Abstract
The suitability of groundwater and agricultural products for human consumption requires determining levels and assessing the health risks associated with potential pollutants. Here, particularly pollution with nitrate still remains a challenge, especially for those urban areas suffering from insufficient sewage collection systems, resulting in contaminating soil, endangering food safety, and deteriorating drinking water quality. In the present study, nitrate concentrations in the commonly consumed fruit and vegetable species were determined, and the results, together with the groundwater nitrate levels, were used to assess the associated health risks for Mashhad city residents. For this assessment, 261 water samples and 16 produce types were used to compute the daily intake of nitrate. Nitrate in groundwater was analyzed using a spectrophotometer, and produce species were examined using High-Performance Liquid Chromatography. Ward’s hierarchical cluster analysis was applied for categorizing produce samples with regard to their nitrate content. Additionally, to account for the sanitation hazards associated with groundwater quality for drinking purposes, total coliform and turbidity were also assessed using the membrane filter (MF) technique and a nephelometer, respectively. Nitrate concentrations exceeded the prescribed permissible limits in 42% of the groundwater wells. The outcomes also exhibit significantly higher nitrate accumulation levels in root-tuber vegetables and leafy vegetables compared to fruit vegetables and fruits. Using cluster analysis, the accumulation of nitrate in vegetables and fruits was categorized into four clusters, specifying that radish contributes to 65.8% of the total content of nitrate in all samples. The Estimated Daily Intake (EDI) of nitrate and Health Risk Index (HRI) associated with consumption of groundwater exceeded the prescribed limit for the children’s target group in Mashhad’s south and central parts. Likewise, EDI and HRI values for produce consumption, in most samples, were found to be in the tolerable range, except for radish, lettuce, and cabbage, potentially posing risks for both children and adult consumers. The total coliforms in groundwater were found to violate the prescribed limit at 78.93% of the sampling locations and were generally much higher over the city’s central and southern areas. A relatively strong correlation (R2 = 0.6307) between total coliform and nitrate concentrations suggests the release of anthropogenic pollution (i.e., sewage and manure) in the central and southern Mashhad.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decades, the rapidly growing trend of urbanization, alongside the obligate agricultural/industrial developments, has inflicted multiple contaminants of urgent concern on the environment (Mostaghelchi et al. 2021). Due to the broad dispersion into the soil and high solubility in water, nitrate is likely the most globally widespread contaminant, posing a detrimental influence on the quality of drinking water resources and agricultural products. It naturally occurs in the soil through the microbial transformation of ammonia under oxic conditions which is released by organic compounds such as organic fertilizer, plant and waste decomposition or by mineral fertilizers. Nitrate is not chemically bound to soil components, making it predisposed to leach through the soil and into groundwater and thus impacting both surface and groundwater quality. The nitrate concentration in groundwater, vegetables, and fruits has been observed to exceed the acceptable levels worldwide as well as in many parts of Iran (Gao et al. 2012; Pastén-Zapata et al. 2014; Bahadoran et al. 2016; Qasemi et al. 2018; Mehri et al. 2019; Zendehbad et al. 2019a; Boumaiza et al. 2020). In urban environments, several anthropogenic factors can be considered as the main sources of nitrate contamination in surface and ground waters, including leakage from sewer collection systems, discharge of industrial effluents and municipal wastewater, landfills, and usage of fertilizers and manure in urban green belts and urban/peri-urban agriculture (Wakida and Lerner, 2005; Zendehbad et al. 2019a). Additionally, septic system leakage and organic matter decomposition can also cause total coliforms to increase in groundwater resources (Ling, 2000; Smoroń, 2016; Mititelu-Ionuș et al. 2019).
Nitrate pollution has become an increasingly critical issue in Iran, where groundwater is the primary source of agricultural, domestic, and drinking water. The nitrate concentrations in groundwater resources of different parts of Iran reported in recent studies are summarized in Table 1. Mashhad is an exemplary case, where its urban groundwater suffers from contamination issues (Zendehbad et al. 2019b). Old wastewater disposal and septic systems, discharging a high level of nitrogen into the groundwater, were found to be the primary source of nitrate pollution in Mashhad urban groundwater (Zendehbad et al. 2019a). In such places where groundwater is the main water supply for both drinking and agricultural purposes, besides potentially threatening food safety, the excessive nitrate content in drinking water may cause considerable health risks to the residents.
Drinking water is not considered as the major source of nitrate intake when the concentration is low. However, by exceeding the World Health Organization (WHO) standard of 50 mg/L (World Health Organization, 2017), it becomes potentially the primary source of intake, unless other high nitrate intake sources such as fruits and vegetables are present (Fan, 2011). Several studies confirmed that fruits and vegetables contain high nitrate content and account for approximately 85% of the dietary nitrate intake across many communities (Bahadoran et al. 2016; Bondonno et al. 2018; Chetty et al. 2019). Among several contamination factors, such as irrigation with untreated wastewaters and pesticide residue that may lead to nitrate accumulation in fruits and vegetables, the excessive application of nitrogenous fertilizers is considered the most critical factor, which has arisen as an alarming public health concern (Fan, 2011). The nitrate accumulation levels in fruits and vegetables also depend on several environmental, nutritional, and physiological factors that can vary from region to region, such as location, soil properties, crop’s biological properties, humidity, light intensity, and daytime temperature, as well as the method of cultivation, crop rotation, vegetation period, the season of harvest, and fertilization (Correia et al. 2010; Parks et al. 2012). Several vegetables (e.g., radish, spinach, lettuce, broccoli, beetroot, celery, and cabbage) were shown to have an augmented nitrate content of >1000 mg/kg. The concentration of nitrate in green leafy vegetables has been observed to be higher (Thomson et al. 2007). Higher nitrate accumulation in leafy vegetables, including spinach and lettuce, and several root crops such as beetroot and radish, may be associated with the accumulation tendency of higher nitrate concentrations in the plant’s leaves as well as stems or roots. Accordingly, nitrate has been usually observed at lower levels in fruity vegetables (or flowers) and fruits (FSAN, 2011).
Still, fruits and vegetables are believed to have a preventing role in health risks caused by micronutrient deficiencies (e.g., cardiovascular disease, cancer, and mortality) (Bahadoran et al. 2016; Bondonno et al. 2018; Chetty et al. 2019). Thus, the WHO and Food and Agriculture Organization (FAO) encourage consuming a minimum of 400 g of fruits and vegetables per day (FAO/WHO, 2005). However, by failing to approximately 200 g/day, Iran’s fruits and vegetables per-capita usage are much lower and is half of WHO norms (Esteghamati et al. 2012).
Nitrate at high concentrations, however, may cause health risks, such as methemoglobinemia (in infants) (Tate and Arnold, 1990); diabetes (Kostraba et al. 1992), and can be a potential contributor to developing chronic diseases (Lundberg et al. 2008) and generating some cancer types in the human body (Chiu et al. 2007). Nitrate converts into nitrite in the human digestive system, which readily combines with the secondary amines and forms the N-nitroso compounds (nitrosamines). These compounds are firmly believed to be carcinogenic (Cross et al. 2011; Loh et al. 2011; Roohparvar et al. 2018). N-nitroso compounds have also increased cancer risk in laboratory animals in different studies (Zhu et al. 2014; Espejo-Herrera et al. 2015). While previous reports have confirmed the positive correlations between acute exposure to excessive nitrate concentrations and certain cancer types, including stomach, esophageal and gastric cancers (Yang et al. 1998; Nowrouz et al. 2012), some reports in contrast suggest potential preventive effects and health benefits of nitrate, including cardiovascular effects (e.g., blood-pressure-lowering effects) (Machha and Schechter, 2012; Kapil et al. 2015; Jonvik et al. 2016; Velmurugan et al. 2016; Kerley et al. 2018; Jackson et al. 2019). Other studies noticed that the formation of nitrosamines could be decreased with the high content of polyphenol and vitamin C in some fruits and vegetables by facilitating the non-enzymatic reduction of toxic nitrite to beneficial nitric oxide (Rocha et al. 2009; Erkekoglu and Baydar, 2010). However, the risk-benefit aspects of the dietary intake of nitrate are yet to be investigated, particularly in the places where fruits and vegetables are not the only sources of nitrate intake for the residents.
