Abstract
Different species of microorganisms colonize the plastic surfaces and form biofilms depending on the aquatic environment. In the current investigation, characteristics of the plastic surface after exposure to three different aquatic environments based on visualization using scanning electron microscopy (SEM) and spectroscopic (diffuse reflectance (DR) and infrared (IR)) techniques were examined in laboratory bioreactors with time. For both materials, there were no differences observed in the ultraviolet (UV) region among the reactors and several peaks were observed with fluctuating intensities and without any trends. For light density polyethylene (LDPE), peaks indicating the presence of biofilm could be observed in the visible region for activated sludge bioreactor, and for polyethylene terephthalate (PET), freshwater algae biofilm was also visible. PET in freshwater bioreactor is the most densely populated sample both under the optical microscope and SEM. Based on the DR spectra, different visible peaks for LDPE and PET were observed but, in both cases, the visible region peaks (~ 450 and 670 nm) correspond to the peaks found in the water samples of the bioreactors. The difference on these surfaces could not be identified with IR but the fluctuations observed in the UV wavelength region were also detectable using indices obtained from the IR spectra such as keto, ester, and vinyl. For instance, the virgin PET sample shows higher values in all the indices than the virgin LDPE sample [(virgin LDPE: ester Index (I) = 0.051, keto I = 0.039, vinyl I = 0.067), (virgin PET: ester I = 3.5, keto I = 19, vinyl I = 0.18)]. This suggests that virgin PET surface is hydrophilic as expected. At the same time, for all the LDPE samples, all the indices demonstrated higher values (especially for R2) than the virgin LDPE. On the other hand, ester and keto indices for PET samples demonstrated lower values than virgin PET. In addition, DRS technique was able to identify the formation of the biofilm both on wet and dry samples. Both DRS and IR can describe changes in the hydrophobicity during the initial formation of biofilm but DRS can better describe the fluctuations of biofilm in the visible spectra region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a relative high number of studies on environmental burden caused by plastic litter (Takada and Karapanagioti 2019; Karapanagioti and Kalavrouziotis 2019). The most widely spread plastic waste are low-density polyethylene (LDPE, LLDPE), high-density polyethylene (HDPE), polypropylene (PP), polystyrene (PS), foamed polystyrene, nylon (PA), polyethylene terephthalate (PET), poly(vinyl chloride) (PVC), and cellulose acetate (CA) (Andrady et al. 2011). They can be observed in different aquatic environments, such as marine environment (Schneider et al. 2018), lakes, rivers, as well as urban areas from which they end up in artificial aquatic environments, i.e., wastewater treatment plants (Blettler et al. 2018). According to a review study (Schwarz et al. 2019) about different plastic types in aquatic environments, a relative high input of PET was mentioned (density class: high), while polyethylene (PE) was detected in all aquatic fields.
A film of microbial diversity called biofilm (Donlan 2002), or plastishere in the case of plastic surface (Rummel et al. 2017; Zettler et al. 2013) is potentially developed on any surface in an aqueous environment. In a recent study by Tziourrou et al. (2020), using diffuse reflectance spectroscopy (DRS) technique, they observed that biofilm in the marine environment does not develop randomly but in a specific way, depending on the type of plastic material. The steps followed by the creation and development of a biofilm on a surface are generally as follows: (a) reversible attachment of bacteria, (b) irreversible attachment of a surface by microorganisms, (c) biofilm maturation, and (d) biofilm dispersion (Khatoon et al. 2018; Donlan 2002). Studies have reported chemical alterations (i.e., functional groups) of plastics due to their exposure to microorganisms [Fotopoulou and Karapanagioti (2017) and references within].
The techniques used to study biofilms can vary from biological, physicochemical (Azeredo et al. 2017), microscopy techniques (Pantanella et al. 2013) to mathematical modeling (Wilson et al. 2017). There are very few publications which study biofilm on plastics directly after sampling from the field or during a laboratory experiment, without any pretreatment or alteration of the sample (e.g., Tziourrou et al. 2020; Sfaelou et al. 2015a); in other words, the study of biofilm as a material that alters polymer surfaces at different periods (e.g., color change of biofilm) needs to be further studied.
UV-visible (UV-vis) spectroscopy can be used as a useful method for microorganism determination (Badri et al. 2015). According to Alupoaei and Garcia-Rubio (2005), UV-vis spectra of microorganisms contain information on their properties (e.g., chemical composition, internal structure, number, size, and shape). Correlating natural and artificial marine environments, the research supported that biofilm patterns were related to DRS peaks; more specifically, the fluctuation of the DR spectra depended on the polymer type and the time period of biofilm formation on plastic surfaces (Tziourrou et al. 2020).
The purpose of the present study was to compare the biofilm formation on two commonly used plastics such as LDPE and PET in three different laboratory aqueous systems with various microorganisms. Specifically, the laboratory bioreactors were used to simulate a marine environment, the aeration tank of a wastewater treatment plant, and a freshwater pond environment. At different residence times, biofilms were characterized (using DRS) in both materials and simultaneous check for changes in their IR spectra. The specific objectives of the present study were (a) to check if the DRS measurements would provide different spectra for biofilm formed by different microorganisms and on various plastic surfaces; (b) to identify changes in the spectra due to the desiccation of samples, e.g., dry plastic samples collected from a beach; (c) to compare information collected with DRS to that collected with IR spectra and determine their qualitative differences.
