesel generator. Fuel Processing Technology. 2008, 89 (12): 1330-1336. 10.1016/j.fuproc.2008.05.024.
Azcan N, Danisman A: Microwave assisted transesterification of rapeseed oil. Fuel. 2008, 87: 1781-1788. 10.1016/j.fuel.2007.12.004.
Hsiao MC, Lin CC, Chang YH: Microwave irradiation-assisted transesterification of soybean oil to biodiesel catalyzed by nanopowder calcium oxide. Fuel. 2011, 90 (5): 1963-1967. 10.1016/j.fuel.2011.01.004.
Kim D, Choi J, Kim G, Seol SK, Ha YC, Vijayan M, Jung S, Kim BH, Lee GD, Park SS: Microwave-accelerated energy-efficient esterification of free fatty acid with a heterogeneous catalyst. Bioresource Technology. 2011, 102: 3639-3641. 10.1016/j.biortech.2010.11.067.
Kamath HV, Regupathi I, Saidutta MB: Optimization of two step karanja biodiesel synthesis under microwave irradiation. Fuel Processing Technology. 2011, 92: 100-105. 10.1016/j.fuproc.2010.09.003.
Shakinaz AES, Refaat AA, Shakinaz TES: Production of biodiesel using the microwave technique. J Adv Res. 2010, 1 (4): 309-314. 10.1016/j.jare.2010.07.003.
Suppalakpanya K, Ratanawilai SB, Tongurai C: Production of ethyl ester from esterified crude palm oil by microwave with dry washing by bleaching earth. Applied Energy. 2010, 87: 2356-2359. 10.1016/j.apenergy.2009.12.006.
Zhang S, Zu YG, Fu YJ, Luo M, Zhang DY, Efferth T: Rapid microwaveassisted transesterification of yellow horn oil to biodiesel using a heteropolyacid solid catalyst. Bioresource Technology. 2010, 101: 931-936. 10.1016/j.biortech.2009.08.069.
Jin L, Zhang Y, Dombrowski JP, Chen C, Pravatas A, Xu L, Perkins C, Suib SL: ZnO/La2O2CO3 layered composite: a new heterogeneous catalyst for the efficient ultra-fast microwave biofuel production. Applied Catalysis B Environmental. 2011, 103 (1–2): 200-205.
Perin G, Alvaro G, Westphal E, Viana LH, Jacob RG, Lenardao EJ, D’Oca MGM: Transesterification of castor oil assisted by microwave irradiation. Fuel. 2008, 87: 2838-2841. 10.1016/j.fuel.2008.01.018.
Leadbeater NE, Stencel LM: Fast, easy preparation of biodiesel using microwave heating. Energy & Fuels. 2006, 20 (5): 2281-2283. 10.1021/ef060163u.
Duz MZ, Saydut A, Öztürk G: Alkali catalyzed transesterification of safflower seed oil assisted by microwave irradiation. Fuel Processing Technology. 2011, 92: 308-313. 10.1016/j.fuproc.2010.09.020.
Hsiao MC, Lin CC, Chang YH, Chen LC: Ultrasonic mixing and closed microwave irradiation-assisted transesterification of soybean oil. Fuel. 2010, 89 (12): 3618-3622. 10.1016/j.fuel.2010.07.044.
Ozturk G, Kafadar AB, Duz MZ, Saydut A, Hamamci C: Microwave assisted transesterification of maize (Zea mays L.) oil as a biodiesel fuel. Energy, Exploration & Exploitation. 2010, 28 (1): 47-58. 10.1260/0144-5987.28.1.47.
Han X, Chen L, Peng Q: Preparation of biodiesel from sunflower oil under microwave irradiation by ionic liquids H2SO4. Journal of Zhengzhou University (Engineering Science). 2008, 4:
Kong J, Han X, Chen L, Huo J: Preparation of biodiesel under microwave irradiation from sunflower Oil by solid super acid TiO2/SO4. Guangzhou Chemical Industry. 2009, 2:
Chai F, Cao FH, Zhai FY, Chen Y, Wang XH, Su ZM: Transesterification of vegetable oil to biodiesel using a heteropolyacid solid catalyst. Adv Synth Catal. 2007, 349: 1057-1065. 10.1002/adsc.200600419.
