Abstract
Microplastic contamination in wastewater treatment plants (WWTPs) is well documented, but only a few studies have investigated plastic pollutants in waste fractions from biogas plants. We have analysed microplastic contaminants in reject water and digestate from biowaste digestion (at three time points) and sewage sludge digestion (at two time points). Samples were collected from a thermophilic biogas plant in Ostrobothnia in the western part of Finland. Plastic particles in the samples were identified with µ-FTIR (Fourier transform infrared) spectroscopy or attenuated total reflection (ATR)-FTIR, and the grain sizes of the investigated particles were > 40 µm and < 5 mm. All investigated sample matrices contained microplastic particles, but the numbers differed significantly at each sampling time. In reject water from biowaste digestion, the numbers of microplastic particles were between 80 and 3320/L. In digestate from biowaste digestion, the number of microplastic particles was between 2290 and 7200/L. Only 20–30 microplastic particles per litre were found in reject water from sewage sludge digestion, but in the digestate, the quantity of microplastic particles was between 1870 and 36,800/L. Seventeen different polymer types were identified from the samples all together. Our results show that digestate from biowaste and sewage sludge digestion can both be carriers of microplastic into the environment and can contain higher numbers of microplastics at specific time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to the numerous applications of plastics and the widespread use, plastics have become a source of pollution all over the world. In recent years, a rising environmental concern has emerged about microplastics—small plastic particles under 5 mm (GESAMP, & Kershaw, 2016). Sources of microplastics can be divided into two main types, primary and secondary. Primary microplastics are for example manufactured plastic beads under 5 mm, used in industry or domestic applications. Secondary plastics are the result of the fragmentation of larger plastic particles, by physical abrasion, ultraviolet (UV) radiation, temperature, and biological degradation (Auta et al., 2017).
Due to their small size and the fact that they are slowly degradable, microplastics are found everywhere in nature. Studies show a global spread of microplastics in the aquatic environment, from densely populated areas to remote areas of Siberia and Antarctica (Ajith et al., 2020; Cunningham et al., 2020; Materić et al., 2022). Microplastics have been found in all forms of marine life from zooplankton to whales. In the marine environment, the plastic waste contributes to 80% of the marine litter found (Landrigan et al., 2020), and microplastics have been reported to have a concentration of 100,000 particles per cubic meter in seawater and sediment (Wright et al., 2013). As much as 4–23 times more microplastics may be present in the soil than in the oceans (Wang et al., 2019). The degradation of larger plastics after improper disposal, the influence of runoff (e.g. tyres and road wear particles, maintenance and wear of artificial turf pitches), and agricultural practice (e.g. application of sewage sludge and plastic mulching) are the major pathways of microplastics into the terrestrial environment (Martinho et al., 2022).
Studies show that microplastics are abundant in wastewater, and the sources are mainly microbeads in industry and in personal care products, fibres from the laundering of synthetic clothes, tyres, and road wear particles from urban runoff. The WWTPs show good removal efficiencies of microplastics from the incoming water. Even around 99% of microplastics are removed at WWTPs (Carr et al., 2011; Lares et al., 2018; Magnusson & Norén, 2014; Murphy et al., 2016). On the other hand, it is estimated that the majority of the microplastic accumulates in the sewage sludge. A Swedish study showed that about 40% of the microplastic was separated into the sludge fraction after cleaning grids (3 mm), whilst other microplastics were assumed to accompany debris, grease, and sand (Ljung et al., 2018). In a Finnish WWTP study, it was estimated that 20% of the separated microlitter was recycled back with the reject water, and 80% was separated to the sludge (Talvitie et al., 2017). There are also a few studies showing that digestate and organic fertilisers from household biowaste fermentation can contain a large number of microplastic fragments, whilst fertilisers from agricultural biogas plants and greenery compost are less contaminated by plastics (Ljung et al., 2018; Steiner et al., 2022a, b; Weithmann et al., 2018).
There are different approaches to disposal or reuse of sewage sludge, i.e. by landfill, incineration, application to agricultural land as fertiliser, or composted into soil. About 50% of the total sewage sludge generated in Europe and North America is used as soil fertiliser (Nizzetto et al., 2016; Sol et al., 2020). During 2019–2020, almost 90% of the sewage sludge was digested in Finland. About 46% of the treated sludge was used in agriculture, 40–45% in green constructions, and 4–7% for landfills (2–3% unknown) (Vilpanen & Seppälä, 2021). Most countries allow a certain quantity of impurities such as plastics, glass, and metals in organic fertilisers. The limits are 0.3% dry weight for each impurity type (size > 2 mm) and 0.5% dry weight for the total sum of these impurities according to regulations of the European Union (European Parliament & European Union, 2019). In Finland, the limits for these impurities are set to 0.2% dry weight in packaged products and 0.5% dry weight in fertilisers sold unpackaged (Ministry of Agriculture & Forestry of Finland, 2011).
