Abstract
Graphite (GR) and graphite/alginate (GRA) composite were synthesized utilizing the thermal annealing technique and used as a new adsorbent material for the selective separation and removal of La(III) and Ce(III) from aqueous solutions. Fourier transform infrared (FTIR) spectroscopy, thermal analysis (DTA, TGA), X-ray diffraction (XRD), surface area, porosity, and scanning electron microscope (SEM) were also used to characterize the generated material. Distinct experiments were performed to test the ability of the GRA to La(III) and Ce(III) removal, which include the effect of pH, shaken time, initial concentration of La(III), and Ce(III) at different temperatures range. After 20 min, both ions have reached equilibrium. The pseudo second-order kinetic model was chosen as one which best fits the experimental evidence and better reflects the chemical sorption process. Adsorption isotherm was studied using the Langmuir, Freundlich, and D-R models. The Langmuir model was used to better fit the results obtained. At 25 °C, Ce(III) and La(III) have maximum monolayer capacities of 200 and 83.3 mg/g, respectively. The sorption was endothermic reaction and spontaneous, as illustrated by the data of thermodynamics studies. GRA has the ability to be used as a novel lanthanide adsorbent material, especially for selective separation between Ce(III) and La(III).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A contaminant material displays a substantial challenge for many scientists to protect humanity and the environment from the risky effect (Allam et al. 2021). The contaminants have multiple sources in the environment as organic dyes, radioisotopes, and heavy elements (Wu et al. 2019; Hassan et al. 2019). The radioisotopes are produced from many applications such as nuclear fuel, medical diagnoses, and treatment (Zhang et al. 2019; Abdel Maksoud et al. 2022). One of the fission products generated by irradiated nuclear fuel is lanthanides elements (Vergas et al. 2021; Lankapati et al. 2021). Also, lanthanide elements have a distinct application in many fields, for example, metallurgy and the ceramic industry. Therefore, the demand for lanthanides will be an utmost in the future, particularly using the high purity lanthanides in high technology applications (Zhu et al. 2015; Wang et al. 2013). The waste containing lanthanide elements is discharged into the environment, so their removal from respective mixtures has considerable importance and their recoveries are very important (Lee et al. 2005; Bonal et al. 1998; Volokh et al. 1992).
Many techniques were used for the separation and extraction of lanthanide elements such as adsorption, liquid–liquid extraction, membrane, ion exchange, and solid–liquid extraction (Gupta and Krishnamurthy 1992; Li and Sun 2005; Hassan and Elmaghraby 2019). One of the most efficient separating techniques is the adsorption process (Ahmadi et al. 2022; Naeini et al. 2022; Moradi and Sharma 2021; Babudurai et al. 2021). This method has a number of advantages, including fewer wastes and a faster solid–liquid separation. Several bio adsorbents have received a lot of attention for adsorption in the previous decade, owing to their low cost and environmental integrity (Moradi et al. 2021; Kim et al. 2011; Ngah and Hanafiah 2008; Zhang et al. 2012; Xiong et al. 2011; Gupta et al. 2015). Alginate acts as a biologically degradable carbohydrate polymer that is often produced from brown seaweed, and it is the sodium salt of alginic acid (Kazemi and Javambakht 2019). It is composed of α–L–G (guluronic acid) with β–D–M (mannuronic acid). The effective functional groups in alginate are carboxylic and hydroxyl groups (Huixue et al. 2016; Zj et al. 2018). Alginate has powerful binding abilities and hydrophilicity properties as well as biologically renewable for many hazardous elements removal (Cajnko et al. 2019; You et al. 2019). Encapsulation of particles with alginate beads via calcium ion cross-linking is a less hazardous impregnating approach (Anisha and Prema 2008; El-Din et al. 2021). Alginate adsorbents have restricted use because of their low mechanical strength and durability. Combining alginates with other materials such as graphene oxide (Mirzaeian et al. 2019; Malinga and Jarvis 2020), activated carbon or graphite with suitable particle size distribution and large pores, high adsorption capacity, and specific surface area (Ahmadi et al. 2021) has improved their mechanical qualities (Rajabiet al. 2017). Rajendran et al. (2021) reported that carbon-based materials and their composites are super capacitors due to their high efficiency, superior energy densities, low cost, and prolonged life span (Rajendran et al. 2021).
Graphite with its considerable surface area and microporous nature is promising adsorbent for the removal of hazardous elements from wastewater. The applications of porous graphite for the removal of hazardous elements can be restricted by slow diffusion kinetics) Pattanayak et al. 2000; Pelekani and Snoeyink, 1999), but graphite nanostructure has found its place as an alternative of the porous graphite materials. The properties of nano graphite make them promise nuclear technology for the removal of hazardous elements (Iuima 1991; Hussain and Iqbal 2011). Babaei et al. (2021) prepared a quantum dots adsorbent using carbon-based materials (grapheme) with particle size less than 10 nm as a super adsorbent for heavy metals removal from waste water (Babaei et al. 2021).
Olatunji et al. (2018) used a sorption approach and a composite of polypyrrole and wood sawdust to remove 139Ce (a strong gamma-emitting radioisotope of cerium with T1/2 = 137.641 day). According to the Langmuir model, the maximal capacity was found to be 6.57 ± 0.54 mg/g. Sert et al. (2008) investigated the biosorption of La(III) and Ce(III) by Platanus oriental leaf. According to the results, the sorption was endothermic in nature and spontaneous, with the adsorbent’s Langmuir capacity of 28.65 mg/g for La(III) and 32.05 mg/g for Ce(III) (Sert et al. 2008).
