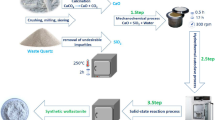
Abstract
For the tendency toward cleaner production and safe conversion of undesired toxic wastes to highly priced advanced products, this work introduces new ceramics/glass composites of Cr2O3/Fe2O3/lead silicate glass (LSG) from industrial LSG wastes. Both chromia Cr2O3 and hematite Fe2O3 ceramics are added equally to the LSG wastes with different percentages (10, 20, and 30 wt.%) via the pressureless sintering method. The competitiveness of this work is dependent on the conversion of undesired waste materials into advanced/smart optical materials with a low cost and an environmentally friendly method. Hence, the influence of both Cr2O3 and Fe2O3 additions on the behavior and the different characteristics of the lead silicate wastes are comprehensively investigated. Evaluation of the final ceramics/glass composites was achieved through their phase composition, microstructure, optical, and magnetic characteristics. The results verified that the insertion of both chromia and hematite together into the glass waste had a key role in improving its morphological properties and optical and magnetic behaviors. Composite with 30% of Cr2O3/Fe2O3 gave the highest optical absorbance of 90%, the lowest and best band gap energy of 1.68 ev, and the highest refractive index of 2.85. Also, it recorded the best magnetic behavior with the highest saturation magnetization of 139.700 × 10−2A m2 kg−1 and the best coercivity of 190.0 Oe. These findings confirmed the successful clean conversion of the hazardous lead silicate waste into advanced products with promising optoelectronic characteristics.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Rapid global manufacturing has not only led to the development and civilization of countries but also to the generation of hazardous industrial waste in huge amounts. Solid industrial wastes generated from the industrial activities of the different chemical and developed plants cause harmful environmental pollution (Hoornweg and Bhada-Tata 2012). Management and recycling of these wastes are considered a serious challenge and inadequate due to the lack of integrated/sustainable management plans and insufficient resources.
Industrial LSG wastes discarded by glass and crystal manufacturing companies, in particular, were classified as extremely hazardous materials (Besisa et al. 2021; Ishchenko 2019). The severity of these glass wastes comes from the presence of a high concentration (30%) of toxic lead compounds (Meng et al. 2016). So, in order to get rid of these undesirable materials, there is a need for new applications.
Various investigations have included the recycling of the lead silicate waste revealed from the cathode ray tube (Meng et al. 2016; Fernandes et al. 2021; Guo et al. 2010a and b). However, very few studies have been conducted on the same wastes released by glass manufacturing companies (ElKersh and El-Haggar 2015; Zhernovaya and Onishchuk 2005). Besides, these studies failed to recognize the exact effect of the lead compounds on the glass properties. They could not successfully convert them into advanced products for new applications.
Furthermore, it was reported that combining glass and ceramic materials together in one composite structure results in a new polycrystalline material with desirable properties and tailored applications (Ahmed et al. 2017; Besisa and Ewais 2019). The type and properties of the inserted ceramic materials can determine the final characteristics and the new applications of the original glass (Sandu et al. 2017a, b). For example, Cr2O3 was added to iron phosphate glass to enhance the optical properties of the parent glass. The obtained glass/ceramic composite was nominated as a highly efficient luminescence material (Šantic et al. 2007). Another interesting thing about this glass/ceramic system is its low processing temperatures compared to those required for engineering ceramic materials.
On the other hand, hematite (α-Fe2O3) and chromia (Cr2O3) are isostructural ceramic oxides with a corundum structure. They form a continuous and homogenous solid solution in the entire concentration range (Grygar et al. 2003). It was reported that the combination of hematite and chromia has great importance in many technological and industrial applications. Together, they can exhibit outstanding optical, magnetic, and catalytic properties. This behavior arose from the attractive characteristics of both Cr2O3 and Fe2O3. Chromia has been considered a p-type semiconductor with wide band gap energy. It has high electrical conductivity, high thermal and optical properties, thermal stability, and high temperature oxidation resistance (Cao et al. 2006; Hammoudeh et al. 2006; Santulli et al. 2011). Also, hematite is a well-known optical/magnetic material with promised photocatalytic behavior (Ohkoshi et al. 2016; Besisa and Ewais 2021). It has a remarkable effect on improving the physical properties of the original glass materials (Ibrahim et al. 2014).