After application of sewage sludge or biowaste compost in agricultural settings, microplastics become part of the soil and can affect its properties, affect animals and plants, or spread into the marine environment. At present, there are more questions than answers about the harmful effects of microplastics in the environment. The most well-known effects are that they can cause great damage to digestive organs and gills in marine life. Microplastic is not just one material but a mixture of many different plastics and substances (e.g. stabilisers, flame-retardants or environmental toxins, and metals adsorbed on its surface) that can be harmful when ingested (Yong et al., 2020). Abnormal behaviour or growth and reproductive problems have been linked to these substances in several fish and mouse studies (Yong et al., 2020). Negative impacts on the microbiological activity in the soil and on earthworms have also been reported (Huerta Lwanga et al., 2016; Yi et al., 2021; Zhu et al., 2019). Furthermore, microplastic pollution in the soil has been observed to change the soil’s particle structure and ability to store water, as well as plant biomass and root formation can be affected (Boots et al., 2019; de Souza Machado et al., 2018, 2019).
Our aim was to collect more information about the quantity of microplastics in reject water and digestate from biogas plants and to monitor their temporal variation. A municipal waste treatment company with two thermophilic biogas digesters was selected for this case study. Reject water and digestate samples were collected at two to three timepoints during a time span of 15 months. The microplastics were extracted and characterised based on size, abundance, and plastic type with FTIR (Fourier transform infrared) spectroscopy. FTIR is a commonly used state-of-the-art analytical method in microplastic research (Käppler et al., 2016; Löder et al., 2015). To the best of our knowledge, this is the first study to monitor the occurrence of microplastic particles in both reject water and digestate from biowaste digestion over several months.
2 Material and Methods
2.1 Biogas Plant Description
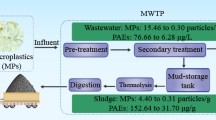
The biogas plant is a municipal waste management company located in Mustasaari municipality, in Ostrobothnia, Finland. The population size in the collection area is 105,000 inhabitants. The biogas plant includes two thermophilic (55 °C) wet anaerobic digesters (total volume 3,200 m3) with stirring and continuous feeding. Biowaste and sewage sludge are digested separately in the two digestion chambers, and the end products are kept separate. The plant processes approximately 15,000 tons biowaste (TS% 31, retention time 20 days) and 15,000 tons sewage sludge (TS% 20, retention time 14 days) per year. Municipal sewage sludge together with grease and sludge from the own treatment plant is processed in the sludge reactor. The biowaste is collected from private households, grocery stores, and commercial kitchens in the nearby municipalities. Only paper bags may be used for the collection of household waste. Large bioplastic bags (Bioska + , manufacturer Walki Plastiroll, Ylöjärvi, Finland) are used in larger garbage collection cans. The Bioska + bioplastic bags meet the European standard for industrial composting EN 13432. According to the manufacturer’s information, Bioska + bioplastic bags consist of 30–50% renewable vegetable starch, and the bags are 90% degraded within 180 days during industrial conditions. The biowaste plant removes plastics and other nonbiodegradable materials by crusher, screw presses, and metal separation before the substrate mixture is transferred into the digester. Additionally, the operators remove contaminating materials from the digestate using a drum sieve (8 mm) before the dewatering step and separation of reject water. The total volume of produced digestate from biowaste is 1577 tons per year, and digestate from sewage sludge is 6859 tons per year (dewatered digestate, TS% 26). The digestate is dewatered by centrifugation with chemical flocculants of the polyacrylamide (PAM) type. The total volume of reject water from the two reactors together is 56,000 m3 per year. The reject water is led to a wastewater treatment facility at the biogas plant before it is discharged to the sewage. Only sludge from the wastewater treatment facility is circulated back to the sludge reactor. The digestate is composted together with wood chips into soil improvement compost. The compost production is divided into two steps, an active compost phase (2 months) and an after-ripening phase (8 months). The temperature requirements are for sewage sludge-based compost 45 °C for 5 days and for biowaste-based compost 60 °C for 7 days followed by 55 °C for 7 days. The company commercialises certified soil improvement compost for gardens from the biowaste process and soil improvement compost for lawns from the sludge process (Finnish Food Authority´s approval number FIB389- 02602/2020NA).
2.2 Wastewater Treatment Plant Description
Sewage sludge used in the biogas plant is produced in a local WWTP in Vaasa, Finland. The treatment process involves multiple steps (pre-treatment, pre-sedimentation, biological treatment, sedimentation, flotation with chemical treatment, sand filtration, and nitrogen reduction). At the WWTP, the sludge is dewatered (centrifugation with polymers) prior to transport (TS% 21.6), since the WWTP is located 13 km from the biogas plant. At the biogas plant, the sludge is diluted again to TS% 7.1. In the dilution step, groundwater is used. The WWTP is treating the wastewater from ca. 70,000 inhabitants from the city of Vaasa and partly from two neighbouring municipalities (Mustasaari and Maalahti). The amount of wastewater is between 6 and 7 million m3 annually, depending on the amount of rain and meltwater.