The main scope of this work is to demonstrate that DRS is a valuable technique, very fast and with low cost, and that it can be applied to give information about the formation of biofilm even in almost not-detectable conditions. Also, it can give this information independably of the biofilm type, bacteria type, and time. It may be used for the identification of the biofilm on polymer surfaces. The proposed technique can be directly applied in the field, demands no special treatment, and even no highly qualified personnel, it is not destructive, which is very important in laboratory experiments, in food industry and in many more applications.
Polyethylene is the most common polymer produced and thus, the most found in the environment. LDPE and PET were chosen in the present study because they are very different in the polarity and the surface groups. It is considered important to investigate if the polarity of the support (polymer) can alter the macro-characteristics and the amount of the biofilm. This study involves many different parameters such as 2 polymers, 3 bioreactors, 2 different analytical techniques, 2 different sample states, dry and wet, and several different sampling points in time.
Materials and methods
Bioreactor setup and operation
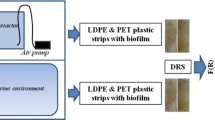
Three glass batch bioreactor systems (1.5 L at 25 ± 2 °C) were developed for the experiment purposes (Fig. S1) (Sfaelou et al. 2016). Each reactor was operated in subsequent cycles of 5 days [more specifically a 4-day operation of bioreactors plus one day of refreshing the liquids based on Tziourrou et al. (2020) and the energy source (C6H12O6) (Imai and Gloyna 1990; Sgountzos et al. 2006)]; a similar procedure was followed by Sfaelou et al. (2015a, b, 2016) and Papadimitriou et al. (2010, 2018)]. There was constant air flow via air pumps in each reactor (dissolved oxygen is consumed by the microorganisms as an electron acceptor during aerobic growth). The total solution volume in each reactor was 1L (Sfaelou et al. 2015a). In Fig. 1, a flow diagram of the steps taken during the experiment is illustrated.
The above process (Fig. 1) was repeated for 50 days and based on our previous study (Tziourrou et al. 2020), measurements were taken on the 1st, 2nd, 5th, 13th, 18th, 33rd, 40th, 45th, and 50th days from the starting day.
Visualization techniques
The visualization of biofilm from R1, R2, and R3 by optical microscope Leica DMLB (Leica, Germany) was carried out only on PET surfaces, due to the transparent nature of the material. For the visualization of biofilm on both plastic materials in different magnifications, scanning electron microscope (SEM) JSM 6300 of the company JEOL was used. For selected samples, X-ray energy-dispersive spectrometer (EDS) after coating by C was used for surface elemental analyses. For all optical observations, day 33 was chosen. The presence of microorganisms were visualized with a FEI QUANTA 250 FEG (SEM), with FE source and analysis under 10 to 4000 Pa pressure.
Spectroscopic characterization of the samples
UV-vis spectrophotometer (Varian Cary 3) equipped with an integration sphere for the DRS measurements was used. The absorption spectra of the liquid phase of the bioreactors were recorded from 200 to 800 nm. Subsequently, for the comparison of the initial and final stages (namely after creating biofilm) spectra were obtained in the UV-vis region (ultraviolet-visible) using DRS (diffuse reflection UV-vis spectroscopy) method. Each time, a virgin plastic strip by the same material was used as blank against plastic strip with biofilm; thus, peaks of DR spectra were related to the biofilm formed on the plastic strips.
The Kubelka-Munk equation (outcome is F(R)) is used for DR spectroscopy. For the solid samples the technique measures the reflectance of the visible radiation spectrum that cannot penetrate the sample. The F(R) which is an expression taking into account the absorption and scattering that result from the sample, rather than the reflectance, can be used. These values are proportional to the absorbance of the sample. This is a method used in the past by our group for the observation of biofilm formation (Tziourrou et al. 2020; Sfaelou et al. 2015a) and polymer degradation (Rtimi et al. 2015).
For functional groups identification for these samples, attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR) (model: “Bruker Optics’ Alpha-P Diamond ATR Spectrometer of Bruker Optics GmbH”) was used. The measurement range was 400–4000 cm−1 with a resolution of 4 cm−1 and 24 scans (Tziourrou et al. 2019). Before the measurements, the samples were air-dried, and biofilm was not removed from the plastic surfaces.
Results
Visualization of the plastic surfaces
Based on optical microscope images, on each PET surface in the bioreactors, microorganism species of different shape, color, size, and abundance were observed (Fig. 2). Differences of LDPE surfaces (SEM images) in R1 (Fig. 3), R2 (Figs. 4 and S2), and R3 (Figs. 5 and S3) were also observed. The sample from R2 seems to be the most diverse in terms of the number of species (Figs. 4 and S2). EDS analysis indicate silicon (Si) on the sample, which is the element of the shell of diatoms (Dugdale and Wilkerson 2001) (Fig. S2). Based on the optical microscope image for PET from R2, a possible common (with circular shape) microorganism can be observed in correlation with the SEM image for LDPE from R2. Shells of microorganisms (diatoms) were observed on PET surfaces both for R1 and R2. For R3, algae were mainly observed on PET surface (Fig. 5).
UV-vis interpretation
Absorption (Abs) spectra of the liquid phase from the three bioreactors are presented in Fig. 6. In Abs spectra, each bioreactor sample demonstrated shoulders as follows: at 275 nm for R1, R2, and R3, at 420 nm for R2, at 225 nm and 330 nm for R3 and peaks at 675 nm for R2, and 687 nm for R3. A similar spectrum for R1 was also recorded by Tziourrou et al. (2020) at an early day and R1 was characterized as low in biodiversity and the number of microorganisms compared to the marine environment. Here, at a later day, only one peak with low intensity was observed since only glucose was used as a carbon source, certain species may grow in favor of others. The other two reactors seem to maintain more microorganisms. The light absorption of the suspensions of R2 and R3 in the visible region (400–800 nm) can be attributed to the colors of the suspensions. In general, the observed spectra were influenced by many parameters such as cell size, internal structure, and chemical composition (Spear et al. 2009).