Hence, it can be believed that the addition of Cr2O3 and Fe2O3 together with varying their compositions to glass materials can produce new ceramic/glass composites with homogenous solid solution/diffusion reaction and promised performance/application. To the best of our knowledge, no work has investigated the addition of chromia and hematite together to lead silicate glass before or investigated its characteristics neither from a pure source nor in waste form.
In this work, we have two challenges to overcome and achieve. The first one is finding a cheap radical environmental solution for the disposal of the accumulated lead silicate glass waste. The second one is producing advanced glass/ceramic materials for new optical and magnetic applications from these wastes. Obtaining this novel material will lead to produce a sustainable high-performance product suitable for new advanced applications. These can be attained by the addition of Cr2O3 and Fe2O3 together with different compositions to the revealed LSG waste by using the pressureless sintering method. Moreover, different properties of the produced glass/ceramic composites will be evaluated, such as physical, morphological, magnetic, and optical properties.
Experimental
Materials and preparation
The parent glass material used in this investigation is the solid waste of lead silicate crystal glass production at the final polishing and finishing stage. It was supported by Asfour Crystal manufacturing company, Shoubra, Egypt. Details of the solid lead silicate glass wastes processing, as well as its chemical and physical analysis, are described in detail (Besisa et al. 2021). Highly pure (99.999%) Fe2O3 and Cr2O3 were added equally with different contents to the parent glass in order to create various glass/ceramics composites. Raw Fe2O3 and Cr2O3 were provided by Loba Chemie Pvt. Ltd., India. Four Fe2O3/Cr2O3/LSG composites with various content of Fe2O3/Cr2O3 (0, 10. 20, and 30 wt%) were obtained. Composite nomenclature with different additions of Fe2O3, Cr2O3, and the lead silicate waste is demonstrated in Table 1. Composite mixtures were uniformly blended by a planetary ball mill for 2 hrs in 100% ethanol with zirconia balls. Green compacts in cylindrical form with dimensions of 13.7 mm in diameter were produced by uniaxial cold pressing (at ≈ 400 MPa). The different sintered Fe2O3/Cr2O3/lead silicate glass composites were obtained by pressureless sintering at a temperature of 600 °C/2 h of the green compacts with a heating rate of 5 °C/min.
Characterization
Phases identification of the waste and the produced composites are recognized by a Bruker D8-advance X-ray powder diffractometer with Cu Ka radiation (k = 1.5406 Å).
Microstructural properties of the obtained composites are examined by using field emission scanning electron microscopy (FESEM; QUANTA FEG250, Holland) connected to an energy-dispersive X-ray microanalyzer (EDX).
Optical absorbance and reflectance of the sintered composites are measured by using a UV-VIS spectrophotometer (Jasco-V-570 spectrophotometer, Japan) fitted with an integrating sphere reflectance unit (ISN) in the wavelength range of 200–800 nm.
Magnetic properties are measured using a vibrating sample magnetometer (VSM) Lake Shore, USA.
Results and discussion
Phase analysis and microstructure examination
The XRD patterns of the sintered Cr2O3/Fe2O3/LSG composites with various Cr2O3+ Fe2O3 content (0–30 wt%) are shown in Fig. 1. It was revealed that adding and increasing the wt% of Cr2O3 and Fe2O3 has led to enhancing the crystallinity of the parent LSG material. It resulted in the formation of highly crystalline Cr2O3/Fe2O3/LSG composites, especially for CF30 composite. In another sense, adding and increasing the content of ceramic materials has transformed the amorphous glass structure into a polycrystalline composite material. For sintered composite without chromia and hematite addition (CF0), the main formed phases were lead silicate (Pb2SiO4), lead oxide (PbO), calcium silicate (Ca2SiO4), and silica (SiO2). Calcium silicate was formed as the result of a direct reaction between calcium carbonate and silica that was found in the parent glass waste (Kale et al. 2012).