Due to the global concerns on water quality, multiple studies investigated the health risks of exposure to different pollutants through drinking water, both worldwide and in many parts of Iran (Mortada and Shokeir, 2018; Moeini and Azhdarpoor, 2021; Badeenezhad et al. 2021a, b; Mohammadpour et al. 2022). In Iran, Moeini and Azhdarpoor (2021) reported that the health risks for Shiraz residents were higher than the threshold values in those locations with the groundwater nitrate concentration exceeding the WHO standard, potentially posing a higher risk to infants and children. Badeenezhad et al. (2021b) also found an increasing trend in the maximum nitrate levels of Shiraz groundwater between 2013 and 2017, and consequently, health risks of nitrate exposure, particularly for the children’s age group, were also estimated to be increasing. The researchers reported that land-use changes in the study area, predominantly urban and residential developments, significantly affected the groundwater nitrate concentration and the degree of the associated health. In another study, children were found to be the significant at-risk group due to exposure to nitrate in drinking water in Behbahan (Badeenezhad et al. 2021a). Mortada and Shokeir (2018) found that nitrate levels in water samples of Mansoura city, Egypt, were within the acceptable limit, and the population’s health risk in these areas was also low. However, further studies on other potential sources of nitrate, such as fruits and vegetables, were recommended to provide a complete profile of the possible impacts.
Given the health risks associated with nitrate intake from fruits and vegetables, regulatory authorities have established the tolerable limit of dietary nitrate termed: Acceptable Daily Intake (ADI). The Scientific Committee for Food (SCF) of the European Commission (EC) stipulated the nitrate ADI of 3.7 mg nitrate/kg body weight/day. (ECETOC, 1988). Later, in 2002, the Joint WHO/FAO Expert Committee on Food Additives (JECFA) reaffirmed the limit for nitrate ADI (EFSA, 2008).
In the pretext of the above-mentioned health hazards and pollution pressures on water supply sources in Mashhad, where the population relies on groundwater for both drinking and agriculture, assessing nitrate health risks using different intake sources (i.e., water, fruits, and vegetables) is of great significance. The current status of nitrate in the Mashhad urban area remains unexplored, with limited investigations giving way to conducting further studies on the aspect. Therefore, the present study aims to determine the nitrate intakes and associated health risks with the consumption of both groundwater and agricultural products in Mashhad, using a high-resolution groundwater nitrate database as well as the measured nitrate concentrations of locally cultivated fruits and vegetables. The health risk evaluation was carried out based upon the established safety limits, Health Risk Index (HRI), and Acceptable Daily Intake (ADI). The outcomes are expected to assist the health professionals, decision-makers, and authorities devise better-detailed health management plans in terms of local and regional produce and drinking water quality protection.
Materials and methods
Description of study area
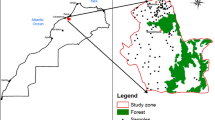
The study area is Mashhad — the second most populous city in Iran — situating in the North East of the country (between 36°.15′–36°.20′ N and 59°.30′–59°.40′ E) (Fig. 1). The city covers 280 km2 and has a population of larger than 3 million, with around 20 million tourists visiting annually. Locating in a semiarid climate, Mashhad region has wet-cold winters and dry-hot summers, with an average temperature of 13.5 °C (min −21 °C, and max 44 °C) and average annual precipitation of 253 mm (Khalili et al. 2016).
The geology of the area is comprised of various sedimentary rocks (e.g., marl, limestone, and clastic rocks) in northern margins and granitic, metamorphic, and ultrabasic outcrops (e.g., slate, phyllite, and schist) in the southern and southwestern parts. The city is positioned on thick deposits of Quaternary, and the Mashhad’s underneath sediments are a combination of ultramafic and metamorphic rocks (Zendehbad et al. 2019a). The aquifer of Mashhad is characterized as unconfined and composed of alluvial gravel-sand sediments of the Quaternary period. Groundwater flows according to the plain general slope, from northwest to southeast, and the industrial estate is located upstream. Approximately 220 million m3 of water is required to meet the yearly domestic demand, mainly supplied by the city’s groundwater (Zendehbad et al. 2019a). Mashhad’s average water usage is over 550,000 m3/d, and it is estimated that nearly 75% of the water (on a yearly basis) returns to groundwater (Ehteshami et al. 2014). Therefore, municipal return flow is the primary source of groundwater recharge. Southern and central areas — the city’s major old core with a higher density of population — suffer from an insufficient sewer collection system compared to the western part, which is newly developed as a result of the city’s expansion.
Mashhad groundwater is also being heavily pumped to meet agricultural water needs, significantly contributing to the area’s groundwater table declination (1.2 m/year). The area’s most common types of crops are cereals (55%), vegetables (21%), orchards (19%), and industrial crops (5%). Mashhad also grows a variety of fruits throughout the year. The gardens embedded in the dense urban fabric take on great relevance to the production of agricultural products. Alandasht garden (9.6 ha, in the city’s central part), Malek Abad garden (299 ha, in the city’s central part), Imam Reza garden (113 ha, in the city’s northern part), Astan-e-Quds gardens (771 ha, in the city’s northern part) are being used for urban agriculture and helping meet the need for local food. Private farms in the suburbs of Mashhad also play a significant role in response to the daily demand of consumers within the city. In recent decades, yields of leafy vegetables (such as lettuce) have increased (82%), and due to vegetable farmers’ use of untreated wastewater for irrigation, contamination of soil and crops (e.g., non-acceptable pathogen levels in leafy vegetables) has also increased (Danso et al. 2018).
Sampling
Water
The nitrate data presented in this study were obtained from a dataset with a high spatial resolution of groundwater wells from a previous study (Zendehbad et al. 2019a). The nitrate levels were determined using water samples collected from 261 groundwater wells. Although the area of study is situated in a semiarid climate, the quality of groundwater was purposely studied in a dry season to avoid water quality fluctuations resulting from potential short-term recharge in Mashhad’s wet season. Prior to the sampling, the wells were purged multiple times to ensure that the samples truly represent the formation of the aquifer. These wells provide the supply of the city’s water for drinking and irrigation needs. Information on the screening length and actual depth of these old drinking water wells is limited as they were mainly installed decades ago. The aquifer from which all the samples were collected was the same. Figure 1 displays sampling locations, covering the entire city. A submersible pump was used to collect groundwater samples around 3 to 5 m beneath the groundwater table. Turbidity measurements were conducted in situ using a turbidimeter (nephelometer). A 0.45 m membrane filter was used to filter the samples before pouring them into 60 ml PET bottles. The samples were stored at 0–4 °C and, quickly after collection, transferred to the lab.
Fruits and vegetables
The fruit and vegetable species were chosen based upon two assumptions: (i) their consumption rate among the residents, and (ii) the nitrate content in vegetables and fruits grown in the region. Due to the different socio-demographic status of the residents and differences in the quality of supplied food items in the different areas, Mashhad was divided into four zones, i.e., south/central, north, west, and east. A random sample of 250 residents aged 20 years and over were requested to respond to a face-to-face survey specifying their household frequent fruit and vegetable items, and 222 subjects agreed. All the samples were randomly collected based on the locals’ and agricultural officers’ opinions and by considering their biological maturity from the main municipal markets and produce shops in each city’s specified area. The samples were also divided into four groups: (i) fruits (banana, apple, grape, orange), (ii) fruit vegetables (tomato, cucumber, cauliflower, eggplant, green bean, green pea, zucchini), (iii) tuber and root vegetables (carrot, potato, radish), and (iv) leafy vegetables (lettuce, cabbage). They were afterward placed into ziplock bags and, after being labeled, transferred to the lab. The prepared samples were stored at below 4 °C until the following day for analysis.
Standard and reagent solutions
Potassium nitrate and hydrochloric acid, all in the analytical grade, and 1-Pentanesulfonic acid sodium salt and methanol, in HPLC grade, were purchased from Merck, Germany. For the preparation of the solutions as well as extraction of the samples, deionized water was used.
Sample preparation
Vegetable and fruit samples from two different locations in each specified area of the city with three replicates were used for analysis. Preparation and extraction of the samples were carried out in accordance with the recommendations by the EC’s Directive 1882/2006 (Commission, 2006). Following the gathering of the samples, non-edible parts were discarded, and subsequently, the samples were cut into smaller pieces and thoroughly homogenized. Directly after, the samples were kept at –15 °C, waiting for the laboratory analysis. The homogenized samples (2 g) were placed into a 100 mL Erlenmeyer flask, and afterward, about 50 mL of deionized water was added. Next, it was kept in a boiling water bath (80 °C) for 15 min, shaken, and left undisturbed to cool down before diluting with deionized water to a final volume of 100 mL. Finally, after discarding the first 2 mL of the filtrate (using a 0.45–l m pore-sized filter), the solution was stored at 4 °C for nitrate determination analysis.
