Abstract
Ambient fine particulate matter (PM2.5) exposures during pregnancy could lead to adverse birth outcomes, including neurobehavioral development defects. However, limited studies explored the effects and potential epigenetic mechanisms of maternal PM2.5 exposure on offspring spatial memory defects. This study aims to explore the effects and underlying epigenetic mechanisms of maternal concentrated ambient PM2.5 exposure in male mice offspring with spatial memory defects. Pregnant female C57BL/6 mice were exposed daily to concentrated ambient PM2.5 (CAP) or filtered air (FA) throughout gestation, with the concentration of particulates (102.99 ± 78.74 μg/m3) and (2.78 ± 1.19 μg/m3), respectively. Adult male mice offspring were subsequently assessed for spatial learning and memory ability using Morris Water Maze tests and locomotor activities in open field tests. The hippocampus of the male mice offspring was harvested to test mRNA expression and DNA methylation. Results from the probe test of Morris Water Maze showed that the mice offspring in the CAP group had shorter swimming distance travelled in the target quadrant, shorter duration in the target quadrant, and less number of entries into the target quadrant (p < 0.05), suggesting spatial memory impairments. The acquisition trials of Morris Water Maze did not show a significant difference in learning ability between the groups. The mRNA level of interleukin 6 (IL-6) in the CAP group hippocampus (10.80 ± 7.03) increased significantly compared to the FA group (1.08 ± 0.43). Interestingly, the methylation levels of the CpG sites in the IL-6 promoter region declined significantly in the CAP group, (5.66 ± 0.83)% vs. (4.79 ± 0.48)%. Prenatal exposure to concentrated ambient PM2.5 induced long-lasting spatial memory defects in male mice offspring. The underlying biological mechanism might be mediated by an inflammatory reaction which is regulated by DNA methylation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Air pollution exposure is a worldwide environmental problem, which is one of the main contributors to the global disease burden (Collaborators 2018). With rapid industrial development and overpopulation, air pollution was one of the serious problems in many developing countries, including China. Air pollution contained mixtures of solid particles and liquid droplets found in the air. Among the key air pollutants, fine particulate matter (PM2.5), with aerodynamic diameters equal to or less than 2.5 μm, could be inhaled and cause adverse health effects in many different aspects. The temporary effects caused by short-term PM2.5 exposure ranged from simple discomfort (wheezing, coughing, etc.) to more serious states (asthma, pneumonia, lung and heart problems, etc.). The long-term PM2.5 exposure was harmful to the neurological, reproductive, respiratory systems, and cardiovascular and respiratory systems and causes cancer (Manisalidis et al. 2020). Studies have also suggested that perinatal exposure to ambient PM2.5 could affect placental development and function (Zanini et al. 2020), thus resulting in short-term adverse outcomes in offspring, including declined birth weight, intrauterine developmental restriction, and premature delivery (Dadvand et al. 2013; Hjortebjerg et al. 2016; Trasande et al. 2016).
The studies on the health impacts of air pollution have long been focused on cardiovascular and pulmonary systems in the past decades. However, an increasing amount of evidence demonstrated that the central nervous system can also be deleterious by air pollution exposure. Neurodevelopment began in the early life within the embryo during pregnancy, which was an important and sensitive time window for the whole lifespan. The development of the neural system contained some complex and critical developmental processes, including proliferation, migration, differentiation, synaptogenesis, myelination, and apoptosis. (Rice and Barone 2000). These processes continued to develop in the postnatal years. As the sensitive period, the nervous development in the embryo and fetus during pregnancy was vulnerable to environmental insults, including air pollutants (Shang et al. 2020), which may have consequences for the nervous system development in offspring.
The developmental origins of health and disease (DOHaD) hypothesis demonstrated that the adverse exposures in early life could permanently shape the molecular programming and contributed to later disease predisposition (Godfrey and Barker 2001, Barker 1997). Multiple studies demonstrated that a high level of PM2.5 exposure in utero and early life was associated with neurodevelopmental disorders, long-lasting behavioral alterations, and cognitive defects in offspring (Patten et al. 2020; Buoli et al. 2018). Epidemiological studies suggested that a higher level of prenatal PM2.5 exposure was associated with the declined function of memory and attention domains (Chiu et al. 2016). Animal studies demonstrated exposure to traffic-related air pollution from approximately gestational day 14 through postnatal days 41–51 affected neurodevelopment in rat offspring experiments (Patten et al. 2020). High dose of PM2.5 exposure through intranasal instillation during postnatal days 3–15 impaired spatial learning and memory abilities in immature rats (Liu et al. 2019). However, some areas of the central nervous system, such as the forebrain, midbrain, and hindbrain began to form with the neurogenesis and migration of cells earlier than the second week of gestation in rodents (since gestational day 7 in mouse, and 9.5 days in rats) (Rice and Barone 2000). The PM2.5 exposure animal models in previous studies were unlike real-world exposure (Liu et al. 2019). In addition, both population (Lertxundi et al. 2019; Chiu et al. 2016) and experimental studies (Allen et al. 2014; Bolton et al. 2017) demonstrated sex-dependent neuropsychological effects due to prenatal air pollution exposure, which is especially detrimental for males.