However, upon the addition of 10% Cr2O3+ Fe2O3 (CF10 composite), the formed calcium silicate was substituted by calcium chromium oxide (CaCrO4). This behavior was attributed to the fact that the reactivity of added chromia was higher than silica. Hence, part of the added chromia diffused through the formed calcium silicate, substituted the silicate position, and formed a solid solution of calcium chromium oxide. The other part existed as a single chromia phase. With increasing chromia and hematite content to 20 and 30%, the crystallinity and content of the formed chromia, calcium chromium oxide, and hematite increased and enhanced, which confirms the successful conversion of the parent glass to polycrystalline ceramic composites. The more interesting thing is that CaCrO4 has outstanding optical, catalytic, and photoluminescent properties. It has been widely used in various technological applications such as photosensitization, catalysis, scintillation, photoluminescence, pigments, and photoconductive dielectric materials (Driscoll and Ozkan 1994; Kobayashi et al. 1998; Yu et al. 2002; Liu et al. 2004; Xiang et al. 2004). Hence, it is expected to have a significant effect on the optical performance of the investigated glass/ceramic composites.
The microstructure of the obtained ceramics/glass composites is illustrated in Fig. 2. It was obviously observed that the grain surface and edges of the CF0 sample without the addition of chromia or hematite are inconspicuous due to the amorphous nature of the parent LSG. However, addition of Cr2O3 and Fe2O3 has enhanced the appearance, homogeneity, and topographic features of all the formed phases through the different sintered ceramic/glass composites. Also, there was a variety in the grain size and morphology of the formed phases. This diversity in the grains shapes between cubic, elongated, and angular structures can be related to the formation of new phases and also to the intimate diffusion between the glass phases and the added chromia+hematite phases in each composite.
Optical properties
The UV-Visible absorption and reflectance spectra of the different sintered Cr2O3/Fe2O3/LSG composites are depicted in Figs. 3 and 4. Noticeably, the various obtained composites from CF0 to CF30 have exhibited absorption and reflectance in the whole investigated light range (200–800 nm). Also, it was confirmed that increasing the ceramic materials content has a significant impact on promoting the optical absorbance and reflectance. In other words, CF30 composite with the highest percentage of Cr2O3+ Fe2O3 (30%) had the best absorbance of almost 90% in the visible light region. However, LSG material without the addition of chromia and hematite (CF0) recorded the best absorbance of 79% in the UV light region only at a wavelength of ≈ 330 nm (Fig. 3). This glass material gave only two absorption bands in the UV light range: one sharp with a maximum at a wavelength of 215 nm and the other is a broad one with a spectral wavelength range of 260–380 nm. However, upon the addition of Cr2O3 and Fe2O3, the second broad band at 260–380 nm was moved toward the visible light range, with a spectral wavelength range of 260–420 nm. Besides, new broad absorption bands were observed at the wavelength ranges of 420–530 nm and 530–600 nm. The release of these strong and enhanced absorption bands resulted from the indirect charge transition of Fe3+ 3d→3d (visible absorption) and the direct charge transition of O2− 2p→Fe3+ 3d (UV absorption) (Zhang et al. 2010; Cao et al. 2006). Absorption in the range of 260–420 nm is caused by the d–d transition in Cr3+ (3d3) of Cr2O3 (Mirhadi and Mehdikhania 2011). Furthermore, absorption bands in the wavelength range of 530–600 nm refer to the peaks of Cr3+ ion in glass and calcium chromate. This behavior confirms the functional role of chromia and hematite in promoting the optical performance of the parent lead silicate glass material.
On the other hand, it was found that smart materials used in optical applications should have high optical performance. Thus, the band gap energy and refractive index of the various sintered Cr2O3/Fe2O3/LSG composites are detected. The band gap energy (Eg) of the sintered composites was calculated according to the Kubelka–Munk function (Feng et al. 2018).
The optical band gap energy of the different Cr2O3/Fe2O3/LSG composites is shown in Fig. 5. Obviously, the band gap energy of the obtained Cr2O3/Fe2O3/LSG composites decreased with increasing Cr2O3+Fe2O3 content. Its value decreased from 2.363 ev for CF0 composite until it reached its lowest and best value of 1.68 ev for CF30 composite with the 30% addition of Cr2O3+Fe2O3. This enhanced behavior can be explained according to the fact that Cr2O3 and Fe2O3 acted as dopants inside the glass structure and led to the generation of new discrete energy levels between its valence and conduction band. Accordingly, they will decrease the transition energy of the transferred electron, and, at the same time, they will act as electron (or hole) traps (Mardare and Hones 1999).