Shibasaki-Kitakawa N, Honda H, Kuribayashi H, Toda T, Fukumura T, Yonemoto T: Biodiesel production using anionic ion-exchange resin as heterogeneous catalyst. Bioresource Technol. 2007, 98: 416-421. 10.1016/j.biortech.2005.12.010.
Brunschwig C, Moussavou W, Blin J: Use of bioethanol for biodiesel production. Progress in Energy and Combustion Science. 2012, 38: 283-301. 10.1016/j.pecs.2011.11.001.
Patil PD, Gude VG, Pinappu S, Deng S: Transesterification kinetics of Camelina sativa oil on metal oxide catalysts under conventional and microwave heating conditions. Chem Eng J. 2011, 168 (3): 1296-1300. 10.1016/j.cej.2011.02.030.
Zabeti M, Wan Daud D, Ashri M, Aroua MK: Activity of solid catalysts for biodiesel production: a review. Fuel Process Technol. 2009, 90 (6): 770-777. 10.1016/j.fuproc.2009.03.010.
Breccia A, Esposito B, Fratadocchi GB, Fini A: Reaction between methanol and commercial seed oils under microwave irradiation. J Microw Power Electromagn Energy. 1999, 34 (1): 3-8.
Schuchardt U, Serchelia R, Vargas RM: Transesterification of vegetable oils: a review. J. Braz. Chem. Soc. 1998, 9 (1): 199-210.
Sridharan R, Mathai IM: Transesterification reactions. Sci Ind Res . 1974, 22: 178-187.
Kulkarni MG, Dalai AK, Bakhshi NN: Transesterification of canola oil in mixed methanol/ethanol system and use of esters as lubricity additive. Bioresour Technol. 2007, 98: 2027-2033. 10.1016/j.biortech.2006.08.025.
Moser BR: Biodiesel production, properties, and feedstocks. In Vitro Cell Dev Biol—Plant. 2009, 45: 229-266. 10.1007/s11627-009-9204-z.
Chisti Y: Biodiesel from microalgae. Biotechnol Adv. 2007, 25 (3): 294-306. 10.1016/j.biotechadv.2007.02.001.
Cooney MJ, Young G, Pate R: Bio-oil from photosynthetic microalgae: case study. Bioresour Technol. 2011, 102 (1): 166-177. 10.1016/j.biortech.2010.06.134.
Lardon L, Helias A, Sialve B, Steyer J, Bernard O: Life-cycle assessment of biodiesel production from microalgae. Environmental science and technology. 2009, 43: 6475-6481. 10.1021/es900705j.
National Algal Biofuels Technology Roadmap: U.S. Department of energy, office of energy efficiency and renewable energy. 2010, USA: Biomass Program
Demirbas A: Production of biodiesel fuels from linseed oil using methanol and ethanol in non-catalytic SCF conditions. Biomass & Bioenergy. 2009, 33: 113-118. 10.1016/j.biombioe.2008.04.018.
Ranjan A, Patil C, Moholkar VS: Mechanistic assessment of microalgal lipid extraction. Ind Eng Chem Res. 2010, 49: 2979-2985. 10.1021/ie9016557.
Mandal V, Mohan Y, Hemalatha S: Microwave assisted extraction – an innovative and promising extraction tool for medicinal plant research. Pharmacognosy Reviews. 2007, 1 (1): 7-18.
Chen W, Sommerfeld M, Hu Q: Microwave assisted Nile red mentod for in vivo quantification of neutral lipids in microalge. Bioresource technology. 2011, 101 (1): 135-141.
Banapurmath NR, Tewari PG, Hosmath RS: Experimental investigations of a four-stroke single cylinder direct injection diesel engine operated on dual fuel mode with producer gas as inducted fuel and Honge oil and its methyl ester (HOME) as injected fuels. Renewable Energy. 2007, 33: 2007-2018.
Bunyakiat K, Makmee S, Sawangkeaw R, Ngamprasertsith S: Continuous production of biodiesel via transesterification from vegetable oils in supercritical methanol. Energy Fuels. 2006, 20: 812-817. 10.1021/ef050329b.
Demirbas A: Biodiesel production via non-catalytic SCF method and biodiesel fuel characteristics. Energy Convers Mgmt. 2006, 47: 2271-2282. 10.1016/j.enconman.2005.11.019.
Demirbas A: Studies on cottonseed oil biodiesel prepared in non-catalytic SCF conditions. Bioresource Technol. 2008, 99: 1125-1130. 10.1016/j.biortech.2007.02.024.