Previous experimental studies using animal models tried to explore the cognitive consequences of air pollution exposure. However, the neurotoxicological mechanism underlying these associations had yet to be clarified. Furthermore, the biological mechanisms were likely to differ depending upon the exposure period underway the brain development period, and the components of the air pollution exposure itself (Allen et al. 2017a). The potential contributory mechanism of PM2.5 exposure affecting cognition and behavior included oxidative stress/inflammation (Zanchi et al. 2010; Gerlofs-Nijland et al. 2010), DNA methylation (Boda et al. 2020), and structural synaptic plasticity disruption (Liu et al. 2019). DNA methylation, a characterized epigenetic modification, might be one of the potential mechanisms of early-life PM2.5 exposure and health effects in adulthood. Epidemiological studies indicated that prenatal environmental conditions resulted in epigenetic changes which persist the whole life (Heijmans et al. 2008). Evidence from animal studies indicated that certain environmental conditions in utero could cause persistent epigenetic dysregulation, resulting in life-long consequences (Anway et al. 2005). Both the global and gene-specific methylation could be affected by particulate matter (Baccarelli et al. 2009; Madrigano et al. 2011).
In our present study, the real-world PM2.5 exposure system was used to explore the long-term effects of maternal exposure to concentrated ambient PM2.5 on male mice offspring spatial learning and memory competence. Furthermore, the hypothesis of potential mechanisms was that methylation modification plays an important role in the effects of prenatal PM2.5 exposure on spatial memory in mice offspring.
Methods
Animal exposure to PM2.5
The study design is summarized in Fig. 1. A total of 40 female and 20 male 6-week-old C57BL/6 mice were obtained from Shanghai Lingchang Biotech Limited Company (certification No. 2013001821608). After a 1-week adaptation period, every two female mice were mated with one male mouse per cage. Seminal plugs were then checked 12–18 h after the breeding pairs were cohoused. Gestation day 0 (GD0) was defined as the day on which upon the appearance of a vaginal plug. There was a total of 30 plug-positive mice which were considered pregnant. The pregnant mice were divided into a concentrated ambient PM2.5 group (CAP) (n = 15) and a filtered air group (FA) (n = 15) randomly.
Animals were respectively exposed to filtered air or concentrated ambient PM2.5 in “Shanghai Meteorological and Environmental Animal Exposure System, Shanghai-METAS” (patent #2015104536008-) (Du et al. 2018; Yang et al. 2019) from GD0 to the delivery day. The “Shanghai-METAS” is located in the center of Shanghai city, and most of the PM2.5 components are attributed to traffic exhausts. The concentrated PM2.5 in the exposure chamber was generated using a versatile aerosol concentration enrichment system, while the air in the control chamber was filtered by a high-efficiency particulate-air filter which removed 98% of ambient particles (Pan et al. 2021; Sun et al. 2005; Maciejczyk et al. 2005). In the living environment of “Shanghai-METAS,” the light circle was 12 h light/12 h dark, the temperature was18–25 °C, and the relative humidity was 40–60%. Food and water were provided ad libitum. The duration of the perinatal exposure was 8 h per day, 7 days per week for about 18 days. The average PM2.5 concentrations in the FA and CAP chambers during the exposure period were (2.78 ± 1.19) μg/m3 and (102.99 ± 78.74) μg/m3, respectively. The average ambient PM2.5 concentration during the exposure period was (36.57 ± 17.46) μg/m3.
In both of CAP and FA groups, the number of dams was 15. Among them, 6 dams from each group were randomly selected and sacrificed for cord blood collection at gestational 16 days. The other 9 dams gave birth 20 male offspring in each group. After giving birth, the maternal mice and pubs were raised in the SPF animal room for 3 weeks, when the pubs were weaned and redistributed by gender. All the male offspring were conducted for the Morris Water Maze test and open field test at postnatal 5 weeks. When finished with the behavior tests, the mice offspring were sacrificed for hippocampus collection. Among them, 10 hippocampus tissues from each group were selected randomly for methylation analysis, and 6 hippocampus tissues were selected randomly from each group for mRNA analysis. The investigators who conducted the Morris Water Maze test, open field test, and tissue samples analysis were all blinded to the group allocation.
The Animal Experimental Ethics Committee of the Department of Laboratory Animal Science, Fudan University, approved all procedures of this study, with an ethics reference number 201805003Z. All animals were treated humanely and with regard for the alleviation of suffering.
Morris water maze test
Spatial learning and memory ability were assessed using the Morris water maze test when the offspring mice were 5 weeks old. The Morris Water Maze device was a cylindrical tank (120 cm in diameter and 50 cm in depth). The pool was filled up with tap water of 30 cm in depth, which was colored opaque with powdered non-fat milk or non-toxic tempera paint. The device was artificially divided into four quadrants (Northwest (NW), Northeast (NE), Southeast (SE), Southwest (SW)), using four points on the rim of the pool, North (N), South (S), East (E), and West (W) (not true magnetic directions). The escape platform (6 cm in diameter) was placed 1 cm below the surface of the water at the southwest quadrants (target quadrant). In the acquisition test phase, each mouse was trained in four trials per day for five consecutive days. In each trial, the mouse was gently placed into the pool facing the wall at one of the four quadrants, randomly. The trial was completed when the mouse swam to and climbed onto the hidden goal platform. If the mouse failed to locate the platform within 60 s, it was guided to the platform by a stick. The mice were left to stay on the platform for 30 s, before they were returned to the cages. The time to reach the platform was recorded as escape latency. The probe test was conducted following the acquisition test. After all the mice had completed the acquisition trials, the platform was removed for probe test 24 h after the last acquisition day. In the probe test, the mouse was released from the opposite quadrant of the target quadrant and was allowed to swim freely for 1 min. The swimming distance within the target quadrant, the time spent in the target quadrant, and the number of target quadrant entries were recorded during the 60 s by the ANY-Maze video tracking system (Stoelting Co., U.S.A). All tracks from all trials were analyzed for several behavioral parameters using SMART software (Panlab).