Absorption (Abs) spectra of each bioreactor liquid. Blue line corresponds to R1 with synthetic seawater and microorganisms from marine environment, red line corresponds to R2 (synthetic fresh water and microorganisms from activated sludge), and green line corresponds to R3 (synthetic fresh water with green algae)
DR spectra observations
Different patterns among the three reactors were captured. Selected DR spectra of LDPE and PET from R1, R2, and R3 are illustrated in Figs. 7, and 8 (wet samples) and Figs. 9, and 10 (dry samples), respectively. The complete set of spectra can be found in the Supplementary Materials (Figs. S4–S7).
Wet samples from R1
For LDPE, low F(R) value peaks were observed at 200–300 nm. The peaks at 200–300 nm demonstrated higher F(R) values from day 18 to day 45. For PET, 3 main peaks at 200–400 were observed with fluctuating values throughout the whole experiment.
Dry samples from R1
No significant qualitative differences between LDPE dry and wet samples were observed. However, for each of the days presented, it seems that one of the two peaks becomes more dominant and the values for dry are higher on days 5 and 45. For PET dry samples, similar peaks (200–400 nm) to wet samples were observed. F(R) values of dry PET samples (~ 0.35) are higher than of wet PET samples (~ 0.15). Also, one of the peaks becomes more dominant.
Wet samples from R2
For LDPE, peaks started with low F(R) values on days 1 and 2 at 200–400 nm. On day 5, a shoulder at 400–700 nm appeared, creating two peaks at 450 and 670 nm on day 13 until day 50. For PET, from day 1 spectrum started from high F(R) value peaks at 200–400 nm. Finally, the peaks at 200–350 nm increased in F(R) value during the last sampling day.
Dry samples from R2
For LDPE, different intensities in DR spectra were observed compared to wet samples. Peaks with similar wavelengths (200–400 nm) were observed while the peaks at 400–700 nm were also observed with lower intensities compared to wet samples. Peaks for PET fluctuated from 200 to 400 nm with increasing intensities compared to wet PET samples.
Wet samples from R3
For LDPE, fluctuations of peaks at UV region (200–400 nm) were observed. For PET samples similar F(R) values (300–400 nm) until day 18 were observed, while from day 33 to day 50, peaks were observed in the visible region (400–700 nm).
Dry samples from R3
For LDPE, similar peaks were observed at 200–300 nm with higher intensities. For PET, peaks were observed in the UV region with increased intensities, while from day 33 to day 50, peaks were observed in the visible region (400–700 nm) with lower intensities compared to the wet PET samples.
Tables S1–S4 summarize all the peaks for wet and dry samples, while the graph in Fig. S8 illustrates the occurrence frequency of peaks for all the wet samples. According to this graph, higher percentage of the peaks were observed in the UV region than were in the visible region in the DR spectra.
IR spectra observations
The ATR-FTIR comparative spectra of the outer surface of the LDPEs and PETs were plotted in a comparative way (Figs. 11 and 12, respectively) for days 0 and 50 whereas the complete set of data are presented in Figs. S10 and S11.
Table S5 shows the extra peaks for the LDPE originating from the three bioreactors (for peaks present in virgin LDPE see Fig. S9). The density and type of these peaks vary greatly mainly from day 5 to day 18 in all three bioreactors. In all three bioreactors, vinyl bond (Albertsson et al. 1987; Harshvardhan and Jha 2013; Fotopoulou and Karapanagioti 2015a) and ester linkage (Fotopoulou and Karapanagioti 2012) were observed. Peak at 1066 cm−1 found in R1 and R2 was also referred by Frost et al. (1998) and Guerrero and Maier (2018) who studied dimethyl sulfoxide intercalated kaolinites and barium sulfate, respectively. In R1, C-O stretching of ether group (Gajendiran et al. 2016) at 1081 cm−1 from day 5 while 1085 cm−1 on day 33 was also observed by Prasad et al. (2011) who studied radiation damage in PET polymer. In R1, C-O stretch (Jung et al. 2018) at 1099 cm−1 on day 45 was also observed. Bond at 1544 cm−1 in R1 and R2 was also found by Riaz et al. (2018) who investigated natural and synthetic collagen. Finally, 1664 cm−1 on day 50 in R1 was observed by Riaz et al. (2018).
For the virgin PET (Fig. S5), five main peaks are identified at wavenumbers 1715, 1245, 1100, 870, and 730 cm−1, corresponding in ketones (C = O), ether aromatic (C-O), ether aliphatic (C-O), aromatic (C-H), and aromatic (C-H) bond (Ioakeimidis et al. 2016) (Table S6). For PET with biofilm, similar peaks were observed. Among the native groups of the polymer (PET), there are various peaks that are decreasing, or even disappearing, or fluctuating and are attributed to the biofilm formation on the plastic surface.