2.3 Sample Collection
At sampling, a 4-L sample was taken from the outlet pipe of the sludge digester (before the centrifuge step for dewatering). The same amount of sample was taken from the biowaste digester’s outlet pipe, before or after the sieve step (8 mm mesh size) but prior to the centrifuge step for dewatering. The reject water samples were collected after the centrifuge step. The sampling was performed under normal running conditions at the biogas plant, and the samples were taken after a certain amount of digestate or reject water had been discharged to avoid clotted residues. A zinc bucket was used for sampling. Immediately after, the samples were divided into smaller batches and stored in glass jars (720 ml) with a screw lid to avoid contamination by plastics. The glass jars had been washed with Millipore water in advance. The digestate and reject water from biowaste digestion were sampled at three time points (in February 2020, September 2020, and in May 2021) and from sewage sludge digestion at two time points (in September 2020 and in May 2021). The February sampling of the biowaste plant was the first sampling to validate the external laboratory’s performance on this type of biological samples. The further sampling timepoints were selected as one in the autumn and one in the spring. The samples were shipped in ambient temperature to the analysing laboratory the same day as sampled, and the courier’s delivery time was 1–3 days.
2.4 FTIR Spectroscopy
Isolation of microplastic particles was performed at the ALS Scandinavia Ab laboratory in Danderyd, Sweden. ALS Scandinavia Ab is a commercial laboratory that offers identification and quantification of microplastics in environmental samples (in-house, non-accredited test) (ALS Scandinavia, n.d.). The laboratory is accredited by the Swedish accreditation body SWEDAC (Reg. No. 2030). The samples have been treated in different cleaning steps (oxidation with the Fenton reaction and addition of peroxide prior to analysis of microplastics) to remove natural organic and mineral particles. The Fenton reaction has been described as a suitable method for the extraction of microplastic particles from environmental matrices (Hurley et al., 2018). The preparation was done in a separate room (plastic-free, preparation in a fume hood, and cotton laboratory coats were used) in the laboratory. Blank samples were routinely done in parallel with an order of samples to monitor contamination. After the preparatory steps, the sample has been filtered through a 40-μm metal filter. Sample volume for filtration varied from 50 to 300 ml for reject water and 17 to 30 ml for digestate samples, depending on the quantity of organic matter remaining in the sample after the preparatory steps have been performed. The digestate samples contained more organic materials than the reject water, and especially the biowaste digestate samples contained a lot of cellulose.
Microplastic particles (including fibres), with a grain sizes > 40 μm and < 5 mm, were identified with a μ-FTIR and reference libraries of PerkinElmer and/or SiMPle (Systematic Identification of Microplastics in the Environment) software. Black particles and rubber particles that cannot be illuminated or give a light scattering reflectance were manually analysed with ATR-FTIR and separated from the analysis of other microplastics. A complementary reference library for styrene butadiene rubber (SBR) and ethylene propylene diene monomer (EPDM) spectra in PerkinElmer’s software was used for black particle and rubber particle identification. Results from FTIR were reported according to polymer type and number of microplastic particles/100 ml or particles/L. The mass of microplastic was reported in µg/100 ml. Limit of reporting (LOR) represents the standard LOR for the respective parameters in each method and may be affected if, e.g. additional dilution was required because of matrix effects, or sample quantity was limited.
2.5 Determination of Dry Weight
The dry weight (DW) of the samples was determined at the ALS Scandinavia Ab laboratory, according to the standard SS-EN 15934:2012 ed 1 and reported as total solids in percent (TS%). TS% was determined once from the different matrices (at sampling 2): reject water from biowaste digestion TS% 1.5; reject water from sewage sludge digestion TS% 0.1; digestate from biowaste digestion before sieve step TS% 2.9 and after sieve step TS% 3.3; digestate from sewage sludge digestion TS% 4.0.
The imaging system of the FTIR detector in combination with the programme SiMPle allowed the calculation of the amount of microplastics in µg and performed at the ALS Scandinavia Ab laboratory. The calculation of microplastics per g/kg DW in digestate samples was calculated according to the following simple equation by the authors:
At the ALS Scandinavia Ab laboratory, samples were analysed in volume, and no total mass of the investigated sample was noted. Therefore, the total mass of the sample was estimated based on aliquot samples of digestate from the specific sampling time.
Statistical analysis has not been performed due to the small dataset and the single-site case study constraints (it is not considered appropriate to generalise from a single case study). The study is presented as an indicator of microplastic presence in reject water and digestate from biogas plants.
3 Results and Discussion
3.1 Quantity of Microplastics Particles in Biowaste and Sewage Sludge
Microplastic load in reject water and digestate from the two digesters were detected with µ-FTIR or ATR-FTIR spectroscopy, and the sizes of the examined microplastic particles (including fibres) were > 40 µm and < 5 mm. All sample matrices collected from the biowaste and sewage sludge digester contained microplastics. Our results showed that digestate from the biowaste digester (before the sieve step) contained 0.13–11.20 g microplastics/kg DW (Table 1), which is significantly more than previously reported in digestate residues from biowaste in a Swedish study (6 mg microplastics/kg DW). The biogas plant in Sweden treated food waste and manure (Ljung et al., 2018). It was not unexpected that a high amount of microplastics in digestate from the biowaste digester would be found at the study cite. In Finland and in many other countries, current practice for collection of organic waste fractions from private households, grocery stores, and commercial kitchens calls for separate collection bins. Therefore, a pure organic fraction for biogas digestion should be obtained. In practice, this is not the case, and most biowaste contains contaminants such as plastics from food packaging. For example, often unsold food items arrive in their packaged form to the biowaste fermentation plant. In addition, biodegradable plastic bags used for collection of biowaste may also still be present in the digestate after anaerobic digestion. There is still great variation in the degradation of bioplastics during anaerobic digestion, and not all bioplastics designed to be aerobically degraded will be biodegradable under anaerobic conditions (Quecholac-Piña et al., 2020; Steiner et al., 2022a, b; Zhang et al., 2018).