Open field test
The open field test was applied to assess the offspring’s ability to cope with escapable stressful situations (Laugeray et al. 2018). To let the mice acclimate to the experimental room, the mice were transported to the test room and left undisturbed for 30 min before the test. The open field was set up according to the experimental manufacturer. Each mouse was placed in a corner of the open field individually and left for 10 min to explore the entire apparatus. The EthoVision video-tracking system (Noldus, The Netherlands) was applied to record and analyze the activities. The travel variables, including the total travelled distance, the time spent in the center, in the intermediary, and the intermediary in the arena as well as the mean speed in each zone, were automatically recorded. We wiped the apparatus clean using a 50% ethanol/water solution and allow time for it to dry between mice.
The mice were sacrificed when both the Morris Water Maze test and open field test were done. The hippocampus was harvested to conduct DNA methylation analysis (10 samples per group) and mRNA analysis (6 samples per group).
Analysis of the DNA methylation status
The genomic DNA of the hippocampus was extracted using the TIANamp Genomic DNA Kit (TIANGEN BIOTECH, China). Genomic DNA bisulfite conversion was performed using the EpiTeck Bisulfite Kit (QIANGEN), which was ready for bisulfite sequencing PCR (BSP) analysis. The primers of IL-6, IL-1β, TNF-α, and BDNF were designed using PyroMark Assay Design 2.0 (BGI, China). The target promoter sequences of IL-6, IL-1β, TNF- α, and BDNF were amplified from the converted DNA performed through touch-down PCR, and then the purified PCR products were cloned into pMD-18 T vector (Takara, China) for sequencing. Pyrosequencing was performed using PyroMark Q96 ID software (QIAGEN), and the CpG Island level was analyzed automatically.
Quantitative real-time PCR
Total RNA was extracted from the hippocampus using TRIzol® reagent (Invitrogen, USA) according to the manufacturer’s instruction. The RNA was then reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit (Takara Bio, Inc.), at 37 ℃ for 15 min and 80℃ for 5 s. PCR amplification was performed on a StepOnePlus™ real-time PCR detection system using TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus) (cat. no. RR820A, Takara Bio, Inc.). The following conditions were used for PCR amplification: initial denaturation at 95℃ for 30 s, followed by 40 cycles of denaturation at 95 ℃ for 30 s, annealing at 60 ℃ for 30 s. The primer sequences for IL-6, interleukin 1β (IL-1β), tumor necrosis factor-a (TNF-a), brain-derived neurotrophic factor (BDNF), and β-actin shown in Table 1 were designed and synthesized by primer Express Software (Beijing Genomic Institute, China. Relative changes of mRNA expression were analyzed using the 2−△△Ct method (Livak and Schmittgen 2001) and normalized to the internal reference gene GAPDH. All experiments were performed in triplicate.
Statistical analysis
Continuous variables were reported as the mean ± standard error (SE) when met normal distribution; otherwise, the median and interquartile range were reported. The Kolmogorov–Smirnov test was applied to determine the normal distribution. The differences between two groups of body weight, tissue weight, metal concentration, open field test data, mRNA, and DNA methylation data were compared using a two-tailed students’ t-test. For the data of probe trials in Morris Water Maze were not normally distributed, Mann–Whitney U test by rank was used to compare the differences between groups. The repeated measurement of 5-day average escape latency in the acquisition trials of Morris Water Maze was analyzed using two-way repeated measures ANOVA with Bonferroni post-hoc test (Vorhees and Williams 2006). Furthermore, the data of acquisition trials in Morris Water Maze at each time point did not fit a normal distribution; the differences between groups were evaluated using Mann–Whitney U test by rank. Data statistical analyses were conducted using SPSS software (SPSS program, version 26.0, Chicago, IL, USA). A p-value less than 0.05 was considered statistically significant. The graphs were generated using GraphPad Prism software for Windows (Version 9.2.0, GraphPad Software, Inc).
Results
PM2.5 exposure concentration
PM2.5 exposure concentrations were estimated by averaging particle weights from multiple Teflon filters across the treatment period. The average PM2.5 concentrations in the FA and CAP chambers were comparable. CAP filters contained almost 43-fold increase in the concentration of particulates (102.99 ± 78.74) μg/m3 compared to FA filters (2.78 ± 1.19) μg/m3and 2.82-fold the concentration found in the ambient air (36.57 ± 17.46) μg/m3. Filters from ambient PM2.5 were collected and further analyzed for particle components including carbons, elements, and ions. Detailed analysis results of particle components can be found in the supplementary materials.