Discussion
DR spectra interpretation
For both materials, there were no differences observed in the UV region among the reactors and several peaks were observed with fluctuating intensities and without any trends. Plastic type is significant for the first days; biomolecules on PET are attached earlier than on LDPE and are less hydrophobic (Tziourrou et al. 2020) as is PET compared to LDPE (Jones et al. 1999). In general, 𝑛–𝜎∗ and/or 𝜋–𝜋∗ electron transitions (peaks at 200 – 220 nm) can be detected in several organic functional groups while 𝜋–𝜋∗ and n–𝜋∗ transitions at 260–280 nm found in aromatic/polyaromatic compounds and conjugated molecules (including proteins and nucleic acids), respectively (Bikova and Treimanis 2004; Jia et al. 2007; Trabelsi et al. 2009; Rtimi et al. 2015).
In a previous study performed by our group (Tziourrou et al. 2020), during field experiments exposing similar plastic strips to the marine environment, it was found that the polymer type is only important for the colonization during the first period. PET is colonized faster during this initial phase than LDPE due to its low hydrophobicity. After this initial colonization has covered the surface, the next phase is similar for both polymers since the surface is at this point covered by similar molecules. This is not totally true for the present study, since the number of organisms in the bioreactors is not infinite and it is regulated by the addition of glucose. Although, the above microorganisms are attached to the surface only in some cases, biofilm formation is observed as indicated by peaks at the visible wavelength region.
In the present study, for LDPE, peaks indicating the presence of biofilm could be observed in the visible region for R2 and for PET, freshwater algae (R3) biofilm was also visible. PET in R3 is also the most densely populated sample both under the optical microscope (Fig. 2) and SEM (Fig. 5). Based on the DR spectra, different visible peaks for LDPE and PET were observed but, in both cases, the visible region peaks correspond to the peaks found in the water samples of the bioreactors (Fig. 6). This indicates that the growth of microorganisms depends not only on the length of contact time, but also on the type of material. Also, the patterns that can be seen for the fluctuation of peaks and their intensities could indicate periodic attachment and detachment of the microorganisms and/or the biofilm (Trulear and Characklis 1982).
Small differences were observed between DRS measurements for the samples that were wet compared to those that were allowed to dry at room temperature for 2 weeks. The peaks found at the UV region remain even after drying the samples and some of them increase in intensity. These peaks correspond to sorbed microorganisms. Peaks at visible wavelength region decrease in intensity suggesting the shrinking of the biofilm and the degradation of its colour but they were still present at the same wavelength. Alupoaei and Garcia-Rubio (2004) observed that the distribution of intensities as a function of wavelength depends on the size, shape, and optical properties of the microorganisms or cells. Another important point is the effect of relative humidity which varied with species (McEldowney and Fletcher 1988); for instance, Pseudomonas sp. increased under desiccation conditions.
IR spectra interpretation
In both cases, different transmittance (%) values of the LDPE and PET samples were observed. This was likely related to biofilm characteristics. Specifically, according to Davis et al. (2011) the relative intensity among live and dead Escherichia coli cells were different using FT-IR spectra (700–4000 cm−1); lower intensity was observed for the dead cells.
Based on the IR spectra, different fluctuations of ester, keto, and vinyl indices were observed (Fig. 13) from day 0 to day 50. Τhe virgin PET sample shows higher values in all the indices than the virgin LDPE sample as expected based on the carbonyl and aromatic groups present in PET monomer; [(virgin LDPE: ester in.= 0.051, keto in.= 0.039, vinyl in.= 0.067 similar indices values were calculated also by Tziourrou et al. (2021)), (virgin PET: ester in.= 3.5, keto in.= 19, vinyl in.= 0.18)]. This suggests that virgin PET surface is hydrophilic as expected.
Ester (up), keto (middle), and vinyl (down) indices graphs of the LDPE (left) and PET (right) samples from day 1 to day 50. For the calculation of these indices, the peaks at 1740, 1715, and 1640 cm−1 are divided with the peak at 1465 cm−1 that corresponds to the invariant absorbance of the -CH2- bond to calculate the ester, keto, and vinyl indices, respectively (Kaberi et al. 2013; Fotopoulou and Karapanagioti 2015b; Tziourrou et al. 2019)
For all the LDPE samples, all the indices demonstrated higher values (especially for R2) than the virgin LDPE. On the other hand, ester and keto indices for PET samples demonstrated lower values than virgin PET while vinyl index values seem to be similar with LDPE. The fluctuations of the values depend on the microorganisms (Albertsson et al. 1987). The patterns that can be seen for the indices could indicate periodic attachment and detachment of the microorganisms and/or the biofilm (Trulear and Characklis 1982). Actually, the indices increase and decrease several times, and actually more times for PET compared to LDPE corroborating previous findings the colonization of PET is faster than of LDPE (Tziourrou et al. 2020) due to its hydrophilicity.
Fotopoulou and Karapanagioti (2015b) suggested the colonization of PET by non-polar organisms assuming that the initial step for PET degradation is the colonization by these organisms. This is corroborated by the lower ester and keto indices of the PET covered by biofilm which however are higher than for LDPE suggesting that they are not that high due to the biofilm but due to PET itself. PET ester and keto indices have decreased due to biofilm covering most of the original carbonyl groups. The carbonyl groups formed due to biofilm are negligible compared to original PET carbonyl groups.
It is obvious that if only IR spectra are considered, the fluctuations observed in the DRS technique at the UV wavelength region is also identified. However, the difference on the surface of HDPE in R2 or PET in R3 cannot be identified. DRS technique was able to identify the formation of the biofilm both on wet and dry samples.