High refractive index is a characteristic of high light dispersion efficiency and hence high optical performance. The refractive index (n) of the pressureless sintered composites is estimated according to Herve and Vandamme’s relation (Herve and Vandamme 1994):
where A and B are constants with values of 13.6 eV and 3.4 eV, respectively. Eg is the optical band gap energy value. Figure 6 illustrates the relationship between the calculated refractive index and band gap energy values of the obtained Cr2O3/Fe2O3/LSG composites. Refractive index values were found to be mainly dependent on the added ceramic material content. Meaningly, increasing the weight percent of the added chromia and hematite to 30% has a significant role in enhancing the refractive index of the original waste material. It gave the highest refractive index value of 2.85. So, we can conclude that ceramics/glass composite with ceramics content of 30 wt% is a promising candidate in optical and optoelectronic applications.
Magnetic properties
Functional magnetic materials are playing a major role in various technological uses such as energy storage, microelectronics, biomedical, and optical applications (Matzui et al. 2019; Kozlovskiy et al. 2021). Specifically, some tailored glass/ceramic materials with high magnetic characteristics have already been used in wide industrial applications (Ferreira et al. 2021; Deng et al. 2019; Worsch et al. 2013). Accordingly, the magnetic properties of our proposed Cr2O3/Fe2O3/LSG composites are investigated at room temperature according to Fig. 7 and Table 2.
The magnetic behavior of the pressureless sintered Cr2O3/Fe2O3/LSG composites is shown in Fig. 7. Moreover, the measured magnetic parameters such as saturation magnetization (Ms), remanent magnetization (Mr), and coercive field (Hc) are listed in Table 2. Noticeably, the original glass material without ceramic addition (CF0 sample) shows very weak magnetic behavior. However, adding and increasing the ceramics content of Cr2O3 and Fe2O3 had an unparalleled influence on promoting the magnetic behavior of the obtained composites. In other words, Cr2O3/Fe2O3/LSG composites with 10 to 30 wt% addition of Cr2O3+Fe2O3 exhibited strong ferromagnetic behavior with improved saturation magnetization. The hysteresis loop area is obviously increased with increasing the wt% of Cr2O3+Fe2O3. The best magnetic behavior with the highest magnetization was recorded by the 30% ceramics/glass composite (Table 2). For example, the addition of 10% Cr2O3+Fe2O3 to the original glass waste resulted in boosting the saturation magnetization from 11.694 × 10−2 to 94.242× 10−2A m2 kg−1 and the coercivity from 149.3 to 183.1 Oe for CF0 and CF10 composites, respectively. Furthermore, increasing the ceramic addition to 30% (CF30 sample) has pointedly increased the saturation magnetization to 139.700 × 10−2A m2 kg−1 and the coercivity to 190.0 Oe. This promotion and improvement in the magnetic properties of the obtained composites is explained by increasing the concentration of the added ferromagnetic ceramic phases to the original glass waste material (Sandu et al. 2017a, b).
Finally, we can report that successful waste management has a significant impact in the economy transition. So we can replace the sentence “waste as a problem” to “waste as a resource for new applications.” According to the characteristics of our investigated glass/ceramic composites, they can be successfully used in highly advanced applications with low-cost production methods. It can be used in optical lenses, laser optics, and electronic and magnetic sensor applications. They can be also used in the construction of materials as a substitute to other ingredients and in radiation shielding applications (Tyagi et al. 2020; Aygün et al. 2021).
Conclusions
In an attempt of disposing and recycling the revealed industrial glass wastes, new advanced glass/ceramics composites were successfully produced. Promising Cr2O3/Fe2O3/LSG composites with various ceramics content (0–30 wt.%) have been produced by the low-cost pressureless sintering method. Different characteristics of the obtained composites such as phase composition, microstructure, and optical and magnetic properties were evaluated. Results indicated that parent LSG extracted from glass manufacturing industrial wastes has been successfully converted into promising advanced products for optoelectronic applications. The addition of chromia and hematite together to the glass waste had a significant effect on improving the morphological properties and optical and magnetic behaviors of the parent LSG material. Composite with the highest ceramic content of 30% Cr2O3/Fe2O3 has recorded the highest optical absorbance of 90%, the lowest and best band gap energy of 1.68 ev, and the highest refractive index of 2.85. Also, it has attained the best magnetic behavior with the highest saturation magnetization of 139.700 × 10−2A m2 kg−1 and coercivity of 190.0 Oe. Accordingly, our produced glass/ceramic composites can be successfully used as highly efficient optical and magnetic products for smart/advanced applications.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request (E.M).