Demirbas A: Biodiesel from waste cooking oil via base-catalytic and supercritical methanol transesterification. Energy Convers Mgmt. 2009, 50: 923-927. 10.1016/j.enconman.2008.12.023.
Hawash S, Kamal N, Zaher F, Kenawi O, Diwani GE: Biodiesel fuel from Jatropha oil via non-catalytic supercritical methanol transesterification. Fuel. 2009, 88: 579-582. 10.1016/j.fuel.2008.09.007.
Madras G, Kolluru C, Kumar R: Synthesis of biodiesel in supercritical fluids. Fuel. 2004, 83: 2029-2033. 10.1016/j.fuel.2004.03.014.
Rathore V, Madras G: Synthesis of biodiesel from edible and non-edible oils in supercritical alcohols and enzymatic synthesis in supercritical carbon dioxide. Fuel. 2007, 86: 2650-2659. 10.1016/j.fuel.2007.03.014.
Song ES, Lim JW, Lee HS, Lee YW: Transesterification of RBD palm oil using supercritical methanol. J Supercrit Fluids. 2008, 44: 356-363. 10.1016/j.supflu.2007.09.010.
Varma MN, Madras G: Synthesis of biodiesel from castor oil and linseed oil in supercritical fluids. Ind Eng Chem Res. 2007, 46: 1-6. 10.1021/ie0607043.
Vieitez I, Silva C, Alckmin I, Borges GR, Corazza FC, Oliveira JV, Grompone MA, Jachmanian I: Effect of temperature on the continuous synthesis of soybean esters under supercritical ethanol. Energy Fuels. 2009, 23: 558-563. 10.1021/ef800640t.
Young G, Nippgen F, Titterbrandt S, Cooney MJ: Lipid extraction from biomass using co-solvent mixtures of ionic liquids and polar covalent molecules. Sep Purification Technol. 2010, 72: 118-121. 10.1016/j.seppur.2010.01.009.
Johnson MB, Wen Z: Production of biodiesel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuels. 2009, 23: 5179-5183. 10.1021/ef900704h.
Aresta M, Dibenedetto A, Carone M, Colonna T, Fragale C: Production of biodiesel from macroalgae by supercritical CO2 extraction and thermochemical liquefaction. Environ Chem Lett. 2005, 3: 136-139. 10.1007/s10311-005-0020-3.
Patil PD, Gude VG, Mannarswamy A, Deng S, Cooke P, Munson-McGee S, Rhodes I, Lammers P, Khandan NN: Optimization of direct conversion of Wet algae to biodiesel under supercritical methanol conditions. Bioresour Technol. 2011, 102 (1): 118-122. 10.1016/j.biortech.2010.06.031.
Patil PD, Gude VG, Mannarswamy A, Deng S, Cooke P, Munson-McGee S, Rhodes I, Lammers P, Khandan NN: Optimization of microwave-assisted transesterification of dry algal biomass using RSM. Bioresour Technol. 2011, 102 (2): 1399-1405. 10.1016/j.biortech.2010.09.046.
Patil PD, Gude VG, Mannarswamy A, Cooke P, Khandan NN, Lammers P, Deng S: Comparison of direct transesterification of algal biomass under supercritical methanol and microwave irradiation conditions. Fuel. 2012, 97: 822-831.
Koberg M, Cohen M, Ben-Amotz A, Gedanken A: Bio-diesel production directly from the microalgae biomass of Nannochloropsis by microwave and ultrasound radiation. Bioresour Technol. 2011, 102 (5): 4265-4269. 10.1016/j.biortech.2010.12.004.
Encinar JM, Gonzalez JF, Martinez G, Sanchez N, Pardal A: Soybean oil transesterification by the use of a microwave flow system. Fuel. 2012, 95: 386-393.
Kumar R, Kumar GR, Chandrashekar N: Microwave assisted alkali-catalyzed transesterification of Pongamia pinnata seed oil for biodiesel production. Bioresour Technol. 2011, 102 (11): 6617-6620. 10.1016/j.biortech.2011.03.024.
Vijayaraghavan K, Hemanathan K: Biodiesel production from freshwater algae. Energy Fuels. 2009, 23: 5448-5453. 10.1021/ef9006033.
Hill J, Nelson E, Tilman D, Polasky S, Tiffany D: Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. PNAS. 2006, 103 (30): 11206-11210. 10.1073/pnas.0604600103.