Implications for plastic pollution
In this study, the first objective was to compare the biofilm formation on two commonly used plastics such as LDPE and PET. Sfaelou et al. (2015a) concluded that the polarity of the surface can influence biofilm quantity; more specifically, the surface with polar groups is more supportive to the formation of biofilm than a hydrophobic surface. Compared to hydrophobic surfaces, hydrophilic surfaces more quickly build up biomolecules that increase surface roughness, which may assist microbial adhesion (Bhagwat et al. 2021).
Indeed, plastic substrate properties affect or select biofilm communities to be initially attached on the biomolecules structure however biofilm formation causes physicochemical properties of the plastic surface to somewhat converge over time (McGivney et al. 2020) as in Fig. 13. This is also observed in the present study as well as in the previous one (Tziourrou et al. 2020). So, if all plastics have a similar surface, similar organisms are more abundant, and this can explain the observation by Krause et al. (2020) and Shen et al. (2021) that lower biodiversity is recorded on plastic biofilms compared to the surrounding environment. Also, according to Shen et al. (2021) plastic biofilms have a preference for pathogenic bacteria which should be true for all plastics since ultimately, they grow biofilms with similar surface properties.
The second parameter to be studied was the effect of the different environments and the various organisms found in these environments on the biofilm formation considering that plastics can be found in many different aquatic environment settings [e.g., marine environment: Baltic Sea (McGivney et al. 2020) and abyssal seafloor (Krause et al. 2020), freshwater: Xuanwu Lake (Shen et al. 2021), wastewater (Sfaelou et al. 2015a)]. Bacteria and algae found in R1 and R3 have a negative charge and varying degrees of hydrophobicity on their surfaces (Pal and Lavanya 2022; Ozkan and Berberoglou 2013). Since these microorganisms have a negative charge, they are attracted to positively charged surfaces. Negatively charged bacteria repel negatively charged surfaces by electrostatic forces. Ozkan and Berberoglou (2013) observed higher negatively surface charge for the saltwater algae than freshwater. This can explain the higher affinity of freshwater algae in R3 for the PET plastic surface compared to the affinity of saltwater algae in R1. The presence of salt in R1 creates an environment with high ionic strength that decreases the surface charge of PET making it more hydrophobic (Jones et al. 1999) causing it to have a similar behavior with HDPE but slightly higher initial attachment.
For the activated sludge system in R2, in our previous study, we have observed that although it contains functional groups that are both positive and negative, around neutral pH, the surface of the microorganisms is also neutral (Sfaelou et al. 2015b). This property makes the activated sludge less hydrophilic, and thus, it is easier to be attached to hydrophobic surfaces or to create flocs. Thus, this corroborates the formation of biofilm in R2 only on the hydrophobic LDPE and not on PET (even though PET had a higher initial colonization that created an initial roughness).
In a wastewater treatment plant, LDPE that floats in the aeration tank will obtain a biofilm with higher mass than PET. However, PET will obtain a coverage of biomolecules that will make it more hydrophobic.
In natural aquatic environments, LDPE floats and this property does not favor biofilm formation since it will mainly attract any hydrophobic microorganisms that are left on the surface. Most of the bacteria that are hydrophobic are benthic or are creating flocs (Fattom and Shilo 1984). Thus, LDPE that is moved to the bottom of the aquatic system is expected to have higher biofilm formation than the floating LDPE due to the presence of hydrophobic microorganisms found there. PET sorbs most aquatic biomolecules while floating and in freshwater after the initial roughness is formed, it can attract microalgae. Based on our previous experience with field experiments (Tziourrou et al. 2020), both LDPE and PET will form biofilm in the open sea because of the higher biodiversity of the sea compared to experimental setups used in the present study. Nevertheless, biofilm formation on LDPE will be higher and faster in benthos whereas on PET will be higher in freshwater systems.
Conclusions
Based on microscopy observations, different species of microorganisms on both plastic surfaces were observed. DR spectra indicate different fluctuations among plastic materials, between the aquatic environments and relative humidity conditions (wet vs dry samples). Biofilm formation on the plastic samples was dependent of the material and type of the aquatic environment due to different biodiversity and differences of the hydrophobicity of the organisms. Alterations of the hydrophobicity of LDPE and PET samples were observed, based on IR spectra which were independent of aquatic environments, the same was true for the DRS peaks at the UV wavelength region. However, IR could not describe the formation of biofilm that created peaks in the visible region of DRS.
We propose future investigations to concentrate to the study (1) of biofilm formation on control degraded plastic samples due to plastic litter in the environment affected by solar UV radiation and (2) the correlation of DRS with microbiological analyses; in addition, more chemical composition information on plastic litter can be investigated, by inactive microorganisms on plastic surfaces (e.g., when membranes of dead microorganisms are broken down). Metagenomic sequencing of the bioreactor biomass (biofilm) DNA is required to analyze the microbial community and provide more meaningful results in the scientific community that is not only interested in the surface chemistry changes. Employing a negative control (sterile test water) with a strain that does not produce biofilm could be used to allow a better comparison with the original community in the three environments used.