Pups body weight, tissue weight, lead and mercury concentrations of dams’ cord blood
As shown in Table 2, maternal exposure to CAP did not significantly alter the mice offspring’s body weight. Significant differences in the weights of offspring’s hippocampus and prefrontal cortex between the CAP and FA groups were not observed. The concentrations of lead and mercury in the dams’ blood were tested. The mercury concentration of the dams’ cord blood in the CAP group (4.77 ± 0.84) μg/kg was higher than that in the FA group (2.63 ± 0.15) μg/kg, with statistical significance (p = 0.030). The lead concentration of the dams’ cord blood in the CAP group was (4.69 ± 0.61) μg/L and (4.47 ± 0.81) μg/L in the FA group, and the difference was not statistically significant (p = 0.837).
Maternal exposure to CAP impaired the spatial memory ability in male mice offspring
Spatial learning and memory ability of mice offspring was evaluated by performing the Morris water maze. The percentage time in each quadrant of the Morris water maze performance on each day during the acquisition trials is shown in Fig. 2A–D. Increasing trends of percentage time were observed in the target quadrant (quadrant SW, Fig. 2D) in both groups, suggesting the mice offspring had the ability of discriminating between the target quadrant and all other quadrants. Changes in escape latency time in 5-day acquisition trials are shown in Fig. 2E. Analysis of two-way repeated measures ANOVA showed that there was no significant interaction between groups and days (interaction effects: F(4, 76) = 0.272, p = 0.895). The escape latency of training mice decreased as training days increased (time main effect: F(4, 76) = 7.956, p < 0.001), indicating that mice in each group had learning abilities. The differences of the escape latency between the two groups were not statistically significant (group main effect: F(1, 19) = 0.663, p = 0.426). Specifically, the results of Mann–Whitney U test showed that the escape latency on each day of acquisition trials did not differ between the groups.
Maternal concentrated ambient PM2.5 exposure impaired the spatial memory ability in male mice offspring tested by Morris Water maze. A–D Percent time in each quadrant of Morris water maze performance on each day during the acquisition trials. A Quadrant NW, B Quadrant NE, C Quadrant SE, D Quadrant SW). E Escape latency during the training days; F Swimming distances within the target quadrant; G time spent in the target quadrant; H number of entries in the target quadrant. (n = 20 per group). Abbreviations: FA, filtered air; CAP, concentrated ambient PM2.5. NW, Northwest; NE, Northeast; SE, Southeast; SW, Southwest
The results from the probe test of Morris Water Maze showed that, compared with the FA group, the swimming distance within the target quadrant was significantly shorter in the CAP group (p < 0.05) (Fig. 2F). In addition, the time spent in the target quadrant and the number of target quadrant entries in CAP group were significantly less than FA group (p < 0.05) (Fig. 2G, H). Collectively, the results showed that maternal exposure to high concentration PM2.5 impaired the spatial memory ability in mice offspring.
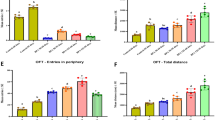
Maternal exposure to CAP did not differ the anxiety-like behavior in male mice offspring
The open field test was conducted to assess whether maternal exposure to concentrated ambient PM2.5 altered the spontaneous locomotor activity and exploratory behavior in mice offspring. No statistically significant differences were detected in total distance travelled (Fig. 3A), total movement duration (Fig. 3D), distance travelled, and duration in the periphery area (Fig. 3C, F) between the two groups of mice offspring. Although the CAP group offspring showed shorter travelling distance and duration in the central area than the FA group (Fig. 3B, E), the differences between the two groups were not statistically significant. The results of the open field test suggested that anxiety-like behavior in mice offspring did not differ.
Maternal concentrated ambient PM2.5 exposure did not impact anxiety-like activity in male mice offspring. A Total distance travelled; B distance travelled in central area; C distance travelled in periphery area; D movement duration; E duration stayed in central area; F duration stayed in periphery (n = 20 per group). Abbreviations: FA, filtered air; CAP, concentrated ambient PM2.5
mRNA expression levels of inflammation related cytokines in mice offspring hippocampus
To investigate the effects of PM2.5 exposure on the expression levels of IL-6, IL-1β, TNF-α, and BDNF in the hippocampus, quantitative real-time PCR was performed. The mRNA level of IL-6 in the CAP group (10.80 ± 7.03) increased significantly in the hippocampus than that in the FA group (1.08 ± 0.43) (Fig. 4A). We did not observe a significant difference in mRNA levels of IL-1β, TNF-α, and BDNF between the CAP and FA groups (Fig. 4B, C, D).
The expressions of inflammation-related mRNA (A–D) and DNA methylation modification (E–H) in the hippocampus. The data are expressed as the (mean ± SE). N = 6 per group for mRNA analysis and three replicates were conducted for each sample. N = 10 per group for DNA methylation analysis. Abbreviations: FA, filtered air; CAP, concentrated ambient PM2.5; SE, standard error. Students’ t-test were used to test the difference between the two groups
Analysis of DNA methylation modification
The effects of PM2.5 exposure during pregnancy on the methylation modification in the IL-6, IL-1β, TNF-α, and BDNF promoter regions in the hippocampus were investigated using bisulfite sequencing PCR. As shown in Fig. 4E, compared with the FA group (5.66 ± 0.83) %, the methylation levels of the CpG sites in the IL-6 promoter region declined significantly in the CAP group, (4.79 ± 0.48) %. The differences in the methylation levels of IL-1β, TNF-α, and BDNF between the two groups were not significant (Fig. 4F, G, H).