Nitrate determination
Nitrate in drinking water samples was determined using a colorimetric method with a UV-visible spectrophotometer measured at 410 nm (Rowell, 2014). Measurement of nitrate in fruits and vegetables was conducted employing a High-Performance Liquid Chromatography (HPLC) (Waters, Milford, USA) equipped with an autosampler (Waters, model 717 plus, Milford, USA) and a photo array detector (Waters, model 996, Milford, USA) and the separation was carried out on a C18 column (4.6 mm × 250 mm, 5 μm; Waters, Ireland).
With certain adjustments, the nitrate in this investigation was evaluated using the method reported by Hsu et al. (2009). A buffer solution consisting of 2 g of 1-Pentanesulfonic acid sodium salt dissolved into 950 mL of deionized water, using a 1-L beaker, was used for analytical procedures. Potassium nitrate (160 mg) was also mixed into deionized water inside a volumetric flask to provide a range between 100 and 500 ppm of standard nitrate solutions for the preparation of the nitrate calibration curve. The obtained calibration curve from plotting the concentration (ppm) against the peak area exhibited satisfactory linearity (R2 ≥ 0.997), and the peak’s Relative Standard Deviation appeared to be less than 1%. Thus, in order to determine the nitrate content in chosen samples of vegetables and fruits, we used the calibration curve and expressed the values in mg/kg.
The mobile phase, which contained a solution of buffer and methanol (70% and 30%, respectively), was set to flow through the column of HPLC until a stable baseline signal was equilibrated. The mobile phase’s pH was adjusted with 2 M of hydrochloric acid to receive pH 3. The flow rate was 1 mL/min, and the column temperature was maintained at 40°C. The injection volume was 10 μL with a run time of 10 min, and the detection wavelength was set at 225 nm. Finally, the sample’s peaks were identified by comparing their retention times and peak areas with those of standard solutions.
Turbidity and coliform determination
So as to account for the sanitation hazards attributed to sustaining the sufficient quality of water for drinking purposes, further parameters, i.e., evaluation of turbidity and coliform, were also incorporated in this research. According to the American Public Health Association’s (APHA) established standard methods (9222 D/B method), the membrane filter (MF) technique was employed to identify and estimate total coliforms. Turbidity measurements were performed employing a nephelometer (EutechTM) as per method 2130 of APHA and expressed in Nephelometric Turbidity Units (NTU) (APHA, 2012).
Exposure estimation
The intake of nitrate from water, vegetables, and fruits over the toxicity level or Acceptable Daily Intake (ADI) limit (3.7 mg nitrate/kg body weight (EFSA, 2008)) can cause health issues or even fatality (Du et al. 2007). The daily nitrate intake was calculated to estimate the average daily accumulation of nitrate in a consumer’s body of specific bodyweight and also to estimate the relative bioavailability of nitrate. It was separately determined for water and the samples of fruits and vegetables using the following Estimated Daily Intake (EDI) equation (Equation (1)), taking into account that the EDI represents only the possible ingestion rate and does not consider the potential metabolic excretion of nitrate (USEPA, 2004).
where AC stands for the average consumption of water per capita (L), and fruits and vegetables (g); C represents the nitrate concentration in water (mg/L), and fruits and vegetables (mg/kg); and, BW is the mean bodyweight of the consumer.
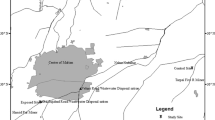
To calculate the EDI of nitrate from water, the city map was divided into four areas (i.e., south/central, north, west, and east), and the average concentration of nitrate was used separately in the calculations for each specified area. Figure 2 shows the zonation of the sampling locations. The average consumption rate of water per person was considered to be 2 L/day. The rate for vegetables and fruits for Iranian consumers was considered to be 286, and 142 g/day, respectively (Esteghamati et al. 2012; Sheikholeslam, 2001). We additionally computed the EDI (and Health Risk Index; HRI) if a person, according to the FAO and WHO guidelines, consumed 400 g of fruits and vegetables (FAO/WHO, 2005). In the calculations, the bodyweight of adult consumers and children was considered 70 kg and 31 kg, respectively (Hosseini et al. 1998). For the evaluation of health risks associated with the consumption of nitrate-containing water, vegetables, and fruits, the calculated EDI was subsequently compared with the ADI.
Different zones in the study area for the calculations of EDI of nitrate; green points represent samples from the northern sector, red points represent samples from central and southern sectors, blue and gray points represent samples from western and eastern parts, respectively. The boxplot shows the nitrate concentrations in each area
With a value of HRI less than 1 for any nitrate in water and food items, the consumer population is assumed to be safe and not exposed to the associated health hazards (USEPA, 2004). The HRI can be determined by the food item’s EDI value together with the oral Reference Dose (Rfd). The oral RfD is an estimation of the daily exposure of the human population, including sensitive subgroups (e.g., children), to dietary nitrate over the course of a lifetime without the significant risk of harmful effects (Barnes et al. 1988). The HRI for exposure to nitrate through consumption was determined applying Equation (2), recommended by earlier studies (Abtahi et al. 2018; Rahmani et al. 2018).
In Equation (2), EDI is in mg/kg body weight/day (expressed as nitrate ion), and Rfd is expressed in mg/kg body weight/day. According to the United States Environmental Protection Agency (USEPA), the oral Rfd of the nitrate-nitrogen is determined to be 1.6 mg/kg body weight/day, which is equivalent to 7.09 mg/kg body weight/day of nitrate (USEPA, 2013).
Statistical analysis
Data processing for groundwater nitrate was conducted using IBM SPSS software v25. The spatial variability of the groundwater nitrate concentrations was conducted by interpolating sampling points applying the algorithmic method ‘Inverse Distance Weighted’ (IDW) integrated into the ArcGIS 10.5 software package.
For nitrate exposure evaluations, the normal distribution of nitrate data and EDI values was determined by applying Shapiro–Wilk test. Due to the not-normally distributed data, the relative nitrate content of different groups was analyzed using the Kruskal–Wallis test, and for the comparison of each group, the Bonferroni correction was applied. The statistical significance level was adjusted as p < 0.05. The analyses were executed using SPSS software (version 28.0, IBM SPSS, Chicago, IL, USA). For categorizing tested samples with regard to their nitrate content, Ward’s hierarchical cluster analysis was applied, using RStudio for macOS (version 1.4.1717).
Results and discussion
Nitrate concentration in groundwater
The concentration of nitrate in the groundwater of the Mashhad urban area is illustrated in Fig. 3, which ranges from 8 to 166.1 mg/L. Nitrate concentrations exceeded the prescribed permissible limits of 50 mg/L (World Health Organization, 2017) for nitrate in drinking water in 42% of the wells. Central and southern parts of the city demonstrate elevated nitrate concentrations (with mean concentration of 119.37 mg/L), whereas the western area shows a significantly lower concentration of nitrate (mean concentration of 35.69 mg/L). The maximum concentration value of nitrate (166.1 mg/L) belongs to the study area’s central and southern parts, representing higher population density. In contrast, the minimum value (8 mg/L) was obtained from newly constructed and less populated western areas (boxplot in Fig. 2). There is no known lithological source for this pollutant, indicating that nitrate mainly originates from anthropogenic sources (Ritzi et al. 1993; Marie and Vengosh, 2001). An earlier study has shown strong correlations between nitrate and other co-migrant ions (i.e., chloride, phosphate, and sulfate), indicating that these pollutants have arisen from the same origin of the sewage leakage into the groundwater of Mashhad. This anthropogenic source of nitrate contamination was also reconfirmed using nitrate isotopic analysis (Zendehbad et al. 2019a). While the western parts have already been equipped with the new sewage collection systems, completion of the construction of such systems in the central and southern parts can help mitigate the nitrate contamination and minimize anthropogenic impacts on the city’s drinking water source.