Abbreviations
- ATR-FTIR:
-
attenuated total reflectance–Fourier transform infrared spectroscopy
- DR:
-
diffuse reflectance
- DRS:
-
diffuse reflectance spectra
- HDPE:
-
high-density polyethylene
- IR:
-
infrared
- LDPE:
-
light density polyethylene
- PET:
-
polyethylene terephthalate
- R1:
-
bioreactor with seawater
- R2:
-
bioreactor with activated sludge
- R3:
-
bioreactor with freshwater algae
- SEM:
-
scanning electron microscopy
- UV-vis:
-
ultraviolet–visible spectrometry
References
Albertsson A-C, Andersson SO, Karlsson S (1987) The mechanism of biodegradation of polyethylene. Polymer Degradation and Stability 18:73–87
Alupoaei CE, García-Rubio LH (2004) Growth behavior of microorganisms using UV-Vis spectroscopy: Escherichia coli. Biotechnology and Bioengineering 86(2):163–167
Alupoaei CE, García-Rubio LH (2005) An interpretation model for the UV-VIS spectra of microorganisms. Chemical Engineering Communications 192:198–218
Andrady AL (2011) Microplastics in the marine environment. Marine Pollution Bulletin 62:1596–1605
Azeredo J, Azevedo NF, Briandet R, Cerca N, Coenye T, Costa AR, Desvaux M, Di Bonaventura G, Hébraud M, Jaglic Z, Kačániová M, Knøchel S, Lourenço A, Mergulhão F, Meyer RL, Nychas G, Simões M, Odile Tresse O, Sternberg C (2017) Critical review on biofilm methods. Critical Reviews in Microbiology 43(3):313–351
Badri C, Bella B, Ibrahim AS, Setiadji S (2015) Assessment method for bacterial virulence based on ultraviolet and visible (UV-Vis) spectroscopy analysis. In: Goh J, Lim CT (eds) IFMBE Proceedings 52, 7th World Congress on Bioengineering. Springer International Publishing Switzerland 2015. https://link.springer.com/chapter/10.1007/978-3-319-19452-3_5
Bhagwat G, O’Connor W, Grainge I, Palanisami Y (2021) Understanding the fundamental basis for biofilm formation on plastic surfaces: role of conditioning films. Frontiers in Microbiology 12:687118
Bikova T, Treimanis A (2004) UV–absorbance of oxidized xylan and monocarboxyl cellulose in alkaline solutions. Carbohydr Polym 55:315–322
Blettler MCM, Abrial E, Khan FR, Sivri N, Espinola LA (2018) Freshwater plastic pollution: recognizing research biases and identifying knowledge gaps. Water Research 143:416–424
Davis R, Deering A, Burgula Y, Mauer LJ, Reuhs BL (2011) Differentiation of live, dead and treated cells of Escherichia coli O157:H7 using FT-IR spectroscopy. Journal of Applied Microbiology 112:743–751
Donlan RM (2002) Biofilms: Microbial Life on Surfaces. Emerging Infectious Diseases 8:881–890
Dugdale RC, Wilkerson F (2001) Sources and fates of silicon in the ocean: The role of diatoms in the climate and glacial cycles. Scientia Marina 65(S2):141–152. https://doi.org/10.3989/scimar.2001.65s2141
Fattom A, Shilo M (1984) Hydrophobicity as an adhesion mechanism of benthic cyanobacteria. Applied and Environmental Microbiology 47:135–143
Fotopoulou KN, Karapanagioti HK (2012) Surface properties of beached plastic pellets. Marine Environmental Research 81:70–77
Fotopoulou K, Karapanagioti HK (2015a) Surface properties of beached plastics. Environmental Science and Pollution Research 22:11022–11032
Fotopoulou KN, Karapanagioti HK (2015b) Surface properties of beached plastics. Environmental Science and Pollution Research 22(14):11022–11032
Fotopoulou K, Karapanagioti HK (2017) Degradation of various plastics in the environment. In: Takada H, Karapanagioti HK (eds) 2019. Hazardous Chemicals Associated with Plastics in the Marine Environment. The Handbook of Environmental Chemistry 78. Springer International Publishing
Frost RL, Kristof J, Paroz GN, Kloprogge JT (1998) Molecular structure of dimethyl sulfoxide intercalated kaolinites. The Journal of Physical Chemistry B 102:8519–8532
Gajendiran A, Krishnamoorthy S, Abraham J (2016) Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech 6:52
Guerrero ACB, Maier MS (2018) Analysis of pictorial materials by Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy. Cadernos do Lepaarq XV(30):267–276. https://go.gale.com/ps/i.do?p=AONE&u=googlescholar&id=GALE|A680186665&v=2.1&it=r&sid=googleScholar&asid=f053776e. https://doi.org/10.15210/lepaarq.v15i30.13157
Harshvardhan K, Jha B (2013) Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea. India. Marine Pollution Bulletin 77:100–106
Imai A, Gloyna EF (1990) Effects of pH and oxidation state of chromium on the behavior of chromium in the activated sludge process. Water Research 24:1143–1150. https://doi.org/10.1016/0043-1354(90)90178-9
Ioakeimidis C, Fotopoulou KN, Karapanagioti HK, Geraga M, Zeri C, Papathanassiou E, Galgani F, Papatheodorou G (2016) The degradation potential of PET bottles in the marine environment: An ATR-FTIR based approach. Scientific Reports 6:23501
Jia S, Yu H, Lin Y, Dai Y (2007) Characterization of extracellular polysaccharides from Nostoc flagelliforme cells in liquid suspension culture. Biotechnol Bioprocess Eng 12:271–275
Jones CR, Adams MR, Shdan PA, Chamberlain AHL (1999) The role of surface physicochemical properties in determining the distribution of the autochthonous microflora in mineral water bottles. Journal of applied microbiology 86:917–927
Jung MR, Horgen FD, Orski SV, Rodriguez CV, Beers KL, Balazs GH, Todd Jones T, Work TM, Brignac KC, Royer S-J, Hyrenbach KD, Jensen BA, Lynch JM (2018) Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organism. Marine Pollution Bulletin 127:704–716