Discussion
Increasing evidence demonstrated that concentrated ambient particulate matter exposure affected the behavioral, neurochemical, and neuropathological development (Allen et al. 2013, 2017b). The relationship between PM2.5 exposure and neurodevelopment has become a great public health concern in recent decades. The conclusions of existing epidemiology studies from different populations and animal models were somewhat inconsistent, which might be owing to the discrepancy in particle components, particle size, exposure duration, and exposure level in the various studies (Kim et al. 2015). The neurotoxicological mechanisms of air pollution impairment had not yet been identified, for it was likely to differ depending on the specific nature of the cognitive impairment, the developmental period of exposure, the components of pollution, and even sex (Allen et al. 2017a). Notably, studies suggested that prenatal air pollution exposure showed sex-specific neuropsychological effects in offspring (Lertxundi et al. 2019; Bolton et al. 2017), which specifically caused stronger effects on males. In particular, we focused on the PM2.5 exposure effects on male offspring.
In the current study, concentrated PM2.5 exposure during pregnancy reduced the male mice offspring spatial memory ability assessed by the Morris water maze test. Our results were consistent with previous reports. Studies reported that neonatal rat exposure to PM2.5 through intranasal instillation impaired spatial learning and memory abilities led to increased anxiety-like symptoms and apparent depressive-like behaviors (Liu et al. 2019). Gestational PM2.5 exposure led to neurobehavioral defects including anxiety- and depression-like behavior, tested by the open field test and tail suspension test (Wang et al. 2020). An epidemiological study reported that prenatal exposure to ambient particulate matter was negatively associated with children’s neurodevelopment throughout the first 24 months of life (Kim et al. 2014). However, Hougaard et al. (2008). found that the cognitive function and levels of biomarkers of prenatal exposure to diesel exhaust particles (DEP) were generally similar in exposed and control offspring. The inconsistent results might be due to the composition of the particulates and exposure methods. Meanwhile, it should be noted that most of the PM2.5 components are attributed to traffic exhausts; therefore, PM2.5 from other sources may not necessarily lead to similar results from this study.
The developing central nervous system was especially susceptible to the toxic effects of metals and metal components, including mercury and lead. Epidemiological studies demonstrated that low levels of prenatal Hg exposure may cause early childhood neurocognitive effects (Karagas et al. 2012). There was convincing experimental evidence that exposure to low levels of neurotoxic substances during vulnerable developmental periods can induce permanent functional disturbances in the central nervous system (Andersen et al. 2000). We found a higher mercury concentration in the dams’ cord blood in the concentrated PM2.5 group. Collectively, the metals consisted in PM2.5 might play an important role in the negative effect on spatial learning of prenatal PM2.5 exposure.
Growing evidence suggested that air pollutants may adversely affect the central nervous system. Peripheral inflammation itself can adversely affect the central nervous system, thus direct translocation of particle pollutants into the brain would not be necessary to elicit neurotoxicity (Allen et al. 2013). Acutely exposed to diesel exhaust showed microglia activation, increased lipid peroxidation, and neuro-inflammation in various mice brain regions, particularly the hippocampus and the olfactory bulb (Costa et al. 2017). Air pollution exposure–induced neuroinflammation might be one of the potential mechanisms of exposure-induced adverse outcomes on the nervous system (Calderon-Garciduenas et al. 2015). In the current study, gestational exposure to concentrated PM2.5 elevated the mRNA expression of the inflammatory cytokine IL-6 in the hippocampus of offspring mice. Significant differences in IL-1β, TNF-α, and BDNF mRNA expression between the two groups were not observed. Zheng et al. (2018) also demonstrated that gestational exposure to PM2.5 was associated with increased secretions of inflammatory proteins (including NF-κB, TNF-α, IL-1β) with a dose–response relationship. A clinical study reported that PM2.5 exposure significantly increased the concentration of IL-6 and TNF-α compared to baseline, while without a significant decrease of BDNF concentration in the sera of adults (Cliff et al. 2016). However, molecular epidemiological evidence from a mother-infant pairs cohort study reported that PM2.5 exposure in utero was negatively associated with the placental expression of BDNF at birth (Saenen et al. 2015). PM2.5 exposure after birth decreased BDNF expression level of BDNF in the hippocampus. These inconsistent results between different studies may be ascribed to differences in neurodevelopmental stage, PM2.5 exposure duration, and the specimen sample. In the central nervous system, IL-6 is an important signaling molecule. IL-6 is produced by resident cells in the brain. Under normal conditions, the expression of IL-6 in the central nervous system is generally low (Gruol 2015). The increasing expression of IL-6 was involved in the deterioration of cognitive functions during aging and neurodegenerative diseases (Weaver et al. 2002). Although the current study could not provide direct evidence of a causal relationship between elevated IL-6 expression and spatial memory impairment, the causal relationship could be supported by previous studies. IL-6 over-expression mice in the central nervous system exhibited inflammatory neurodegeneration and learning impairment (Heyser et al. 1997). Moreover, IL-6 deficiency mice (IL-6 knockout) presented better spatial reference memory, slower age-related memory decline (Bialuk et al. 2018), and improvement in long-term memory (Bialuk and Winnicka 2018).