In digestate from sewage sludge fermentation, the microplastic load ranged between 0.20 and 11.86 g microplastics/kg DW (Table 1) in our study. A low amount of microplastics has been reported from a sewage treatment plant in Sweden, where the amount was 0.42 g microplastics/kg DW (Ljung et al., 2018). Several other studies have analysed the occurrence of microplastic particles in sewage sludge, and the average concentrations were very variable, 1500–170,000 particles/kg DW (Sun et al., 2019). The variation in previously reported studies is mainly caused by different types of treatment processes at the WWTPs, accompanied by different detection sizes, sample preparations, and identification methods (Hartmann et al., 2019).
In our current study, a number of microplastic particles were 1870–36,800/L in digestate and 20–30/L in reject water from the sewage sludge digestion (Tables 1–2), demonstrating that a major part of the microplastic particles end up in the sludge. A similar trend was seen for the biowaste digestion in our dataset, the number of microplastics were 2290–7200/L in digestate and 80–3320/L in reject water from biowaste digestion (Tables 1–2). This is in line with the results from other WWTPs, where microplastic particles have been described to accumulate to the sludge fraction (Magnusson & Norén, 2014; Michielssen et al., 2016; Talvitie et al., 2017). For example, at Viikinmäki WWTP in Helsinki, Finland, the number of microlitter in excess and raw sludge mixture was 63,611 (± 3544)/L and in reject water 12,867 (± 275)/L (Talvitie et al., 2017). Notably, the Viikinmäki WWTP was treating 99–101 million m3 of wastewater from ca. 800,000 inhabitants at the time the study was done, compared to 6–7 million m3 of wastewater from ca. 70,000 inhabitants in our study. The study by Talvitie et al. included microlitter from excess and raw sludge, and our results included only microplastics found in digested sludge.
3.2 Plastic Particles Above 500 µm
Plastic particles above 500 µm in size were also reported but were not considered in the summary tables of microplastic abundance (Tables 1–2). Two polyethylene (PE) particles sized 0.8–1.0 cm were found in 125-ml reject water from biowaste digestion at second sampling, and five PE particles above 500 µm were detected in 20-ml digestate from biowaste (before the sieve step) at third sampling. A relatively low quantity of plastic particles above 500 µm was present in the samples.
3.3 Variations of Microplastic Concentrations
We detected very variable results during the 15 months that the monitoring continued. The number of microplastic particles in biowaste is mostly depending on the incoming type of substrates and the quantity of contaminants in the biowaste. The biowaste fermented at the study site was collected from private households, grocery stores, and commercial kitchens in the nearby municipalities. This kind of biowaste is typically contaminated by plastic bags and foils from food packaging in variable quantities (Steiner et al., 2022a, b; Weithmann et al., 2018). The polymer types found in the biowaste digestate samples were common plastic types used for packaging (Fig. 1a) and are discussed further in section “Polymer Diversity of the Detected Microplastic Particles”. However, the sewage sludge could be susceptible for seasonal variations. Seasonal variations of microplastic concentrations have been described, as being higher in hot periods, caused by higher levels of UV-radiation which help fragmentation and degradation of plastics. As well as larger numbers of microplastics are detected after rainfall due to entrance into the sewage system washed from the ground (Sol et al., 2020). This could explain the higher number of microplastics in digested sludge detected in September 2020 (36,800 particles/L) compared to May 2021 (1870 particles/L) (Table 1) in our dataset. The month of September is usually a period of high precipitation in this area of Finland that is preceded with a period of higher UV-radiation (June–August). The monthly precipitation in Vaasa in September 2020 was 111 mm and 64 mm in May 2021, which could have contributed to a higher quantity of plastics entering the sewage system (Finnish Meteorological Institute, n.d.).
3.4 The Impact of the Sieve Step Remains Unclear
Microplastic abundance in digestate from biowaste fermentation after the final sieve step ranged between 2290 and 7200 particles/L. The first sample taken before the sieve step showed a very high number of microplastic particles (107,080 particles/L) and a 15-times lower number of microplastic particles after the sieve step (7200 particles/L). At the next (third) sampling, the same effect could not be seen, 1200 microplastic particles/L were detected before the sieve step and 2290 particles/L after the sieve step (Table 1). The efficiency of the sieve step remains unclear, and more samples need to be analysed before any conclusions can be drawn. Sieving has been reported to efficiently remove bigger fragments (> 5 mm) in compost or solid organic fertilisers but is less efficient for the removal of smaller fragments (1–5 mm) (Steiner et al., 2022a, b).
3.5 Polymer Diversity of the Detected Microplastic Particles
Altogether, 17 different polymer types were identified from the study samples, 12 polymer types with µ-FTIR, and 8 polymer types with ATR-FTIR of which 3 were of the same polymer type. The polymer types identified in the samples with µ-FTIR were EPDM, ethylene vinyl acetate (EVA), polyamide (PA), kraton (trade name for high performance styrenic block copolymers), PE, polypropylene (PP), polyester, polylactic acid (PLA), polyoxymethylene (POM), polystyrene (PS), polyurethane (PUR), and polyvinylchloride (PVC).