Coliform and turbidity
Coliform and turbidity tests were also conducted on the above samples as they are indicators of the sanitary quality of water. The presence of total coliform in the drinking water can be a sign of the contamination of water with urban and human wastes due to effluent from septic systems and untreated sewage discharge, which can contribute amounts considerably above those naturally present in groundwater (Lang et al. 2006; Marshall et al. 2019). Drinking water samples should have zero total coliform count per 100 mL, according to the Institute of Standards and Industrial Research of Iran (ISIRI) and WHO (World Health Organization, 2017). The high amount of coliforms in groundwater may induce intestinal diseases, including typhoid, hepatitis A and E, dysentery, cryptosporidiosis, and diarrhea (Ling, 2000).
The present study witnessed the total coliform value ranging from 0 to 127 CFU/100 mL (55 wells < 1, including 32 wells = 0 CFU/100 mL). Total coliforms are violating the prescribed limit at 78.93% of the sampling locations. As with nitrate, total coliforms were generally much higher over the city’s central and southern areas. Figure 4 shows that the most affected parts by nitrate pollution from septic systems have also the highest total coliform concentrations. The groundwater is affected due to the insufficient sewer system and household waste infiltration caused by septic or adsorption wells, mainly in older areas of the city.
Furthermore, concentrations of nitrate and total coliform counts confirmed relatively strong correlations (R2 = 0.63), specifying that they have arisen from the same origins (Fig. 5). Hence, it can be concluded that nitrates are released in the system by anthropogenic sources (i.e., manure and sewage), which is consistent with the previous study’s findings (Zendehbad et al. 2019a). Based on the data, it would appear that nitrate measurements in the locations affected by a single sewage source of contamination can be a good indicator of coliform and bacterial contamination levels.
Turbidity, a measure of the light refractiveness of water, is an evaluation of the degree to which water loses its transparency due to the presence of suspended matters, either organic or inorganic, chemical or biological particulates. Leaching of industrial and domestic wastes can contribute to turbidity in groundwater samples. The turbidity in the water samples is an indication of water pollution, particularly due to the source near the adsorbing wells or cesspools. Inorganic nutrients such as nitrogen and phosphorus present in agricultural runoff stimulate the growth of algae, which also contributes to turbidity. There are chances for the pathogenic organisms to be enclosed in the turbidity-causing particles, thus leading to health hazards (Prakash and Somashekar, 2006). Turbidity can protect pathogenic microorganisms from the effects of the disinfection process and thus stimulate the growth of bacteria during storage. As presented in Table 2, our samples have turbidity ranging from 0 to 29 NTU. The majority (74.32%) of turbidity values are below the permissible limit of 5 NTU.
Nitrate concentration in fruits and vegetables
Vegetables and fruits are known to contribute to bringing a substantial amount of nitrates to the population’s diet. The samples tested in this work were exhibited with their respective nitrate concentrations and categorized in the earlier-mentioned categories of four. The nitrate concentration of the samples was detected, and the findings exhibit a substantial difference between the specified groups. As seen in Fig. 6, a significantly higher nitrate content was found in tuber and root vegetables (1193 mg/kg) and leafy vegetables (1173 mg/L) compared to fruit vegetables (160.13 mg/kg). Also, when compared to vegetables, the fruit samples were found to have a lower nitrate content (102.37 mg/kg).
As summarized in Fig. 7, among the individual type of root and tuber vegetables, radish demonstrates the highest mean nitrate concentration (2881.27 mg/kg), followed by carrot (355.88 mg/kg) and potato (341.56 mg/kg). The leafy vegetables of lettuce and cabbage were measured to have high nitrate contents (1227.63 and 1118.30 mg/kg, respectively). Among fruit vegetables, the highest nitrate content was determined in eggplant, and cauliflower (>250 mg/kg), followed by cucumber and zucchini (150–250 mg/kg). Other fruit vegetables have been observed to have a nitrate content of below 100 mg/kg. Among fruits, the highest nitrate content appears in bananas (279 mg/kg), while other fruits of apple, orange, and grape show mean nitrate concentration of below 45 mg/kg.
Figure 8 shows the hierarchical clustering for nitrate accumulation in fruits and vegetables, where the samples are grouped into four clusters. The first cluster consists of banana, eggplant, cauliflower, potato, carrot, zucchini and cucumber; this group is accountable for 6.2% of the total nitrate content. Fruits such as apple, orange, and grape as well as green beans, green pea and tomato comprise the second cluster, contributing to 1.21% of the total nitrate content. The third cluster, which contributes to 26.79% of the total nitrate content, includes leafy vegetables of lettuce, and cabbage. The fourth cluster is only comprised of radish by contributing to 65.8% of the total nitrate content.
The findings suggest that root and tuber vegetables, and leafy vegetables have the highest nitrate levels, followed by fruit vegetables, and then fruits. This order is in line with those of some other studies (Sušin et al. 2006; Bahadoran et al. 2016). The use of fewer fertilizers in fruit farming could be a reason for reduced nitrate contents in fruits.
The mean nitrate concentrations detected in fruits and vegetables (mg/kg) in Mashhad clearly demonstrate lower values compared to the reported values from other studies (De Martin and Restani, 2003; Feng et al. 2006; Temme et al. 2011; Bahadoran et al. 2016; Stavroulakis et al. 2018; Roila et al. 2018). The concentrations of nitrate in the eggplant, cauliflower, zucchini, green bean and green pea were found to be comparable to the other Iranian studies’ findings (Bahadoran et al. 2016; Mehri et al. 2019; Taghipour et al. 2019). As summarized in Table 3, the vegetable nitrate contents of this study are comparable to the reported values from other countries such as Japan, UK, and Italy. Previous studies have linked lower nitrate concentrations in some fruits and vegetables to longer durations of sunshine and higher temperatures (Escobar-Gutierrez et al. 2002). Mashhad climate is also characterized by high temperatures in summer along with long periods of sunshine. Such contributing factors could be an explanation for the overall lower nitrate levels found in present research, particularly in fruits. On the other hand, high nitrate levels and relatively high variability between the individual types of fruit and vegetable samples, which lead to some samples’ large standard deviation, might be attributable to the soil type, application of fertilizers, agricultural practices, time of harvesting and groundwater nitrate contamination (Amr and Hadidi, 2001; Mahvi et al. 2005; Abdulrazak et al. 2014). However, in this regard, further investigation is required.
Health risk assessment
The degree of nitrate toxicity is directly proportional to the amount consumed on a daily basis. In this study, the estimated EDI and HRI of nitrate for water, fruits, and vegetables were computed using Equations (1) and (2). The results are shown in Table 4. While groundwater of the south and central part of the city shows the highest EDI value of nitrate, the western region has the lowest, followed by eastern and northern parts. The south and central parts’ EDI value for adults is marginally within the acceptable limit; however, children’s estimated daily nitrate intake from drinking water of these regions is more than 7 mg/kg body weight, far higher than the prescribed limit. The ADI for children in the area is determined to be higher than the maximum allowable ADI limit (3.7 mg/kg body weight/day: WHO). These regions’ HRI value is also above the acceptable level of 1 for the children target group. Therefore, based on the calculations, it could be inferred that potentially there is no significant health risk from nitrate through the daily intake of drinking water in the children and adult population in the west, east, and north. In contrast, the potential health risks of nitrate intake can be assumed for children in the south and central Mashhad, given that they consume more water per kilogram of body weight. In similar studies in the south and east of Iran, children were reported to be more significantly exposed to the health risks of drinking water contaminants compared to adults (Rezaei et al. 2017; Qasemi et al. 2018). Likewise, the health risks associated with groundwater consumption in other parts of the world, such as China, were found to be more significant in children in comparison to adult consumers by having a higher risk index for infants and children (Su et al. 2013; Chen et al. 2017). The deterioration of water quality by the injection of nitrate to the groundwater through inefficient sewer systems can be the reason for getting relatively higher HRI and EDI values in the south and central Mashhad.
Among the individual type of root and tuber vegetables, the highest nitrate EDI is found in radish for consumers, followed by carrot and potato, based on both current and WHO-recommended consumption rates. Likewise, leafy vegetables of lettuce and cabbage also exceeded the EDI limits. Only in the case of radish, lettuce, and cabbage does the HRI exceed the acceptable level of 1 for children and adults. Adults’ daily nitrate intake from fruit vegetables such as eggplant, cauliflower, and cucumber is estimated to be approximately 1 mg/kg body weight (expressed as nitrate ion), whereas children’s intake appears to be around 2 mg/kg body weight. If the consumption considers being according to the WHO recommendation, the value for children ranges from ~3 to 4 mg/kg body weight (expressed as nitrate ion). Adults’ current estimated daily intake of nitrate from other fruit vegetables is less than 1 mg/kg body weight, except for bananas. In bananas, the EDI ranges from 0.5 to 3.5 mg/kg body weight for adults and children. Additionally, the HRI for all of the fruit vegetables, and fruits analyzed is less than 1, which is within the acceptable range.