Epigenetic regulation of gene transcription, including DNA methylation, is sensitive to environmental pollution. Environmental pollution not only affects the total methylation modification but also affects the methylation of specific genes. Gestational exposure to air pollutants can lead to locus-specific changes in gene methylation, which is involved in cellular responses to inflammation and early-life development, in newborn cord blood and placenta (Isaevska et al. 2021). In the present study, high-level PM2.5 exposure during pregnancy significantly elevated the mRNA levels of IL-6 in the hippocampus tissue. Interestingly, PM2.5 exposure during pregnancy induced lower methylation levels of the CpG sites in the IL-6 promoter region. Previous evidence suggested that methylation modification was an essential epigenetic mechanism regulating IL-6 expression (Poplutz et al. 2014), and the DNA methylation levels of the CpG islands in the IL-6 promoters were inversely significantly correlated to the corresponding mRNA expression (Tekpli et al. 2013). In addition, increasing studies demonstrated that epigenetic mechanisms, including DNA methylation, play important roles in neurogenesis (Wang et al. 2016). Our results suggested that the hypomethylation level in the IL-6 gene was associated with higher mRNA levels of IL-6 proteins in the hippocampus, consequently resulting in spatial memory impairment.
To our knowledge, the current study was the first one to explore the epigenetic mechanism of DNA methylation, for the male offspring memory impairment induced by prenatal particulate matter exposure. Notably, a whole-body inhalational system, “Shanghai METAS,” was used to establish the animal exposure model. As the key part of “Shanghai METAS,” the versatile aerosol concentration enrichment system (VACES) had been modified, assembled, tested, and validated by researchers (Maciejczyk et al. 2005). Compared with the intratracheal instillation exposure way, the “Shanghai METAS” can mimic human’s real-world exposure to the environmentally relevant PM2.5 or filtered air to the maximum extent (Pan et al. 2021; Yang et al. 2019; Ying et al. 2014).
Despite the novelty of the findings, there were still several limitations in this study. Although the PM2.5 exposure duration might be considered real-world, the concentrations used were not likely real-world. The current experiment did not expose the dams with PM2.5 of ambient concentrations, which may constitute a more reasonable exposure gradient and reflect real-world exposure scenarios. The PM2.5 concentration in concentrated PM2.5 exposure was higher than the clinic and epidemiology reports, which might not reflect the effects on spatial memory of conventional PM2.5 concentration in the atmospheric environment. Therefore, the results should be interpreted with certain environmental implications, such as pregnant women living in heavily polluted regions. Neural development was a developmental process and the effects of particulate matter exposure during pregnancy on the memory ability of offspring at different growth stages were not measured. Morris Water maze was used to assess the spatial learning and memory ability. However, the minor time spent in the target quadrant in the probe test of Morris Water Maze performance could be attributed to less persevering behavior or perhaps an adaptive advantage. Although the compositions of the ambient particulate matter were measured, it was not possible to further analyze the specific components that caused the impairment of the offspring’s memory ability. Instead of measuring the global methylation, selected genes were methylated based on previous studies and hypotheses. The inflammatory factors and BDNF were tested only in the hippocampus but other brain regions probably involve in the memory deficits, such as the prefrontal cortex. Although we found the changes in mRNA and DNA methylation of IL-6, the results could not provide direct evidence for a causal relationship between these and the spatial memory impairment. Finally, we did not explore the neurodevelopment effects on female mice offspring of gestational PM2.5 exposure. Further studies will be warranted to illustrate these problems in the future.
Conclusion
Together, our results demonstrated that maternal exposure to concentrated ambient PM2.5 induced long-lasting spatial memory development defects in male mice offspring. The underlying biological mechanism might be mediated by the inflammatory reaction which is regulated by DNA methylation. Future studies are needed to explore the effects of high PM2.5 exposure during pregnancy on offspring spatial memory ability and to clarify the biological mechanisms involved.
Data availability statement
The data supporting the conclusions of this article are available from the corresponding author upon reasonable request.
References
Allen JL, Conrad K, Oberdorster G, Johnston CJ, Sleezer B, Cory-Slechta DA (2013) Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environ Health Perspect 121:32–38
Allen JL, Klocke C, Morris-Schaffer K, Conrad K, Sobolewski M, Cory-Slechta DA (2017a) Cognitive effects of air pollution exposures and potential mechanistic underpinnings. Curr Environ Health Rep 4:180–191
Allen JL, Liu X, Weston D, Prince L, Oberdorster G, Finkelstein JN, Johnston CJ, Cory-Slechta DA (2014) Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol Sci 140:160–178
Allen JL, Oberdorster G, Morris-Schaffer K, Wong C, Klocke C, Sobolewski M, Conrad K, Mayer-Proschel M, Cory-Slechta DA (2017b) Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology 59:140–154