Kaberi H, Tsangaris C, Zeri C, Mousdis G, Papadopoulos A, Streftaris N (2013) Microplastics along the shoreline of a Greek island (Kea isl., Aegean Sea): types and densities in relation to beach orientation, characteristics and proximity to sources. 4th International Conference on Environmental Management, Engineering, Planning and Economics (CEMEPE) and SECOTOX Conference, Mykonos Island, 24 – 28 June 2013. Greece, pp 197–202
Karapanagioti HK, Kalavrouziotis IK (2019) Microplastics in water and wastewater. IWA Publishing
Khatoon Z, McTiernan CD, Suuronen EJ, Mah T-F, Alarcon EI (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4:e01067
Krause S, Molari M, Gorb EV, Gorb SN, Kossel E, Haeckel M (2020) Persistence of plastic debris and its colonization by bacterial communities after two decades on the abyssal seafloor. Scientific Reports 10:9484. https://www.nature.com/articles/s41598-020-66361-7
McEldowney S, Fletcher M (1988) The effect of temperature and relative humidity on the survival of bacteria attached to dry solid surfaces. Letters in Applied Microbiology 7:83–86
McGivney E, Cederholm L, Barth A, Hakkarainen M, Hamacher-Barth E, Ogonowski M, Gorokhova E (2020) Rapid physicochemical changes in microplastic induced by biofilm formation. Frontiers in Bioengineering and Biotechnology 8:205. https://doi.org/10.3389/fbioe.2020.00205/full
Ozkan A, Berberoglu H (2013) Physico-chemical surface properties of microalgae. Colloids and Surfaces B: Biointerfaces. 112:287–293
Pal MK, Lavanya M (2022) Microbial influenced corrosion: understanding bioadhesion and biofilm formation. Journal of Bio- and Tribo-Corrosion 8:76. https://doi.org/10.1007/s40735-022-00677-x
Pantanella F, Valenti P, Natalizi T, Passeri D, Berlutti F (2013) Analytical techniques to study microbial biofilm on abiotic surfaces: pros and cons of the main techniques currently in use. Annali di Igiene 25:31–42
Papadimitriou CA, Karapanagioti HK, Samaras P, Sakellaropoulos GP (2010) Treatment efficiency and sludge characteristics in conventional and suspended PVA gel beads activated sludge treating Cr (VI) containing wastewater. Desalination Publications 23:199–205. https://doi.org/10.5004/dwt.2010.1998
Papadimitriou CA, Rouse JD, Karapanagioti HK (2018) Treatment efficiency and sludge characteristics in conventional and suspended PVA gel beads activated sludge treating phenol containing wastewater. Global Nest Journal 20(1):42–48
Prasad SG, De A, De U (2011) Structural and optical investigations of radiation damage in transparent PET polymer films. International Journal of Spectroscopy 2011:810936. https://doi.org/10.1155/2011/810936
Riaz T, Zeeshan R, Zarif F, Ilyas K, Muhammad N, Safi SZ, Rahim A, Syed AA, Rizvi SAA, Rehman IU (2018) FTIR analysis of natural and synthetic collagen. Appl Spectrosc Rev 53(9):703–746
Rtimi S, Pulgarin C, Sanjines R, Kiwi J (2015) Novel FeOx–polyethylene transparent films: synthesis and mechanism of surface regeneration. Royal Society of Chemistry Advances 5:80203–80211
Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt-Jansen M (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environmental Science and Pollution Research Letters 4:258–267
Schneider F, Parsons S, Clift S, Stotle A, McManus MC (2018) Collected marine litter - a growing waste challenge. Marine Pollution Bulletin 128:162–174
Schwarz AE, Ligthart TN, Boukris E, van Harmelen T (2019) Sources, transport, and accumulation of different types of plastic litter in aquatic environments: A review study. Marine Pollution Bulletin 143:92–100
Sfaelou S, Karapanagioti HK, Vakros J (2015a) Studying the formation of biofilms on supports with different polarity and their efficiency to treat wastewater. Journal of Chemistry 2015:7
Sfaelou S, Karapanagioti HK, Vakros J (2015b) The use of potentiometric mass titration (PMT) technique for determining the acid-base behavior of activated sludge. Global NEST Journal 17:397–405
Sfaelou S, Papadimitriou CA, Manariotis ID, Rouse JD, Vakros J, Karapanagioti HK (2016) Treatment of low-strength municipal wastewater containing phenanthrene using activated sludge and biofilm process. Desalination and Water Treatment 57:12047–12057. https://doi.org/10.1080/19443994.2015.1048735
Sgountzos IN, Pavlou S, Paraskeva CH, Payatakes AC (2006) Growth kinetics of Pseudomonas fluorescens in sand beds during biodegradation of phenol. Biochemical Engineering Journal 30:164–173
Shen C, Huang L, Xie G, Wang Y, Ma Z, Yao Y, Yang H (2021) Effects of plastic debris on the biofilm bacterial communities in lake water. Water. 13:1465. https://www.mdpi.com/2073-4441/13/11/1465
Spear AH, Daly K, Huffman D, Rubio LG (2009) Progress in developing a new detection method for the harmful algal bloom species, Karenia brevis, through multiwavelength spectroscopy. Harmful Algae 8:189–195
Takada H, Karapanagioti HK (2019) Hazardous chemicals associated with plastics in the marine environment. In: The Handbook of Environmental Chemistry 78. Springer International Publishing. https://doi.org/10.1007/978-3-319-95568-1
Trabelsi L, Msakni NH, Ouada HB, Bacha H, Roudesli S (2009) Partialcharacterization of extracellular polysaccharides produced by cyanobacteriumArthrospira platensis. Biotechnol Bioprocess Eng 14:27–31