The results obtained in our study for the EDI of cauliflower, cucumber, carrot, tomato, and potato appeared to be higher compared to the values reported in a study from Poland (0.03, 0.16, 0.03, 0.04, 0.84 mg NO3−/kg body weight, respectively) (Gruszecka-Kosowska and Baran, 2017). More similar to our results, the values of EDI for tomato (0.17 mg NO3−/kg body weight) were reported by Mehri et al. (2019). However, our obtained EDI values appear to be higher for potato and carrot samples than those reported in their study (0.13 and 0.19 mg NO3−/kg body weight, respectively).
Radish, lettuce and cabbage were detected to exceed the acceptable limit and potentially pose risks for both children and adult consumers, when taking the value of 3.7 mg NO3−/kg body weight/day as the benchmark of ADI threshold (Hambridge, 2003); and HRI < 1 as the HRI threshold benchmark. Conversely, several studies have reported that none of their samples exceeds the standard ADI value (Suh et al. 2013; Gruszecka-Kosowska and Baran, 2017; Sebaei and Refai, 2021). Nitrate-contaminated irrigation water usage and fertilization intensity could be a reason why radish, lettuce, and cabbage samples exceed the threshold ADI limit in our study.
The health risk index and estimated daily intake for each fruit and vegetable sample were determined based on the 400 g daily consumption rate. Accordingly, radish, lettuce, and cabbage for adult consumers and radish, lettuce, cabbage, carrot, and potato for children were found to exceed the maximum allowable ADI limit (3.7 mg/kg body weight/day: WHO). Whereas, in the case of HRI, only radish, lettuce, and cabbage exceeded the permissible limit. Therefore, the present study concludes that nitrate pollution’s adverse effects in fruits and vegetables in Mashhad are relatively tolerable. However, the lower values of EDI and HRI could be in connection with a lower than the recommended norm of consumption for fruits and vegetables (400 g/person/day: WHO, 2008) as well as lower nitrate contamination of fruits and vegetables.
It is important to take into account that drinking water, fruits and vegetables consumptions are not considered the only (but major) exposure ways to nitrate and that an HRI value below 1 cannot solely reflect a healthy and safe level of nitrate intake. Other potential ways of exposure, including meat and other food products, are beneficial in further investigating the health risks of nitrate intake.
In our study, several commonly consumed fruit and vegetable samples available during our research period were investigated for their nitrate levels. To present a broader picture of nitrate content in fruits and vegetables in Mashhad, further research with a larger sample size is also recommended.
Conclusion
The major concerns regarding human exposure to nitrate through a nitrate-rich diet are the endogenous generation of carcinogenic nitrosamines. Drinking water, fruits, and vegetables are regarded as the primary contributing factors to dietary nitrate. The present study shows that the mean nitrate concentration in the groundwater of the west, north, and east of the Mashhad city, as well as the majority of fruit and vegetable samples, were found to be comparatively lower than that of the standard threshold. The maximum concentration value of nitrate (166.1 mg/L) belonged to the study area’s central and southern parts, representing higher population density. In contrast, the minimum value (8 mg/L) was obtained from newly constructed and less populated western areas. Increased levels of nitrate in some fruits and vegetables can be attributable to prolonged and ineffective storage and excessive application of chemical fertilizers along with nitrate-contaminated irrigation water. Significantly higher nitrate content was found in the root and tuber vegetables (1193 mg/kg) and leafy vegetables (1173 mg/L) compared to fruit vegetables (160.13 mg/kg). Also, when compared to vegetables, the fruit samples were found to have a lower nitrate content (102.37 mg/kg). With the exception of radish, lettuce, and cabbage, as well as south and central groundwater of Mashhad, the values of HRI for nitrate in all the other samples were calculated to be less than 1, which signifies that the associated health risks with nitrate exposure can be considered not significant. Hence, the nitrate intake through the majority of groundwater and vegetables and all the fruits analyzed in this study can be regarded as safe for consumers.
Concerning water quality, the coliform and nitrate levels are significant factors constraining the suitability of groundwater for human consumption in the area. Confirming with relatively strong correlations (R2 = 0.63), the high total coliform found in most samples was linked to the concentrations of nitrate, and both are likely derived from infiltration of household wastes through septic or adsorption wells, particularly in the old south and central parts. Total coliforms violated the prescribed limit at 78.93% of the sampling locations, ranging from 0 to 127 CFU/100 mL (55 wells < 1, including 32 wells = 0 CFU/100 mL). The majority (74.32%) of turbidity values were also found to be below the permissible limit.
The findings of our study are expected to provide a better picture of the residents’ exposure to nitrate through different routes (i.e., water and agricultural products) by quantifying their nitrate levels, combining both routes’ data, and assessing the associated health risks in Mashhad, in order to assist public health providers and governments’ regulators in developing better-detailed management plans.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdulrazak S, Otie D, Oniwapele YA (2014) Concentration of nitrate and nitrite in some selected cereals sourced within Kaduna state, Nigeria. Online J. Anim. Feed Res 4:37–41
Abtahi M, Fakhri Y, Oliveri Conti G, Ferrante M, Taghavi M, Tavakoli J, Heshmati A, Keramati H, Moradi B, Amanidaz N (2018) The concentration of BTEX in the air of Tehran: a systematic review-meta analysis and risk assessment. Intl J Environ Res Pub Health 15:1837. https://doi.org/10.3390/ijerph15091837
Amr A, Hadidi N (2001) Effect of cultivar and harvest date on nitrate (NO3) and nitrite (NO2) content of selected vegetables grown under open field and greenhouse conditions in Jordan. J Food Compos Anal 14:59–67. https://doi.org/10.1006/jfca.2000.0950
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, San Francisco
Ayaz A, Topcu A, Yurttagul M (2007) Survey of nitrate and nitrite levels of fresh vegetables in Turkey. J Food Technol 5:177–179
Badeenezhad A, Darabi K, Heydari M, Amrane A, Ghelichi-Ghojogh M, Parseh I, Darvishmotevalli M, Azadbakht O, Javanmardi P (2021a) Temporal distribution and zoning of nitrate and fluoride concentrations in Behbahan drinking water distribution network and health risk assessment by using sensitivity analysis and Monte Carlo simulation. Intl J Environ Anal Chem 0:1–18. https://doi.org/10.1080/03067319.2021.1903455
Badeenezhad A, Radfard M, Abbasi F, Jurado A, Bozorginia M, Jalili M, Soleimani H (2021b) Effect of land use changes on non-carcinogenic health risks due to nitrate exposure to drinking groundwater. Environ Sci Pollut Res 28:41937–41947. https://doi.org/10.1007/s11356-021-13753-5
Bahadoran Z, Mirmiran P, Jeddi S, Azizi F, Ghasemi A, Hadaegh F (2016) Nitrate and nitrite content of vegetables, fruits, grains, legumes, dairy products, meats and processed meats. J Food Compos Anal 51:93–105. https://doi.org/10.1016/j.jfca.2016.06.006
Bahrami M, Zarei AR, Rostami F (2020) Temporal and spatial assessment of groundwater contamination with nitrate by nitrate pollution index (NPI) and GIS (case study: Fasarud Plain, southern Iran). Environ Geochem Health 42:3119–3130. https://doi.org/10.1007/s10653-020-00546-x
Barnes DG, Dourson M, Preuss P, Bellin J, Derosa C, Engler R, Erdreich L, Farber T, Fenner-Crisp P, Francis E (1988) Reference dose (RfD): description and use in health risk assessments. Reg Toxicol Pharmacol 8:471–486
Barzegar R, Moghaddam AA, Tziritis E, Fakhri MS, Soltani S (2017) Identification of hydrogeochemical processes and pollution sources of groundwater resources in the Marand plain, northwest of Iran. Environ Earth Sci 76:297. https://doi.org/10.1007/s12665-017-6612-y
Bondonno CP, Blekkenhorst LC, Liu AH, Bondonno NP, Ward NC, Croft KD, Hodgson JM (2018) Vegetable-derived bioactive nitrate and cardiovascular health. Mol Asp Med Bioact Impact Human Health 61:83–91. https://doi.org/10.1016/j.mam.2017.08.001
Boumaiza L, Chesnaux R, Drias T, Walter J, Huneau F, Garel E, Knoeller K, Stumpp C (2020) Identifying groundwater degradation sources in a Mediterranean coastal area experiencing significant multi-origin stresses. Sci Total Environ 746:141203. https://doi.org/10.1016/j.scitotenv.2020.141203
Chen J, Wu H, Qian H, Gao Y (2017) Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of Northwest China. Expo Health 9:183–195. https://doi.org/10.1007/s12403-016-0231-9
Chetty AA, Prasad S, Pinho OC, de Morais CM (2019) Estimated dietary intake of nitrate and nitrite from meat consumed in Fiji. Food Chem 278:630–635. https://doi.org/10.1016/j.foodchem.2018.11.081
Chitsazan M, Mohammad Rezapour Tabari M, Eilbeigi M (2017) Analysis of temporal and spatial variations in groundwater nitrate and development of its pollution plume: a case study in Karaj aquifer. Environ Earth Sci 76:391. https://doi.org/10.1007/s12665-017-6677-7
Chiu H-F, Tsai S-S, Yang C-Y (2007) Nitrate in drinking water and risk of death from bladder cancer: an ecological case-control study in Taiwan. J Toxicol Enviro Health, Part A 70:1000–1004. https://doi.org/10.1080/15287390601171801
Chung SW, Tran JC, Tong KS, Chen MY, Xiao Y, Ho YY, Chan CH (2011) Nitrate and nitrite levels in commonly consumed vegetables in Hong Kong. Food Additives and Contaminants 4:34–41. https://doi.org/10.1080/19393210.2011.557784
Chung SY, Kim JS, Kim M, Hong MK, Lee JO, Kim CM, Song IS (2003) Survey of nitrate and nitrite contents of vegetables grown in Korea. Food Additives & Contaminants 20:621–628. https://doi.org/10.1080/0265203031000124146
Commission, E, 2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union 364.