Andersen HR, Nielsen JB, Grandjean P (2000) Toxicologic evidence of developmental neurotoxicity of environmental chemicals. Toxicology 144:121–127
Anway MD, Cupp AS, Uzumcu M, Skinner MK (2005) Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308:1466–1469
Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J (2009) Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med 179:572–578
Barker DJ (1997) Fetal nutrition and cardiovascular disease in later life. Br Med Bull 53:96–108
Bialuk I, Taranta A, Winnicka MM (2018) IL-6 deficiency alters spatial memory in 4- and 24-month-old mice. Neurobiol Learn Mem 155:21–29
Bialuk I, Winnicka MM (2018) Facilitatory effect of IL-6 deficiency on long-term spatial memory in young adult mice. Behav Genet 48:236–246
Boda E, Rigamonti AE, Bollati V (2020) Understanding the effects of air pollution on neurogenesis and gliogenesis in the growing and adult brain. Curr Opin Pharmacol 50:61–66
Bolton JL, Marinero S, Hassanzadeh T, Natesan D, Le D, Belliveau C, Mason SN, Auten RL, Bilbo SD (2017) Gestational exposure to air pollution alters cortical volume, microglial morphology, and microglia-neuron interactions in a sex-specific manner. Front Synaptic Neurosci 9:10
Buoli M, Grassi S, Caldiroli A, Carnevali GS, Mucci F, Iodice S, Cantone L, Pergoli L, Bollati V (2018) Is there a link between air pollution and mental disorders? Environ Int 118:154–168
Calderon-Garciduenas L, Calderon-Garciduenas A, Torres-Jardon R, Avila-Ramirez J, Kulesza RJ, Angiulli AD (2015) Air pollution and your brain: what do you need to know right now. Prim Health Care Res Dev 16:329–345
Chiu YH, Hsu HH, Coull BA, Bellinger DC, Kloog I, Schwartz J, Wright RO, Wright RJ (2016) Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ Int 87:56–65
Cliff R, Curran J, Hirota JA, Brauer M, Feldman H, Carlsten C (2016) Effect of diesel exhaust inhalation on blood markers of inflammation and neurotoxicity: a controlled, blinded crossover study. Inhal Toxicol 28:145–153
Collaborators GBD 2017 Risk Factor (2018) Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1923–1994
Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque PJ (2017) Neurotoxicity of traffic-related air pollution. Neurotoxicology 59:133–139
Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, Gehring U, Glinianaia SV, Gouveia N, Ha EH, Leem JH, van den Hooven EH, Jalaludin B, Jesdale BM, Lepeule J, Morello-Frosch R, Morgan GG, Pesatori AC, Pierik FH, Pless-Mulloli T, Rich DQ, Sathyanarayana S, Seo J, Slama R, Strickland M, Tamburic L, Wartenberg D, Nieuwenhuijsen MJ, Woodruff TJ (2013) Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect 121:367–373
Du XH, Jiang S, Zeng XJ, Zhang J, Pan K, Zhou J, Xie YQ, Kan HD, Song WM, Sun QH, Zhao JZ (2018) Air pollution is associated with the development of atherosclerosis via the cooperation of CD36 and NLRP3 inflammasome in ApoE(-/-) mice. Toxicol Lett 290:123–132
Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RP, Wang K, Campbell A (2010) Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol 7:12
Godfrey KM, Barker DJ (2001) Fetal programming and adult health. Public Health Nutr 4:611–624
Gruol DL (2015) IL-6 regulation of synaptic function in the CNS. Neuropharmacology 96:42–54
Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH (2008) Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 105:17046–17049
Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH (1997) Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A 94:1500–1505
Hjortebjerg D, Andersen AMN, Ketzel M, Pedersen M, Raaschou-Nielsen O, Sorensen M (2016) Associations between maternal exposure to air pollution and traffic noise and newborn’s size at birth: a cohort study. Environ Int 95:1–7
Hougaard KS, Jensen KA, Nordly P, Taxvig C, Vogel U, Saber AT, Wallin H (2008) Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Part Fibre Toxicol 5:3
Isaevska E, Moccia C, Asta F, Cibella F, Gagliardi L, Ronfani L, Rusconi F, Stazi MA, Richiardi L (2021) Exposure to ambient air pollution in the first 1000 days of life and alterations in the DNA methylome and telomere length in children: a systematic review. Environ Res 193:110504
Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, Cowell W, Grandjean P, Korrick S (2012) Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect 120:799–806
Kim E, Park H, Hong YC, Ha M, Kim Y, Kim BN, Kim Y, Roh YM, Lee BE, Ryu JM, Kim BM, Ha EH (2014) Prenatal exposure to PM(1)(0) and NO(2) and children’s neurodevelopment from birth to 24 months of age: mothers and children’s environmental health (MOCEH) study. Sci Total Environ 481:439–445
Kim KH, Kabir E, Kabir S (2015) A review on the human health impact of airborne particulate matter. Environ Int 74:136–143
Laugeray A, Oummadi A, Jourdain C, Feat J, Meyer-Dilhet G, Menuet A, Ple K, Gay M, Routier S, Mortaud S, Guillemin GJ (2018) Perinatal Exposure to the Cyanotoxin beta-N-M, thylamino-L-alanine (BMAA) results in long-lasting behavioral changes in offspring-potential involvement of DNA damage and oxidative stress. Neurotox Res 33:87–112
Lertxundi A, Andiarena A, Martinez MD, Ayerdi M, Murcia M, Estarlich M, Guxens M, Sunyer J, Julvez J, Ibarluzea J (2019) Prenatal exposure to PM2.5 and NO2 and sex-dependent infant cognitive and motor development. Environ Res 174:114–121
Liu J, Yang C, Yang J, Song X, Han W, Xie M, Cheng L, Xie L, Chen H, Jiang L (2019) Effects of early postnatal exposure to fine particulate matter on emotional and cognitive development and structural synaptic plasticity in immature and mature rats. Brain Behav 9:e01453