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG: Biodiesel from algae: challenges and prospects. Current Opinion in Biotechnology. 2010, 21: 277-286. 10.1016/j.copbio.2010.03.005.
USDA agricultural economic report number 845. Energy life-cycle assessment of soybean biodiesel. 2009, USA
Pimentel D, Patzek TW: Ethanol production using corn, Switchgrass, and wood; biodiesel production using soybean and sunflower. Nat Resour Res. 2005, 14 (1): 65-76. 10.1007/s11053-005-4679-8.
Janulis P: Reduction of energy consumption in biodiesel fuel life cycle. Renewable Energy. 2004, 29: 861-871. 10.1016/j.renene.2003.10.004.
Jorquera O, Kiperstok A, Sales Emerson A, Embiruc¸u M, Ghirardi Maria L: Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Renew Bioresour Technol. 2010, 101: 1406-1413. 10.1016/j.biortech.2009.09.038.
Singh J, Gu S: Commercialization potential of microalgae for biofuels production. Renewable and Sustainable. Energy Rev. 2010, 14: 2596-2610.
Illman AM, Scragg AH, Shales SW: Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol. 2000, 27: 631-635. 10.1016/S0141-0229(00)00266-0.
Xu L, Brilman DWF, Withag JAM, Brem G, Kersten S: Assessment of a dry and a wet route for the production of biofuels from microalgae: energy balance analysis. Bioresource Technol. 2011, 102 (8): 5113-5122. 10.1016/j.biortech.2011.01.066.
Brune DE, Lundquist TJ, Benemann JR: Microalgae biomass for greenhouse gas reductions: potential for replacement of fossil fuels and animal feeds. J Environ Eng. 2009, 135 (11): 1136-1144. 10.1061/(ASCE)EE.1943-7870.0000100.
Patil PD, Gude VG, Reddy HK, Muppaneni T, Deng S: Biodiesel production from waste cooking Oil using sulfuric acid and microwave irradiation processes. J Environm Prot. 2012, 3: 107-113. 10.4236/jep.2012.31013.
Chand P, Chintareddy VR, Verkade JG, Grewell D: Enhancing biodiesel production from soybean Oil using ultrasonics. Energy Fuels. 2010, 24 (3): 2010-2015. 10.1021/ef9011752.
Hill JM, Marchant TR: Modelling microwave heating. App Math Model. 1996, 20 (1): 3-15. 10.1016/0307-904X(95)00107-U.
Reimbert CG, Minzoni AA, Smyth NF: Effect of radiation losses on hotspot formation and propagation in microwave heating. IMA J Applied Math. 1996, 57 (2): 165-179. 10.1093/imamat/57.2.165.
Kappe CO, Stadler A: Microwaves in organic and medicinal chemistry. 2005, KGaA, Weinheim: Wiley-VCH Verlag GmbH & Co
Kappe CO, Dallinger D, Murphree SS: Practical microwave synthesis for organic chemists: strategies, instruments, and protocols. 2009, Wiley, John & Sons
Leadbeater NE: Microwave heating as a tool for sustainable chemistry. 2010, CRC Press
Boldor D, Balasubramanian S, Purohit S, Rusch KA: Design and implementation of a continuous microwave heating system for ballast water treatment. Environ Sci Technol. 2008, 42 (11): 4121-4127. 10.1021/es7024752.
Glasnov TN, Kappe CO: Microwave-assisted synthesis under continuous-flow conditions. Macromol Rapid Commun. 2007, 28: 395-410. 10.1002/marc.200600665.
Baxendale IR, Hayward JJ, Ley SV: Microwave reactions under continuous flow conditions. Comb Chem High Throughput Screen. 2007, 10 (10): 802-836. 10.2174/138620707783220374.
Salvi D, Boldor D, Ortego J, Aita GM, Sabliov CM: Numerical modeling of continuous flow microwave heating: a critical comparison of COMSOL and ANSYS. J Microw Power Electromagn Energy. 2010, 44 (4): 187-197.
Moseley JD, Lenden P, Lockwood M, Ruda K, Sherlock J, Thomson AD, Gilday JP: A comparison of commercial microwave reactors for scale-Up within process chemistry. Org Process Res Dev. 2008, 12: 30-40. 10.1021/op700186z.