Correia M, Barroso Â, Barroso MF, Soares D, Oliveira MBPP, Delerue-Matos C (2010) Contribution of different vegetable types to exogenous nitrate and nitrite exposure. Food Chemistry 120:960–966. https://doi.org/10.1016/j.foodchem.2009.11.030
Cross AJ, Freedman ND, Ren J, Ward MH, Hollenbeck AR, Schatzkin A, Sinha R, Abnet CC (2011) Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol 106:432–442. https://doi.org/10.1038/ajg.2010.415
Danso GK, Hanjra MA, Drechsel P (2018) Fixed wastewater-freshwater swap (Mashhad Plain, Iran) - case study
De Martin S, Restani P (2003) Determination of nitrates by a novel ion chromatographic method: occurrence in leafy vegetables (organic and conventional) and exposure assessment for Italian consumers. Food Additives & Contaminants 20:787–792. https://doi.org/10.1080/0265203031000152415
Du S, Zhang Y, Lin X (2007) Accumulation of nitrate in vegetables and its possible implications to human health. Agri Sci China 6:1246–1255. https://doi.org/10.1016/S1671-2927(07)60169-2
ECETOC (1988) Nitrate and drinking water. Brussels, European Centre for Ecotoxicology and Toxicology of Chemicals (Technical Report No. 27)
EFSA, E.F.S.A (2008) Nitrate in vegetables-scientific opinion of the panel on contaminants in the food chain. EFSA Journal 6:689
Ehteshami M, Aghassi A, Tavassoli S, Moghadam S (2014) Simulation of Mashhad aquifer: a successful assessment strategy to control groundwater contamination. Int J Geol 8:39–46
Erkekoglu P, Baydar T (2010) Evaluation of the protective effect of ascorbic acid on nitrite-and nitrosamine-induced cytotoxicity and genotoxicity in human hepatoma line. Toxicol Mech Meth 20:45–52. https://doi.org/10.3109/15376510903583711
Escobar-Gutierrez AJ, Burns IG, Lee A, Edmondson RN (2002) Screening lettuce cultivars for low nitrate content during summer and winter production. J Horticult Sci Biotechnol 77:232–237. https://doi.org/10.1080/14620316.2002.11511485
Esmaeili S, Asghari Moghaddam A, Barzegar R, Tziritis E (2018) Multivariate statistics and hydrogeochemical modeling for source identification of major elements and heavy metals in the groundwater of Qareh-Ziaeddin plain, NW Iran. Arab J Geosci 11:5. https://doi.org/10.1007/s12517-017-3317-1
Espejo-Herrera N, Cantor KP, Malats N, Silverman DT, Tardón A, García-Closas R, Serra C, Kogevinas M, Villanueva CM (2015) Nitrate in drinking water and bladder cancer risk in Spain. Environ Res 137:299–307. https://doi.org/10.1016/j.envres.2014.10.034
Esteghamati A, Noshad S, Nazeri A, Khalilzadeh O, Khalili M, Nakhjavani M (2012) Patterns of fruit and vegetable consumption among Iranian adults: a SuRFNCD-2007 study. British J Nutri 108:177–181. https://doi.org/10.1017/S0007114511005393
Fan AM (2011) Nitrate and nitrite in drinking water: a toxicological review. In: Nriagu JO (ed) Encyclopedia of Environmental Health. Elsevier, Burlington, pp 137–145. https://doi.org/10.1016/B978-0-444-52272-6.00563-8
FAO/WHO, F, 2005. Fruit and vegetables for health: report of the joint FAO/WHO workshop on fruit and vegetables for health, 1-3 September 2004, Kobe, Japan, in: Fruit and Vegetables for Health: Report of the Joint FAO/WHO Workshop on Fruit and Vegetables for Health, 1-3 September 2004, Kobe, Japan.
Feng J, Shi Z, Wu Y, Wu H, Zhao Y (2006) Assessment of nitrate exposure in Beijing residents via consumption of vegetables. Chinese J Food Hygiene 18:514–516
FSAN, F.S.A.N.Z., 2011. Survey of nitrates and nitrites in food and beverages in Australia. Food Standards Australia New Zealand Canberra (Australia).