References
Ahmed YMZ, Zaki ZI, Besisa DHA, Amin AMM, Bordia RK (2017) Effect of zirconia and iron on the mechanical properties of Al2O3/TiC composites processed using combined self-propagating synthesis and direct consolidation technique. Mater Sci Eng A 696:182–189
Aygün B, Şakar E, Agar O, Sayyed MI, Karabulut A, Singh VP (2021) ‘Development of new heavy concretes containing chrome-ore for nuclear radiation shielding applications’ Prog. Nucl Energy 133:103645
Besisa DHA, Ewais EMM (2021) Black zirconia composites with enhanced thermal, optical and mechanical performance for solar energy applications. Sol Energy Mater Sol Cells 225:111063
Besisa DHA, Ewais EMM, Ahmed YMZ (2021) Optical, magnetic and electrical properties of new ceramics/lead silicate glass composites recycled from lead crystal wastes. J Environ Manag 285:112094
Besisa DHA, Ewais EMM (2019) Investigation of mechanical strength of the functionally graded zirconia-mullite/alumina ceramics tailored for high temperature applications. Mater Res Express 6(7):075516
Cao HQ, Qiu XQ, Liang Y, Zhao MJ, Zhu QM (2006) Sol–gel synthesis and photoluminescence of p-type semiconductor Cr2O3 nanowires. Appl Phys Lett 88(24):241112
Deng LB, Zhang XF, Zhang MX, Jia XL, Zhang Z et al (2019) Structure and properties of in situ synthesized FeSi2-diopside glass ceramic composites from Bayan Obo tailings, blast furnace slag, and fly ash. J Alloys Compd 785:932–943
Driscoll S, Ozkan US (1994) Isotopic labeling studies on oxidative coupling of methane over alkali promoted molybdate catalysts. Stud Surf Sci Catal 82:367
ElKersh H, El-Haggar S (2015) Recycling 1 of lead crystal glass sludge to produce foam glass. Int J Environ Tech Manag 18(5/6):448–464
Ferreira PHD, Fabris DCN, Boas MOCV et al (2021) Transparent glass-ceramic waveguides made by femtosecond laser writing. Opt Laser Technol 136
Feng F, Yang W, Gao S, Sun C, Li Q (2018) Postillumination activity in a single phase photocatalyst of Mo-Doped TiO2 nanotube array from its photocatalytic “memory,”. ACS Sustain Chem Eng 6:6166–6174
Fernandes HR, Andreola F, Barbieri L, Lancellotti I, Pascual MJ, Ferreira P.H.D. F, Fabris DCN, Boas MOCV et al (2021) Transparent glass-ceramic waveguides made by femtosecond laser writing. Opt Laser Technol 136:106742
Grygar T, Bezdička P, Dědeček J, Petrovský E, Schneeweiss O (2003) Fe2O3-Cr2O3 System Revised. Ceramics − Silikáty 47(1):32–39
Guo HW, Gong YX, Gao SY (2010a) Preparation of high strength foam glass-ceramics from waste cathode ray tube. Mater Lett 64(8):997–999
Guo HW, Gong YX, Liu XN, Li YH (2010b) Elaboration and mechanical behavior of high strength foam glass. J Shaanxi Univ Sci Technol 28(3):1–6
Hammoudeh A, Mahmoud S, Humaidi J, Wahed HA (2006) Catalytic hydrolysis of CFC-12 over Cr2O3 catalysts. Jordan J Chem 1(2):129–142
Herve PJL, Vandamme LKJ (1994) General relation between refractive index and energy gap in semiconductors. Infrared Phys Technol 35:609–615
Hoornweg D, Bhada-Tata P (2012) What a waste. A global review of solid waste management. Urban Development and Local Government Unit, World Bank, Washington, DC. Report Document, No. 15, Www.Worldbank.Org/Urban
Ibrahim S, Gomaa MM, Darwish H (2014) Influence of Fe2O3 on the physical, structural and electrical properties of sodium lead borate glasses. J Advanced Ceramics 3(2):155–164
Ishchenko V (2019) Heavy metals in municipal waste: the content and leaching ability by waste fraction. J Environ Sci Health A 54(14):1448–1456
Kale KB, Raskar RY, Rane VH, Gaikwad AG (2012) Preparation and characterization of calcium silicate for CO2 sorption. J Adsorption Sci 31 Technol 30(10):817–830