The most abundant synthetic polymers in reject water from biowaste digestion were EVA (44%), PE (31%), and PP (14%) (Figs. 1b, 2a). The most abundant polymer in digested biowaste was EVA (61% before the sieve step; 32% after the sieve step), but also particles of PUR type were present at 25% in samples taken before the sieve step. In samples taken after the sieve step, particles of PE type were present at 28% (Figs. 1a, 2b). PE and PP are both very common types of plastic. PE is the most widely used household plastic today and is used in plastic bags, plastic film, cans, buckets, and in e.g., agricultural plastic. PP is used in bread packs, microwave packs, and jars (Plastics Europe, 2021), whilst PUR is used in paints, foam, elastic fibres, and liquid coatings (Liong et al., 2021). EVA plastic is a mixture of ethylene and vinyl acetate and is a soft and flexible plastic. EVA is used in plastic film and bags for frozen foods, but is also used in many other packaging and plastic goods industries (Mekonnen et al., 2020; Polymerdatabase.com, n.d.). Dominance of EVA particles has not been described in prior microplastic studies from digestate, to our knowledge, but has been detected in river water samples recently (Liong et al., 2021). Our results are in line with the other studies done about microplastics in organic fertilisers, in which PE and PP polymers were commonly found as well as polyester (Ljung et al., 2018; Steiner et al., 2022a, b; Weithmann et al., 2018).
Type of microplastic (%) is displayed in reject water from biowaste (n = 3) or sewage sludge (n = 2) (a), in digestate samples before sieve step (n = 2) or after sieve step from biowaste digestion (n = 3), and from sewage sludge digestion (n = 2) (b), in c, type of black particles and rubber particles (%) are displayed in digestate samples before sieve step (n = 2) from biowaste digestion and from sewage sludge (n = 2)
EVA (57%), PUR (25%), and PP (9%) were the most abundant polymers in digested sludge (Figs. 1a, 2b). The few polymer types found in reject water samples from sewage sludge digestion were PP, EVA, polyester, and kraton (Fig. 2a). Polyester, PA, PE, PP, and PS have been frequently reported in solid effluent fraction from WWTPs or digested sludge by others (Hansen & Vollertsen, 2017; Lares et al., 2018; Mintenig et al., 2017; Murphy et al., 2016). Polyester has been frequently detected in effluent samples from WWTPs (Murphy et al., 2016; Talvitie et al., 2017), with clothes washing as the most likely source. On contrary, polyester was not detected that frequent in our samples. It is possible that polyester fibres are mostly accumulated in the liquid fraction and not the solid fraction at the WWTP in our case study. Because of a limited sample size, it cannot be excluded that the observed differences are due to random variability.
Furthermore, black particles and rubber particles (such as wear of car tyres and granules used on artificial turf) were manually analysed with ATR-FTIR in our study. Black particles cannot be illuminated or give a light scattering reflectance for detection and therefore difficult to analyse with µ-FTIR. The black particles identified with ATR-FTIR were EPDM, PE, polyester urethan, polyacrolyonitrile (PAN), polybutyl methacrylate (PBMA), polyethylene terephthalate (PET), PUR, and SBR.
All sample matrices were analysed for black particles at least once, and digested sludge and biowaste (before sieve step) were analysed twice. Black particles were more abundant in digestate samples from sewage sludge (1200 and 1340 particles/L) than in digestate samples (before the sieve) from biowaste (510 and 200 particles/L) (Table 1). In the reject water sample from sewage sludge digestion, the detected black particles (40 particles/L) were of PE, PAN, PBMA, and PUR type, and in the reject water from biowaste fermentation, only 10 black PE particles/L were found. In digested biowaste (before the sieve step), the identified black particles were of either PE or SBR type (52% and 48%, respectively) (Fig. 2c). Most of the black particles in digested sludge represented SBR (31%), EPDM (31%), and PE (19%) polymers (Fig. 2c). SBR occur in car tyres, and both SBR and EPDM rubber granules are used in ground cover on playgrounds, schoolyards, and as filling material in artificial turf (Magnusson et al., 2016). Both these types of polymers are expected to be transported with the stormwater to sewage treatment plants, but the source of SBR black particles in digestate from biowaste fermentation is unclear. SBR and EPDM are relatively new kind of materials, and they have not been described in digested sludge samples before, as far as we could ascertain.
4 Conclusion
The recycling of digestate as fertilisers in agriculture is a valuable source of nutrients for plant and soil quality. Nonetheless, there are barriers to the agricultural use of digestate in terms of organic and inorganic contaminants. Our results indicate that digestate from both biowaste and sewage sludge produced by a biogas plant can contain a significant amount of microplastics and act as a transporter of microplastics into the environment. The results also show that most of the microplastic particles end up in the digestate fraction and not in the liquid fraction during biowaste treatment, which mainly have been described in WWTPs previously. Furthermore, the results demonstrate substantial variation of microplastic concentration that could be linked to seasonal variation for the sewage sludge. Meanwhile, the biowaste treatment is more dependent on how well the biowaste from households and commercial kitchens have been sorted. Decreasing the plastic content in the incoming biowaste is thus the most important measure for reducing the discharge of plastics and microplastics into the environment via digestate from biowaste. This single-site case and a small dataset are limiting factors to this study. Also, a more detailed description of the size of microplastics would have been more informative but was not available from the commercial laboratory contracted for this study. More quantification analyses are needed to gain more insight on the amount of microplastic in waste fractions from biogas plants and will increase the awareness for microplastic pollution into the terrestrial environment. A consensus about the standardised analytical process of microplastic detection in these kinds of environmental matrices needs also to be established for risk assessments to be carried out.