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC (2005) Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal Toxicol 17:189–197
Madrigano J, Baccarelli A, Mittleman MA, Wright RO, Sparrow D, Vokonas PS, Tarantini L, Schwartz J (2011) Prolonged exposure to particulate pollution, genes associated with glutathione pathways, and DNA methylation in a cohort of older men. Environ Health Perspect 119:977–982
Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E (2020) Environmental and health impacts of air pollution: a review. Front Public Health 8:14
Pan K, Jiang S, Du X, Zeng X, Zhang J, Song L, Lei L, Zhou J, Kan H, Sun Q, Xie Y, Dong C, Zhao J (2021) Parental PM2.5 exposure changes Th17/Treg cells in offspring, is associated with the elevation of blood pressure. Environ Toxicol 36:1152–1161
Patten KT, Gonzalez EA, Valenzuela A, Berg E, Wallis C, Garbow JR, Silverman JL, Bein KJ, Wexler AS, Lein PJ (2020) Effects of early life exposure to traffic-related air pollution on brain development in juvenile Sprague-Dawley rats. Transl Psychiatry 10:166
Poplutz MK, Wessels I, Rink L, Uciechowski P (2014) Regulation of the interleukin-6 gene expression during monocytic differentiation of HL-60 cells by chromatin remodeling and methylation. Immunobiology 219:619–626
Rice D, BaroneJr S (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108(Suppl 3):511–533
Saenen ND, Plusquin M, Bijnens E, Janssen BG, Gyselaers W, Cox B, Fierens F, Molenberghs G, Penders J, Vrijens K, de Boever P, Nawrot TS (2015) In utero fine particle air pollution and placental expression of genes in the brain-derived neurotrophic factor signaling pathway: an environage birth cohort study. Environ Health Perspect 123:834–840
Shang L, Yang L, Yang W, Huang L, Qi C, Yang Z, Fu Z, Chung MC (2020) Effects of prenatal exposure to NO2 on children’s neurodevelopment: a systematic review and meta-analysis. Environ Sci Pollut Res Int 27:24786–24798
Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S (2005) Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 294:3003–3010
Tekpli X, Landvik NE, Anmarkud KH, Skaug V, Haugen A, Zienolddiny S (2013) DNA methylation at promoter regions of interleukin 1B, interleukin 6, and interleukin 8 in non-small cell lung cancer. Cancer Immunol Immunother 62:337–345
Trasande L, Malecha P, Attina TM (2016) Particulate matter exposure and preterm birth: estimates of U. S. attributable burden and economic costs. Environ Health Perspect 124:1913–1918
Vorhees CV, Williams MT (2006) Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1:848–858
Wang X, Wang TT, Sun LJ, Zhang HY, Liu C, Zhang C, Yu L (2020) B-vitamin supplementation ameliorates anxiety- and depression-like behavior induced by gestational urban PM2.5 exposure through suppressing neuroinflammation in mice offspring. Environ Pollut 266(Pt 2):115146
Wang Z, Tang B, He Y, Jin P (2016) DNA methylation dynamics in neurogenesis. Epigenomics 8:401–414
Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE (2002) Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 59:371–378
Yang Y, Yang T, Liu S, Cao Z, Zhao Y, Su X, Liao Z, Teng X, Hua J (2019) Concentrated ambient PM2.5 exposure affects mice sperm quality and testosterone biosynthesis. PeerJ 7:e8109
Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, Zhao J, Liu D, Morishita M, Sun Q, Spino C, Brook RD, Harkema JR, Rajagopalan S (2014) Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect 122:79–86
Zanchi AC, Fagundes LS, Barbosa Jr F, Bernardi R, Rhoden CR, Saldiva PH, do Valle AC (2010) Pre and post-natal exposure to ambient level of air pollution impairs memory of rats: the role of oxidative stress. Inhal Toxicol 22:910–918
Zanini MJ, Dominguez C, Fernandez-Oliva T, Sanchez O, Toda MT, Foraster M, Dadvand P, Llurba E (2020) Urban-related environmental exposures during pregnancy and placental development and preeclampsia: a review. Curr Hypertens Rep 22:81
Zheng X, Wang X, Wang T, Zhang H, Wu H, Zhang C, Yu L, Guan Y (2018) Gestational exposure to particulate matter 2.5 (PM2.5) leads to spatial memory dysfunction and neurodevelopmental impairment in hippocampus of mice offspring. Front Neurosci 12:1000
Funding
This study was supported by the National Natural Science Foundation of China (grant number: 81673179, 81402687, 81602860, 81703233), Shanghai Science and Technology Committee (grant number: 18140903100, 19140903100, 18140903102, 22Y11906200), the Shanghai Sailing Program (grant number: 18Y1419600), Shanghai Municipal Health Commission (grant number: 20174Y0010, 20184Y0078).
Author information
Authors and Affiliations
Contributions
Yingying Yang, Jia He, and Jing Hua contributed to the conception and design. Material preparation, data collection, and analysis were performed by Yingying Yang, Tingting Yang, Zhijuan Cao, Yan Zhao, and Ji Zhou, Zehuan Liao, and Xiujuan Su. The first draft of the manuscript was written by Yingying Yang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The experimental protocol was approved by the Animal Experimental Ethics Committee of the Department of Laboratory Animal Science, Fudan University (ethics reference number 201805003Z).
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, Y., Yang, T., Zhou, J. et al. Prenatal exposure to concentrated ambient PM2.5 results in spatial memory defects regulated by DNA methylation in male mice offspring. Environ Sci Pollut Res 30, 35142–35152 (2023). https://doi.org/10.1007/s11356-022-24663-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24663-5