Gao Y, Yu G, Luo C, Zhou P (2012) Groundwater nitrogen pollution and assessment of its health risks: a case study of a typical village in rural-urban continuum, China. PLoS One 7:e33982. https://doi.org/10.1371/journal.pone.0033982
Ghahremanzadeh H, Noori R, Baghvand A, Nasrabadi T (2018) Evaluating the main sources of groundwater pollution in the southern Tehran aquifer using principal component factor analysis. Environ Geochem Health 40:1317–1328. https://doi.org/10.1007/s10653-017-0058-8
Gruszecka-Kosowska A, Baran A (2017) Concentration and health risk assessment of nitrates in vegetables from conventional and organic farming. Human Ecol Risk Assess Intl J 23:727–740. https://doi.org/10.1080/10807039.2016.1277414
Hambridge T (2003) Nitrate and nitrite: intake assessment. WHO Food Additives Series 50:1053–1071
Himeno M, Ishibashi T, Nakano S, Furuya K, Kigoshi T, Uchida K, Nishio M (2003) A practical procedure for achieving a steady state of NOx concentration in plasma: with special reference to the NOx content of Japanese daily food. Tohoku J Exp Med 199:95–110. https://doi.org/10.1620/tjem.199.95
Hosseini M, Carpenter RG, Mohammad K (1998) Growth charts for Iran. Ann Human Biol 25:237–247. https://doi.org/10.1080/03014469800005602
Hsu J, Arcot J, Alice Lee N (2009) Nitrate and nitrite quantification from cured meat and vegetables and their estimated dietary intake in Australians. Food Chem 115:334–339. https://doi.org/10.1016/j.foodchem.2008.11.081
Jackson JK, Patterson AJ, MacDonald-Wicks LK, Forder PM, Blekkenhorst LC, Bondonno CP, Hodgson JM, Ward NC, Holder C, Oldmeadow C, Byles JE, McEvoy MA (2019) Vegetable nitrate intakes are associated with reduced self-reported cardiovascular-related complications within a representative sample of middle-aged Australian women, prospectively followed up for 15 years. Nutrients 11:240. https://doi.org/10.3390/nu11020240
Jalili D, RadFard M, Soleimani H, Nabavi S, Akbari H, Akbari H, Kavosi A, Abasnia A, Adibzadeh A (2018) Data on nitrate–nitrite pollution in the groundwater resources a Sonqor plain in Iran. Data in Brief 20:394–401. https://doi.org/10.1016/j.dib.2018.08.023
Jonvik KL, Nyakayiru J, Pinckaers PJ, Senden JM, van Loon LJ, Verdijk LB (2016) Nitrate-rich vegetables increase plasma nitrate and nitrite concentrations and lower blood pressure in healthy adults. J Nutri 146:986–993. https://doi.org/10.3945/jn.116.229807
Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A (2015) Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65:320–327. https://doi.org/10.1161/HYPERTENSIONAHA.114.04675
Keeton J, Osburn W, Hardin M, Longnecker M, Bryan N (2009) A national survey of the nitrite/nitrate concentrations in cured meat products and non-meat foods available at retail | National Agricultural Library [WWW Document]. URL https://www.nal.usda.gov/fsrio/research-projects/national-survey-nitritenitrate-concentrations-cured-meat-products-and-non-meat-foods-available (accessed 7.24.21)
Kerley CP, Dolan E, James PE, Cormican L (2018) Dietary nitrate lowers ambulatory blood pressure in treated, uncontrolled hypertension: a 7-d, double-blind, randomised, placebo-controlled, cross-over trial. British J Nutri 119:658–663. https://doi.org/10.1017/S0007114518000144
Khalili N, Khodashenas SR, Davary K, Baygi MM, Karimaldini F (2016) Prediction of rainfall using artificial neural networks for synoptic station of Mashhad: a case study. Arab J Geosci 9:624. https://doi.org/10.1007/s12517-016-2633-1
Kostraba JN, Gay EC, Rewers M, Hamman RF (1992) Nitrate levels in community drinking waters and risk of IDDM: an ecological analysis. Diabetes Care 15:1505–1508. https://doi.org/10.2337/diacare.15.11.1505
Lang Y-C, Liu C-Q, Zhao Z-Q, Li S-L, Han G-L (2006) Geochemistry of surface and ground water in Guiyang, China: water/rock interaction and pollution in a karst hydrological system. Appl Geochem 21:887–903
Ling B (2000) Health impairments arising from drinking water polluted with domestic sewage and excreta in China. Schriftenreihe des Vereins fur Wasser-, Boden-und Lufthygiene 105:43–46
Loh YH, Jakszyn P, Luben RN, Mulligan AA, Mitrou PN, Khaw K-T (2011) N-nitroso compounds and cancer incidence: the European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk Study. Am J Clin Nutri 93:1053–1061. https://doi.org/10.3945/ajcn.111.012377
Lundberg JO, Weitzberg E, Gladwin MT (2008) The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7:156–167. https://doi.org/10.1038/nrd2466
Machha A, Schechter AN (2012) Inorganic nitrate: a major player in the cardiovascular health benefits of vegetables? Nutrition Reviews 70:367–372
Mahvi AH, Nouri J, Babaei AA, Nabizadeh R (2005) Agricultural activities impact on groundwater nitrate pollution. Intl J Environ Sci Technol 2:41–47. https://doi.org/10.1007/BF03325856
Maleki A, Jari H (2021) Evaluation of drinking water quality and non-carcinogenic and carcinogenic risk assessment of heavy metals in rural areas of Kurdistan, Iran. Environm Technol Innov 23:101668. https://doi.org/10.1016/j.eti.2021.101668
Marie A, Vengosh A (2001) Sources of salinity in ground water from Jericho area, Jordan valley. Groundwater 39:240–248. https://doi.org/10.1111/j.1745-6584.2001.tb02305.x
Marshall RE, Levison J, McBean EA, Parker B (2019) Wastewater impacts on groundwater at a fractured sedimentary bedrock site in Ontario, Canada: implications for First Nations’ source-water protection. Hydrogeol J 27:2739–2753. https://doi.org/10.1007/s10040-019-02019-7
Meah MN, Harrison N, Davies A (1994) Nitrate and nitrite in foods and the diet. Food Additives & Contaminants 11:519–532. https://doi.org/10.1080/02652039409374250
Mehri F, Heshmati A, Moradi M, Khaneghah AM (2019) The concentration and health risk assessment of nitrate in vegetables and fruits samples of Iran. Toxin Reviews 40(4):1215–22. https://doi.org/10.1080/15569543.2019.1673424
Menard C, Heraud F, Volatier J-L, Leblanc J-C (2008) Assessment of dietary exposure of nitrate and nitrite in France. Food Additives and Contaminants 25:971–988. https://doi.org/10.1080/02652030801946561
Mititelu-Ionuș O, Simulescu D, Popescu SM (2019) Environmental assessment of agricultural activities and groundwater nitrate pollution susceptibility: a regional case study (Southwestern Romania). Environ Monit Assess 191:501. https://doi.org/10.1007/s10661-019-7648-0
Moeini Z, Azhdarpoor A (2021) Health risk assessment of nitrate in drinking water in Shiraz using probabilistic and deterministic approaches and impact of water supply. Environ Challenges 5:100326. https://doi.org/10.1016/j.envc.2021.100326
Mohammadpour A, Tabatabaee Z, Dehbandi R, Khaksefidi R, Golaki M, Gharechahi E, Samaei MR, Mohammadpour R, Sheibani A, Badeenezhad A (2022) Concentration, distribution and probabilistic health risk assessment of exposure to fluoride in drinking water of Hormozgan province, Iran. Stoch Environ Res Risk Assess 36:1035–1047. https://doi.org/10.1007/s00477-021-02090-1
Mor F, Sahindokuyucu F, Erdogan N (2010) Nitrate and nitrite contents of some vegetables consumed in south province of Turkey. Agri J 5:142–145
Mortada WI, Shokeir AA (2018) Does nitrite and nitrate levels in drinking water impact the health of people in Dakahlia governorate, Egypt? Environ Sci Pollut Res 25:19728–19738. https://doi.org/10.1007/s11356-018-2156-2
Mostaghelchi M, Zendehbad M, Lengauer CL (2021) Small hematite nanoparticles from the Kiruna-type ore; evaluation of declined balance limit of the attrition process and their catalytic properties. J Nanosci Nanotechnol 21:6000–6006. https://doi.org/10.1166/jnn.2021.19502
Nowrouz P, Taghipour H, Dastgiri S, Bafandeh Y, Hashemimajd K (2012) Nitrate determination of vegetables in Varzeghan City, North-western Iran. Health Promot Perspect 2:244–250. https://doi.org/10.5681/hpp.2012.030
Parks SE, Irving DE, Milham PJ (2012) A critical evaluation of on-farm rapid tests for measuring nitrate in leafy vegetables. Scientia Horticulturae 134:1–6. https://doi.org/10.1016/j.scienta.2011.10.015
Pastén-Zapata E, Ledesma-Ruiz R, Harter T, Ramírez AI, Mahlknecht J (2014) Assessment of sources and fate of nitrate in shallow groundwater of an agricultural area by using a multi-tracer approach. Sci Total Environ 470:855–864. https://doi.org/10.1016/j.scitotenv.2013.10.043
Prakash KL, Somashekar RK (2006) Groundwater quality- assessment on Anekal Taluk, Bangalore Urban district, India. J Environ Biol 27:633–637