Data Availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Ajith, N., Arumugam, S., Parthasarathy, S., Manupoori, S., & Janakiraman, S. (2020). Global distribution of microplastics and its impact on marine environment—A review. Environmental Science and Pollution Research, 27(21), 25970–25986. https://doi.org/10.1007/s11356-020-09015-5
ALS Scandinavia. (n.d.). Microplastic analysis. https://www.alsglobal.se/en/environment/analysis-of-microplastics
Auta, H. S., Emenike, C. U., & Fauziah, S. H. (2017). Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environment International, 102, 165–176. https://doi.org/10.1016/j.envint.2017.02.013
Boots, B., Russell, C. W., & Green, D. S. (2019). Effects of microplastics in soil ecosystems: Above and below ground. Environmental Science & Technology, 53(19), 11496–11506. https://doi.org/10.1021/acs.est.9b03304
Carr, D. L., Morse, A. N., Zak, J. C., & Anderson, T. A. (2011). Biological degradation of common pharmaceuticals and personal care products in soils with high water content. Water, Air, & Soil Pollution, 217(1–4), 127–134. https://doi.org/10.1007/s11270-010-0573-z
Cunningham, E. M., Ehlers, S. M., Dick, J. T. A., Sigwart, J. D., Linse, K., Dick, J. J., & Kiriakoulakis, K. (2020). High abundances of microplastic pollution in deep-sea sediments: Evidence from Antarctica and the Southern Ocean. Environmental Science & Technology, 54(21), 13661–13671. https://doi.org/10.1021/acs.est.0c03441
de Souza Machado, A. A., Lau, C. W., Till, J., Kloas, W., Lehmann, A., Becker, R., & Rillig, M. C. (2018). Impacts of microplastics on the soil biophysical environment. Environmental Science & Technology, 52(17), 9656–9665. https://doi.org/10.1021/acs.est.8b02212
de Souza Machado, A. A., Lau, C. W., Kloas, W., Bergmann, J., Bachelier, J. B., Faltin, E., Becker, R., Görlich, A. S., & Rillig, M. C. (2019). Microplastics can change soil properties and affect plant performance. Environmental Science & Technology, 53(10), 6044–6052. https://doi.org/10.1021/acs.est.9b01339
European Parliament & European Union. (2019). Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1009&from=EN
Finnish Meteorological Institute. (n.d.). Temperature and precipitation statistics from 1961 onwards. Retrieved August 5, 2022, from https://en.ilmatieteenlaitos.fi/statistics-from-1961-onwards
GESAMP, & Kershaw, P. J., ed. (2016). “Sources, fate and effects of microplastics in the marine environment: Part two of a global assessment (Rep. Stud. GESAMP No. 93; p. 220 p). http://www.gesamp.org/publications/microplastics-in-the-marine-environment-part-2
Hansen, A. A., & Vollertsen, J. (2017). Microplastics in Danish wastewater: Sources, occurrences and fate. Technical Report. The Danish Environmental Protection Agency. Copenhagen. https://www.researchgate.net/publication/316966942_Microplastic_in_Danish_wastewater_Sources_occurrences_and_fate. Accessed 10 Aug 2022
Hartmann, N. B., Hüffer, T., Thompson, R. C., Hassellöv, M., Verschoor, A., Daugaard, A. E., Rist, S., Karlsson, T., Brennholt, N., Cole, M., Herrling, M. P., Hess, M. C., Ivleva, N. P., Lusher, A. L., & Wagner, M. (2019). Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environmental Science & Technology, 53(3), 1039–1047. https://doi.org/10.1021/acs.est.8b05297
Huerta Lwanga, E., Gertsen, H., Gooren, H., Peters, P., Salánki, T., van der Ploeg, M., Besseling, E., Koelmans, A. A., & Geissen, V. (2016). Microplastics in the terrestrial ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environmental Science & Technology, 50(5), 2685–2691. https://doi.org/10.1021/acs.est.5b05478
Hurley, R. R., Lusher, A. L., Olsen, M., & Nizzetto, L. (2018). Validation of a method for extracting microplastics from complex, organic-rich, environmental Matrices. Environmental Science & Technology, 52(13), 7409–7417. https://doi.org/10.1021/acs.est.8b01517
Käppler, A., Fischer, D., Oberbeckmann, S., Schernewski, G., Labrenz, M., Eichhorn, K.-J., & Voit, B. (2016). Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Analytical and Bioanalytical Chemistry, 408(29), 8377–8391. https://doi.org/10.1007/s00216-016-9956-3
Landrigan, P. J., Stegeman, J. J., Fleming, L. E., Allemand, D., Anderson, D. M., Backer, L. C., Brucker-Davis, F., Chevalier, N., Corra, L., Czerucka, D., Bottein, M.-Y.D., Demeneix, B., Depledge, M., Deheyn, D. D., Dorman, C. J., Fénichel, P., Fisher, S., Gaill, F., Galgani, F., & Rampal, P. (2020). Human health and ocean pollution. Annals of Global Health, 86(1), 151. https://doi.org/10.5334/aogh.2831