Trulear MG, Characklis WG (1982) Dynamics of biofilm processes. Water Pollut Control Fed 54:1288–1301. http://www.jstor.org/stable/25041684
Tziourrou P, Megalovasilis P, Tsounia M, Karapanagioti HK (2019) Characteristics of microplastics on two beaches affected by different land uses in Salamina Island in Saronikos Gulf, east Mediterranean. Marine Pollution Bulletin. 149:110531
Tziourrou P, Vakros J, Karapanagioti HK (2020) Using diffuse reflectance spectroscopy (DRS) technique for studying biofilm formation on LDPE and PET surfaces: laboratory and field experiments. Environmental Science and Pollution Research 27:12055–12064
Tziourrou P, Kordella S, Ardali Y, Papatheodorou G, Karapanagioti HK (2021) Microplastics formation based on degradation characteristics of beached plastic bags. Marine Pollution Bulletin 169:112470
Wilson C, Lukowicz R, Merchant S, Valquier-Flynn H, Caballero J, Sandoval J, Okuom M, Huber C, Brooks TD, Wilson E, Clement B, Wentworth CD, Holmes AE (2017) Quantitative and qualitative assessment methods for biofilm growth: a mini-review. Research and Reviews: Journal of Engineering and Technology 6:4. http://www.rroij.com/open-access/quantitative-and-qualitative-assessment-methods-for-biofilm-growth-a-minireview-.pdf
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the “plastisphere”: microbial communities on plastic marine debris. Environmental Science and Technology 47:7137–7146
Acknowledgements
The authors would like to thank Prof. J.K. Kallitsis of Polymer Research Group Laboratory, Department of Chemistry, for access to the “Bruker Optics’ Alpha-P Diamond ATR Spectrometer”; Prof. Ch. Kordulis and Dr. Kalliopi Fotopoulou of Department of Chemistry for helpful discussions; Dr. Andreas Seferlis of the Laboratory of Electron Microscopy and Microanalysis (L.E.M.M.) of University of Patras; and Prof. I.D. Manariotis and Dr. Andriana Aravantinou of the Department of Civil Engineering for technical assistance.
Availability of data and materials
All necessary data are provided in the Supplementary materials.
Funding
Open access funding provided by HEAL-Link Greece. This research was supported by the General Secretariat for Research and Technology (GSRT) and Hellenic Foundation for Research and Innovation (HFRI) for Pavlos Tziourrou scholarship (12671). Partial financial support was received from the University of Patras.
Author information
Authors and Affiliations
Contributions
John Vakros and Hrissi K. Karapanagioti contributed to the study conception and design. Material preparation and data collection were performed by Pavlos Tziourrou, data analysis was performed by John Vakros and Hrissi K. Karapanagioti. The first draft of the manuscript was written by Pavlos Tziourrou, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors gave explicit consent to participate both in the research and publication.
Consent for publication
All authors gave explicit consent to submit. Consent from the responsible authorities at the institute/organization where the work has been carried out, before the work is submitted, is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Figure S1: The three glass batch reactor systems were developed for the experiment purposes (photograph from the 1st day of the experiment). Figure S2: SEM image of LDPE surface was in R2 for 33 days (up). Photomicrographs and elemental analysis (“spectrum 6” area) of the sample (LDPE in R2 bioreactor with synthetic fresh water / activate sludge / 33th day); the short arrow shows silicon (Si) which is element of the shell of diatoms (Dugdale and Wilkerson 2001). Figure S3: SEM images of LDPE surface was in R3 for 33 days. Figure S4: DR spectra of wet biofilm on LDPE from R1, R2 and R3. Figure S5: DR spectra of wet biofilm on PET from R1, R2 and R3. Figure S6: DR spectra of dry biofilm on LDPE from R1, R2 and R3. Figure S7: DR spectra of dry biofilm on PET from R1, R2 and R3. Table S1: The F(R) intensity and the wavelength (nm) for selected peaks for wet LDPE samples from R1, R2 and R3. Table S2: The F(R) intensity and the wavelength (nm) for selected peaks for wet PET samples from R1, R2 and R3. Figure S8: The graphs illustrate the occurrence frequency of peaks (wet samples). Table S3: The F(R) intensity and the wavelength (nm) for selected peaks for dry LDPE samples from R1, R2 and R3. Table S4: The F(R) intensity and the wavelength (nm) for selected peaks for dry PET samples from R1, R2 and R3. Figure S9: According to IR spectra 718, 1462, 2847 and 2915 cm−1 are the IR peaks for virgin LDPE while for IR peaks for virgin PET see Table S5. Figure S10:IR spectra of LDPE samples from the three bioreactors. Figure S11: IR spectra of PET samples from the three bioreactors. Table S5: The main of the extra IR peaks of the LDPE sample. Table S6:The table below shows the main peaks for virgin PET.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tziourrou, P., Vakros, J. & Karapanagioti, H.K. Diffuse reflectance spectroscopy (DRS) and infrared (IR) measurements for studying biofilm formation on common plastic litter polymer (LDPE and PET) surfaces in three different laboratory aquatic environments. Environ Sci Pollut Res 30, 67499–67512 (2023). https://doi.org/10.1007/s11356-023-27163-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27163-2