Cleophax J, Liagre M, Loupy A, Petit A: Application of focused microwaves to the scale-Up of solvent-free organic reactions. Org Proc Res Dev. 2000, 4 (6): 498-504. 10.1021/op000031e.
Letellier M, Budzinski H, Garrigues P, Wise S: Focused microwave-assisted extraction of polycyclic aromatic hydrocarbons in open cell from reference materials (sediment, soil, air particulates). Spectroscopy. 13: 71-80. 1996/1997
Leonelli C, Mason TJ: Microwave and ultrasonic processing: now a realistic option for industry. Chem Eng Process. 2010, 49: 885-900. 10.1016/j.cep.2010.05.006.
Bohlmann JT, Lorth CM, Drews A, Buchholz R: Microwave high pressure thermo-chemical conversion of sewage sludge as an alternative to incineration. Chem Engr Technol. 1999, 21: 404-409.
Domínguez A, Menéndez JA, Inguanzo M, Pis JJ: Gas chromatographic–mass spectrometric study of the oils fractions produced by microwave-assisted pyrolysis of different sewage sludges. J Chromatogr A. 2003, 1012: 193-206. 10.1016/S0021-9673(03)01176-2.
Dominguez A, Menendez JA, Inguanzo M, Pis JJ: Investigations into the characteristics of oils produced from microwave pyrolysis of sewage sludge. Fuel Process Technol. 2005, 86: 1007-1020. 10.1016/j.fuproc.2004.11.009.
Tian Y, Zuo W, Ren Z, Chen D: Estimation of a novel method to produce bio-oil from sewage sludge by microwave pyrolysis with the consideration of efficiency and safety. Bioresource Technol. 2011, 102: 2053-2061. 10.1016/j.biortech.2010.09.082.
Zuo W, Tian Y, Ren N: The important role of microwave receptors in bio-fuel production by microwave-induced pyrolysis of sewage sludge. Waste Manage. 2011, 31: 1321-1326. 10.1016/j.wasman.2011.02.001.
Ozcimen D, Yucel S: Biofuel’s Engineering process technology. Novel methods in biodiesel production. Edited by: DosSantos MA. 2011, Intech, 354-384.
Microwave·Uv·Us synthesis/extraction reactor UWave-1000. http://www.sineo.cn/en/Products_px.asp?pid=47,
Li H, Pordesimo LO, Weiss J, Wilhelm LR: Microwave and ultrasound assisted extraction of soybean oil. Trans ASAE. 2004, 47: 1187-1194.
Cravotto G, Boffa L, Mantegna S, Perego P, Avogadro M, Cintas P: Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason Sonochem. 2008, 15 (5): 898-902. 10.1016/j.ultsonch.2007.10.009.
Lagha A, Chemat S, Bartels PV, Chemat F: Microwave - ultrasound combined reactor suitable for atmospheric sample preparation procedure of biological and chemical products. Analusis. 1999, 27: 452-457. 10.1051/analusis:1999124.
Cravotto G, Cintas P: The combined use of microwaves and ultrasound: improved tools in process chemistry and organic synthesis. Chemistry. 2007, 3 (7): 1902-1909.
Liu S, Wang Y, McDonald T, Taylor SE: Efficient production of biodiesel using radio frequency heating. Energy & Fuels. 2008, 22: 2116-2120. 10.1021/ef800038g.
Toukoniitty B, Mikkola J, Murzin DY, Salmi T: Utilization of electromagnetic and acoustic irradiation in enhancing heterogeneous catalytic reactions. Applied Catalysis A: General. 2005, 279: 1-22. 10.1016/j.apcata.2004.10.044.
Metaxas AC: Foundations of electroheat: a unified approach. 1996, John & Sons: Wiley
Acknowledgements
VGG and EMG wish to acknowledge the financial support provided by the Office of Research and Economic Development (ORED), Bagley College of Engineering (BCoE), and Department of Civil and Environmental Engineering (CEE) at Mississippi State University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
VGG and PP conceptualized, researched and wrote most of the manuscript. EMG, SD, and NNK researched and wrote parts of the manuscript. VGG conceptualized and critically revised the manuscript, and made most of the figures. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gude, V.G., Patil, P., Martinez-Guerra, E. et al. Microwave energy potential for biodiesel production. sustain chem process 1, 5 (2013). https://doi.org/10.1186/2043-7129-1-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2043-7129-1-5