Lares, M., Ncibi, M. C., Sillanpää, M., & Sillanpää, M. (2018). Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Research, 133, 236–246. https://doi.org/10.1016/j.watres.2018.01.049
Liong, R. M. Y., Hadibarata, T., Yuniarto, A., Tang, K. H. D., & Khamidun, M. H. (2021). Microplastic occurrence in the water and sediment of Miri River estuary, Borneo Island. Water, Air, & Soil Pollution, 232(8), 342. https://doi.org/10.1007/s11270-021-05297-8
Ljung, E., Borg Olesen, K., Andersson, P.-G., Fältström, E., Vollertsen, J., Wittgren, H. B., & Hagman, M. (2018). Mikroplaster i kretsloppet (No. 2018–13; p. 48 p). Svenskt Vatten Utveckling. https://www.svensktvatten.se/contentassets/7be8e202754e4011a400bcff4ed89b1c/mikSVu-rap-8-13.pdf. Accessed 18 June 2022
Löder, M. G. J., Kuczera, M., Mintenig, S., Lorenz, C., & Gerdts, G. (2015). Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environmental Chemistry, 12(5), 563. https://doi.org/10.1071/EN14205
Magnusson, K., & Norén, F. (2014). Screening of microplastic particles in and down-stream a wastewater treatment plant (IVL Report C; 55). Naturvårdsverket. https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A773505&dswid=-4314. Accessed 4 Aug 2022
Magnusson, K., Eliasson, K., Fråne, A., Haikonen, K., Hultén, J., Olshammar, M., Stadmark, J., & Voisin, A. (2016). Swedish sources and pathways for microplastics to the marine environment. A review of existing data. (C 183; p. 89 p). IVL Swedish Environmental Research Institute 2. https://www.ivl.se/download/18.694ca0617a1de98f473b16/1628417679619/FULLTEXT01.pdf. Accessed 18 June 2022
Martinho, S. D., Fernandes, V. C., Figueiredo, S. A., & Delerue-Matos, C. (2022). Microplastic pollution focused on sources, distribution, contaminant interactions, analytical methods, and wastewater removal strategies: A review. International Journal of Environmental Research and Public Health, 19(9), 5610. https://doi.org/10.3390/ijerph19095610
Materić, D., Peacock, M., Dean, J., Futter, M., Maximov, T., Moldan, F., Röckmann, T., & Holzinger, R. (2022). Presence of nanoplastics in rural and remote surface waters. Environmental Research Letters, 17(5), 054036. https://doi.org/10.1088/1748-9326/ac68f7
Mekonnen, T. H., Behabtu, N., & Lenges, C. (2020). Enzymatic polymerization derived engineered polysaccharides as reinforcing fillers of ethylene vinyl acetate composites. Carbohydrate Polymers, 241, 116252. https://doi.org/10.1016/j.carbpol.2020.116252
Michielssen, M. R., Michielssen, E. R., Ni, J., & Duhaime, M. B. (2016). Fate of microplastics and other small anthropogenic litter (SAL) in wastewater treatment plants depends on unit processes employed. Environmental Science: Water Research & Technology, 2(6), 1064–1073. https://doi.org/10.1039/C6EW00207B
Ministry of Agriculture and Forestry of Finland. (2011). Maa- ja metsätalousministeriön asetus lannoitevalmisteista (No. 1784/14/2011). https://www.finlex.fi/fi/viranomaiset/normi/400001/37638. Accessed 16 Sept 2022
Mintenig, S. M., Int-Veen, I., Löder, M. G. J., Primpke, S., & Gerdts, G. (2017). Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Research, 108, 365–372. https://doi.org/10.1016/j.watres.2016.11.015
Murphy, F., Ewins, C., Carbonnier, F., & Quinn, B. (2016). Wastewater treatment works (WwTW) as a source of microplastics in the aquatic environment. Environmental Science & Technology, 50(11), 5800–5808. https://doi.org/10.1021/acs.est.5b05416
Nizzetto, L., Futter, M., & Langaas, S. (2016). Are agricultural soils dumps for microplastics of urban origin? Environmental Science & Technology, 50(20), 10777–10779. https://doi.org/10.1021/acs.est.6b04140
Plastics Europe. (2021). Plastics—The Facts 2021. An analysis of European plastics production, demand and wasste data. https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf. Accessed 18 June 2022
Polymerdatabase.com. (n.d.). Ethylene vinyl acetate copolymer (EVA) properties and applications. Polymer properties database. Free polymer information source. http://polymerdatabase.com/Polymer%20Brands/EVA.html. Accessed 11 Aug 2022
Quecholac-Piña, X., del Consuelo Hernández-Berriel, M., del Consuelo Mañón-Salas, M., Espinosa-Valdemar, R. M., & Vázquez-Morillas, A. (2020). Degradation of plastics under anaerobic conditions: A short review. Polymers, 12(1), 109. https://doi.org/10.3390/polym12010109
Sol, D., Laca, A., Laca, A., & Díaz, M. (2020). Approaching the environmental problem of microplastics: Importance of WWTP treatments. Science of The Total Environment, 740, 140016. https://doi.org/10.1016/j.scitotenv.2020.140016