Qasemi M, Afsharnia M, Farhang M, Bakhshizadeh A, Allahdadi M, Zarei A (2018) Health risk assessment of nitrate exposure in groundwater of rural areas of Gonabad and Bajestan, Iran. Environ Earth Sci 77:551. https://doi.org/10.1007/s12665-018-7732-8
Rahmani J, Miri A, Mohseni-Bandpei A, Fakhri Y, Bjørklund G, Keramati H, Moradi B, Amanidaz N, Shariatifar N, Khaneghah AM (2018) Contamination and prevalence of histamine in canned tuna from Iran: a systematic review, meta-analysis, and health risk assessment. J Food Protect 81:2019–2027
Rasuli R, Darzi A, Mashadi-Kholerdi F (2018) Analyzing temporal and spatial variations of nitrate concentration and its health risks in groundwater in Mazandaran province. Iran J Irrigation Drain 12:376–386
Razgallah N, Chikh-Rouhou H, Boughattas I, M’hamdi M (2016) Nitrate contents in some vegetables in Tunisia. Arch Agron Soil Sci 62:473–483. https://doi.org/10.1080/03650340.2015.1072623
Reinik M, Tamme T, Roasto M, (1990) Naturally occurring nitrates and nitrites in foods. Bioact Compd Foods 225–253
Rezaei M, Nikbakht M, Shakeri A (2017) Geochemistry and sources of fluoride and nitrate contamination of groundwater in Lar area, south Iran. Environ Sci Pollut Res 24:15471–15487. https://doi.org/10.1007/s11356-017-9108-0
Ritzi RW, Wright SL, Mann B, Chen M (1993) Analysis of temporal variability in hydrogeochemical data used for multivariate analyses. Groundwater 31:221–229. https://doi.org/10.1111/j.1745-6584.1993.tb01814.x
Rocha BS, Gago B, Barbosa RM, Laranjinha J (2009) Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology 265:41–48. https://doi.org/10.1016/j.tox.2009.09.008
Roila R, Branciari R, Staccini B, Ranucci D, Miraglia D, Altissimi MS, Mercuri ML, Haouet NM (2018) Contribution of vegetables and cured meat to dietary nitrate and nitrite intake in Italian population: safe level for cured meat and controversial role of vegetables. Ital J Food Saf 7:7692. https://doi.org/10.4081/ijfs.2018.7692
Roohparvar R, Shamspur T, Mostafavi A, Bagheri H (2018) Indirect ultra-trace determination of nitrate and nitrite in food samples by in-syringe liquid microextraction and electrothermal atomic absorption spectrometry. Microchem J 142:135–139. https://doi.org/10.1016/j.microc.2018.06.027
Rowell DL (2014) Soil science: Methods & applications. Routledge
Santamaria P, Elia A, Serio F, Todaro E (1999) A survey of nitrate and oxalate content in fresh vegetables. J Sci Food Agri 79:1882–1888
Sebaei AS, Refai HM (2021) Hazard index: probabilistic risk exposure of nitrate and nitrite in Egyptian fruits and vegetables. Intl J Environ Anal Chem 101:1477–1484. https://doi.org/10.1080/03067319.2019.1685666
Sheikholeslam R (2001) Food consumption pattern and nutritional problems in the Islamic Republic of Iran. Nutri Health 15:69–70
Smoroń S (2016) Quality of shallow groundwater and manure effluents in a livestock farm. J Water Land Dev
Solgi E, Jalili M (2021) Zoning and human health risk assessment of arsenic and nitrate contamination in groundwater of agricultural areas of the twenty two village with geostatistics (Case study: Chahardoli Plain of Qorveh, Kurdistan Province, Iran). Agri Water Manage 255:107023. https://doi.org/10.1016/j.agwat.2021.107023
Stavroulakis G, Kafouris D, Christofidou M, Paikousis L, Christou E, Christodoulidou M, Kanari P, Ioannou-Kakouri E (2018) Occurrence of nitrate in vegetables and dietary exposure assessment for the Cypriot adolescent consumers. Accred Qual Assur 23:115–122. https://doi.org/10.1007/s00769-018-1311-2
Su X, Wang H, Zhang Y (2013) Health risk assessment of nitrate contamination in groundwater: a case study of an agricultural area in Northeast China. Water Resour Manage 27:3025–3034. https://doi.org/10.1007/s11269-013-0330-3
Suh J, Paek OJ, Kang Y, Ahn JE, Jung JS, An YS, Park S-H, Lee S-J, Lee K-H (2013) Risk assessment on nitrate and nitrite in vegetables available in Korean diet. J Appl Biol Chem 56:205–211
Sušin J, Kmecl V, Gregorčič A (2006) A survey of nitrate and nitrite content of fruit and vegetables grown in Slovenia during 1996–2002. Food additives and Contaminants 23:385–390. https://doi.org/10.1080/02652030600573715
Taghipour H, Hemmati S, Faramarzi E, Somi MH, Dastgiri S, Nowrouze P (2019) Determination of nitrate concentration in consumed vegetables and estimation of that’s dietary intake in Shabestar and Khameneh City, northwest of Iran: Azar Cohort study. Progr. Nutr 21:336–340
Tate CH, Arnold KF (1990) Health and aesthetic aspects of water quality. MCGRAW-HILL, INC.,(USA). 1194 1990
Temme EH, Vandevijvere S, Vinkx C, Huybrechts I, Goeyens L, Van Oyen H (2011) Average daily nitrate and nitrite intake in the Belgian population older than 15 years. Food Additives & Contaminants: Part A 28:1193–1204. https://doi.org/10.1080/19440049.2011.584072
Thomson BM, Nokes CJ, Cressey PJ (2007) Intake and risk assessment of nitrate and nitrite from New Zealand foods and drinking water. Food Additives & Contaminants 24:113–121. https://doi.org/10.1080/02652030600934206
USEPA (2013) Integrated Risk Information System (IRIS) (United States Environment Protection Agency, Washington, DC)
USEPA (2004) Risk Assessment Guidance for Superfund (Human Health Evaluation Manual). USA. Environmental Protection Agency, Washington, DC (USA). Office of Solid Waste
Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, Van Eijl S, Sagi-Kiss V, Chowdhury TA, Curtis M (2016) Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutri 103:25–38. https://doi.org/10.3945/ajcn.115.116244
Wakida FT, Lerner DN (2005) Non-agricultural sources of groundwater nitrate: a review and case study. Water Research 39:3–16. https://doi.org/10.1016/j.watres.2004.07.026
WHO W.H.O (2008) WHO European action plan for food and nutrition policy 2007–2012. WHO Regional Office for Europe, Copenhagen
World Health Organization (2017) Guidelines for drinking-water quality: first addendum to the fourth edition.
Yang C-Y, Cheng M-F, Tsai S-S, Hsieh Y-L (1998) Calcium, magnesium, and nitrate in drinking water and gastric cancer mortality. Japanese J Cancer Res 89:124–130
Ysart G, Miller P, Barrett G, Farrington D, Lawrance P, Harrison N (1999) Dietary exposures to nitrate in the UK. Food Additives & Contaminants 16:521–532. https://doi.org/10.1080/026520399283669
Zendehbad M, Cepuder P, Loiskandl W, Stumpp C (2019a) Source identification of nitrate contamination in the urban aquifer of Mashhad, Iran. J Hydrol Region Stud 25:100618. https://doi.org/10.1016/j.ejrh.2019.100618
Zendehbad SM, Cepuder P, Loiskandl W, Stumpp C (2019b) Nitrate source identification in urban groundwater, in: EGU General Assembly Conference Abstracts. 8798
Zhou Z-Y, Wang M-J, Wang J-S (2000) Nitrate and nitrite contamination in vegetables in China. Food Rev Intl 16:61–76
Zhu Y, Wang PP, Zhao J, Green R, Sun Z, Roebothan B, Squires J, Buehler S, Dicks E, Zhao J, Cotterchio M, Campbell PT, Jain M, Parfrey PS, Mclaughlin JR (2014) Dietary N-nitroso compounds and risk of colorectal cancer: a case–control study in Newfoundland and Labrador and Ontario, Canada. British J Nutri 111:1109–1117. https://doi.org/10.1017/S0007114513003462
Acknowledgements
The authors would like to thank Dorkav Toos Mining Company (DMC) and Masoud Bameshgi, P.E., for their support, and Univ. Prof. Dr. Christine Stumpp, and Lamine Boumaiza, Ph.D., for valuable comments and suggestions, allowing us to improve the work.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s design and conception. Material preparation, data collection, and analysis were performed by Seyed Mohammad Zendehbad with the support of Mohsen Mojganfar and Majid Mostaghelchi. The first draft of the manuscript was written by Seyed Mohammad Zendehbad. All authors commented, read, and approved the previous versions of the manuscript. Peter Cepuder and Willibald Loiskandl supervised the work, provided critical feedback, and helped shape the analysis and manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Xianliang Yi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zendehbad, M., Mostaghelchi, M., Mojganfar, M. et al. Nitrate in groundwater and agricultural products: intake and risk assessment in northeastern Iran. Environ Sci Pollut Res 29, 78603–78619 (2022). https://doi.org/10.1007/s11356-022-20831-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20831-9