Kobayashi M, Ishii M, Usuki Y (1998) Nucl Instrum Methods Phys Res Sect A 406:442
Kozlovskiy AL, Shlimas DI, Zdorovets MV (2021) Synthesis, structural properties and shielding efficiency of glasses based on TeO2-(1-x) ZnO-xSm2O3. J Mater Sci Mater Electron 32:12111–12120
Liu B, Yu S-H, Li LJ, Zhang Q, Zhang F, Jiang K (2004) Angew Chem Int Ed 43:4745
Mardare D, Hones (1999) Optical dispersion analysis of TiO2 thin films based on variable angle spectroscopic ellipsometry measurements. Mater Sci Eng B, 68, Materials 13(9):1067–1070
Matzui LY, Trukhanov AV, Yakovenko OS et al (2019) Functional magnetic composites based on hexaferrites: correlation of the composition, magnetic and high-frequency properties. Nanomaterials 9:1720
Meng W, Wang X, Yuan W, Wang J, Songa G (2016) The recycling of leaded glass in cathode ray tube (CRT). Procedia Environ Sci 31:954–960
Mirhadi B, Mehdikhania B (2011) The effect of chromium oxide on optical spectroscopy of sodium silicate glasses. J Optoelectron Adv Mater 13(9):1067–1070
Ohkoshi S-i, Namai A, Yamaoka T, Yoshikiyo M, Imoto K, Nasu T, Anan S, Umeta Y, Nakagawa K, Tokoro H (2016) Mesoscopic bar magnet based on ε-Fe2O3 hard ferrite. Sci Rep 6(1):42
Šantic A, Moguš-Milankovic A, Furic K, Bermanec V, Kim CW, Day DE (2007) Structural properties of Cr2O3–Fe2O3–P2O5 glasses, Part I. J Non-Cryst Solids 353:1070–1077
Sandu V, Cimpoiasu E, Kuncser A, Nicolescu MS (2017a) Magnetic properties of glass-ceramics obtained by crystallization of iron-rich borosilicate glasses. J Advan Ceram 6[3]:251–261
Sandu V, Greculeas S, Kuncser A, Nicolescu MS, Kuncser V (2017b) Effect of Cr2O3 on the magnetic properties of magnetite-based glass-ceramics obtained by controlled crystallization of Fe-containing aluminoborosilicate glass. J Eur Ceram Soc 37(9):3089–3099
Santulli AC, Feygenson M, Camino FE, Aronson MC, Wong SS (2011) Synthesis and characterization of one-dimensional Cr2O3 nanostructures. Chem Mater 23(4):1000–1008
Tyagi G, Singhal A, Routroy S, Bhunia D, Lahoti M (2020) A review on sustainable utilization of industrial wastes in radiation shielding concrete. Mater Today Proc 32:746–751
Worsch C, Buttner M, Schaaf P, Harizanova R et al (2013) Magnetic properties of multicore magnetite nanoparticles prepared by glass crystallisation. J Mater Sci 48:2299–2307
Xiang JH, Yu S-H, Xu ZL (2004) Polymorph and phase discrimination of lead chromate pigments by a facile room temperature precipitation reaction. Cryst Growth Des 6:1311
Yu S-H, Antonietti M, Colfen H, Giersig M (2002) Angew Chem Int Ed 41:2356
Zhang Z, Takahashi T, Hossain MF (2010) Fabrication of shape-controlled Fe2O3 nanostructures by sonoelectrochemical anodization for visible light photocatalytic application. Mater Lett 64(3):435–438
Zhernovaya NF, Onishchuk VI (2005) Possible directions in recycling crystal production waste. Glas Ceram 62(1–2):13–15
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Project administration, investigation, material preparation, data collection, and analysis were performed by Dina Besisa. The first draft of the manuscript was written by Dina Besisa. Yasser Ahmed provides the LSG waste. Yasser Ahmed and Emad Ewais revised the manuscript. Hanan Mohamed and Amira Amin contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: George Z. Kyzas
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Besisa, D.H.A., Mohamed, H.H., Ewais, E.M.M. et al. Synthesis of new Cr2O3/Fe2O3/glass composites from industrial wastes; from undesired to advanced optical products. Environ Sci Pollut Res 29, 84357–84365 (2022). https://doi.org/10.1007/s11356-022-21694-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-21694-w