Steiner, T., Möller, J. N., Löder, M. G. J., Hilbrig, F., Laforsch, C., & Freitag, R. (2022a). Microplastic contamination of composts and liquid fertilizers from municipal biowaste treatment plants: Effects of the operating conditions. Waste and Biomass Valorization. https://doi.org/10.1007/s12649-022-01870-2
Steiner, T., Zhang, Y., Möller, J. N., Agarwal, S., Löder, M. G. J., Greiner, A., Laforsch, C., & Freitag, R. (2022b). Municipal biowaste treatment plants contribute to the contamination of the environment with residues of biodegradable plastics with putative higher persistence potential. Scientific Reports, 12(1), 9021. https://doi.org/10.1038/s41598-022-12912-z
Sun, J., Dai, X., Wang, Q., van Loosdrecht, M. C. M., & Ni, B.-J. (2019). Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Research, 152, 21–37. https://doi.org/10.1016/j.watres.2018.12.050
Talvitie, J., Mikola, A., Setälä, O., Heinonen, M., & Koistinen, A. (2017). How well is microlitter purified from wastewater?–A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Research, 109, 164–172. https://doi.org/10.1016/j.watres.2016.11.046
Vilpanen, M., & Seppälä, P. (2021). Yhdyskuntalietteen käsittelyn ja hyödyntämisen nykytilannekatsaus vuosilta 2019–2020 (Vesilaitosyhdistyksen monistesarja nro 71). Finnish Water Utilities Association. https://www.vvy.fi/site/assets/files/4691/yhdyskuntalietteen_kasittelyn_ja_hyodyntamisen_nykytilannekatsaus_2021.pdf. Accessed 14 Feb 2023
Wang, J., Liu, X., Li, Y., Powell, T., Wang, X., Wang, G., & Zhang, P. (2019). Microplastics as contaminants in the soil environment: A mini-review. Science of the Total Environment, 691, 848–857. https://doi.org/10.1016/j.scitotenv.2019.07.209
Weithmann, N., Möller, J. N., Löder, M. G. J., Piehl, S., Laforsch, C., & Freitag, R. (2018). Organic fertilizer as a vehicle for the entry of microplastic into the environment. Science Advances, 4(4), eaap8060. https://doi.org/10.1126/sciadv.aap8060
Wright, S. L., Thompson, R. C., & Galloway, T. S. (2013). The physical impacts of microplastics on marine organisms: A review. Environmental Pollution, 178, 483–492. https://doi.org/10.1016/j.envpol.2013.02.031
Yi, M., Zhou, S., Zhang, L., & Ding, S. (2021). The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environment Research, 93(1), 24–32. https://doi.org/10.1002/wer.1327
Yong, C., Valiyaveettil, S., & Tang, B. (2020). Toxicity of microplastics and nanoplastics in mammalian systems. International Journal of Environmental Research and Public Health, 17(5), 1509. https://doi.org/10.3390/ijerph17051509
Zhang, W., Heaven, S., & Banks, C. J. (2018). Degradation of some EN13432 compliant plastics in simulated mesophilic anaerobic digestion of food waste. Polymer Degradation and Stability, 147, 76–88. https://doi.org/10.1016/j.polymdegradstab.2017.11.005
Zhu, F., Zhu, C., Wang, C., & Gu, C. (2019). Occurrence and ecological impacts of microplastics in soil systems: A review. Bulletin of Environmental Contamination and Toxicology, 102(6), 741–749. https://doi.org/10.1007/s00128-019-02623-z
Acknowledgements
This work has been carried out within the research and development project Bothnia Nutrient Recycling (Project partners: Ab Stormossen Oy, Novia University of Applied Sciences, Swedish University of Agricultural Science, and BioFuel Region). We thank all project partners in Bothnia Nutrient Recycling. Especially Emelia Holmström, Magnus Söderberg, and Johan Saarela are gratefully acknowledged for their help with sampling at the study cite and critical review of the paper. Tom Lillhonga at Novia University of Applied Sciences is acknowledged for assistance with the mathematical equation.
Funding
Open Access funding provided by Novia University of Applied Sciences. This work was supported by the European Regional Development Fund via the Interreg Botnia-Atlantica programme, the Regional Council of Ostrobothnia, Ab Stormossen Oy, Novia University of Applied Sciences, Swedish University of Agricultural Science, BioFuel Region, Region Västerbotten, Region Västernorrland, Härnösand Energi & Miljö, and VAKIN.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Viveka Öling-Wärnå. The first draft of the manuscript was written by Viveka Öling-Wärnå, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Öling-Wärnå, V., Åkerback, N. & Engblom, S. Digestate from Biowaste and Sewage Sludge as Carriers of Microplastic into the Environment: Case Study of a Thermophilic Biogas Plant in Ostrobothnia, Finland. Water Air Soil Pollut 234, 432 (2023). https://doi.org/10.1007/s11270-023-06436-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06436-z