The main objective of this study is to prepare high performance and high sorption capacity adsorbent material (graphite/alginate) for selective separation and removal of some lanthanides (Ce III and La III) from aqueous solution. The sorption was discussed in terms of pH, contact time, concentration, temperature, and interfering ion. Kinetic, thermodynamic parameters, and isotherm studies related to the process were performed.
Experimental
Materials and methods
All used reagents in the present study are analytical grade and used without any additional purification. The used cerium chloride and lanthanum chloride were purchased from Fluka Germany Company. Sodium alginate, C5H7O4COONa, with a molecular weight of 216.12, and calcium chloride were purchased from Sigma-Aldrich. The pH was adjusted by the addition of NaOH/HCl acquired from Fluka.
Instrumentation
The initial and final concentrations of Ce (III) and La (III) were determined by a Shimadzu UV–visible spectrophotometer model UV-160 A, Japan. The pH of the solution was measured by a pH meter (Thermo Scientific). The morphological features of graphite/alginate (GRA) adsorbent before and after adsorption of CeIII and LaIII were recorded by scanning electron microscope (Philips XL 30). FT-IR characterization was carried out by spectrometer model 2000 FTIR, Perkin Elmer Company, USA, covered the range from 400 to 4000 cm−1. The crystal structures of pure graphite, alginate, and graphite/alginate (GRA) were analyzed by Shimadzu X-ray diffractometer (XRD) model XD-Dl, Kyoto, Japan. The measurements of the phase changes and weight losses of GRA were performed with the Shimadzu DTA–TGA system of type DTA-TGA-60 H, Japan.
Preparation of graphite (GR)
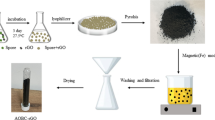
Synthetic routes of GR in detail, pure acrylamide (AA) powder, and ammonium persulfate (APS) were used in the preparation of the organo-sulfonicacids. Each 56.9 g (0.8 mol) of AA monomer was dissolved in double-distilled water up to 200 mL. A hot plate stirrer was used to dissolve the monomer in water at room temperature at a low frequency. The stirrer was adjusted to heat the solution within the reaction flask to a temperature of 80 °C. The initiator was dissolved in water so that 0.1 mol/L of APS was heated to 80 °C. Mixing of the APS solution and the AAmonomer solution took place at 80 °C. The blend was stirred heating for 10 min to ensure complete mixing. In the present work, 1 mL (0.001 mol) of APS solution was added to 20 mL (0.08 mol) of AA solution, i.e., to have a molecular monomer to initiator ratio of 80:1. Only 20 mL of the monomer was used in the preparation of the gel to avoid the overheating that occurs during the exothermic polymerization reaction. Using large amounts of monomer solution would require special attention and the use of a heat sink setup. Polymerization occurs with complete consumption of the monomer to form the polymers, i.e., there are no effective termination reactions. After the preparation of the gel, the reaction process continued for a long time to degrade the properties of the prepared samples. After preparation of the gel, samples were left to dry at 60 °C for 5 days to ensure the full consumption of the monomer and solidification of the gel. Samples were treated thermally at 250 °C for 3 h at ambient temperature. After annealing, samples were left overnight to complete the thermolytic reaction at room temperature. Then the samples were smashed and annealed at 350 °C for an extra 3 h and with extra annealing at 460 °C for 3 h to stimulate the formation of graphite structures.
Preparation of Ca-alginate/graphite (GRA) adsorbent
The graphite nanostructures were made by thermolysis of a synthesized poly-acryl amide gel (Hassan and Elmaghraby 2012). The alginate 2% (w/v) was synthesized by dissolving Na-alginate)2 g) in distilled water (100 ml) with constant stirring strength (300 rpm) until a gel solution was obtained. (GRA) beads were syntheses by blending 1 g graphite with (100 ml) of Na-alginate gel 2% (w/v); the solution was kept under agitation until a homogeneous gel was obtained which was then slowly added drop wise through a peristaltic pump at 3.5–4.0 rpm into 200 ml of 2% (or 0.2 mol/L) aqueous calcium chloride solution at 25 °C with gentle stirring. Then, the sphere became compact after 2 h of drying, and the size of the resultant beads was measured by a sieve (1.5 + 0.2 mm). The gel beads were rinsed with distilled water several times to remove free Ca(II) and dried in the air at 25 °C for 24 h.
Adsorption experiment
Adsorption experiments of La(III) and Ce(III) on GRA and GR were carried out in 50-mL conical tubes at a 100 rpm mixing rate and 25 °C in a water bath shaker. A total of 0.01 g of adsorbent was blended with 10 mL solutions containing known concentrations of La(III) and Ce(III) in the tubes to perform adsorption kinetic studies. Samples were collected and filtered with a 0.45-mm syringe filter (Pall Co., USA) at predetermined time intervals to evaluate the dissolved ionic concentrations of the residual metal ions in the solutions. Adsorption isotherm experiments were performed at different temperatures with an initial concentration of 50–250 mg/L. The removal % (R%) (Mozaffari et al. 2020a, b, c) and the adsorption capacity (Sheshdeh et al. 2014) (qe (mg/g)) were determined according to the following Eqs. (1) and (2), respectively:
The starting and equilibrium concentrations of adsorbate (mg/L) are C0 and Ce, respectively. V (L) and m (g) denote the volume of the aqueous solution and the mass of the adsorbent, respectively. The concentration of lanthanide metal ion was determined spectrophotometrically by Arsenazo (III) method. The samples were analyzed at wavelength 650 using a Shimadzu model 160A double-beam UV spectrophotometer.
Determination of lanthanides elements
Initial and equilibrium lanthanide concentrations in the aqueous phase were estimated by a spectrophotometric method using arsenazo (III) as a reagent. It is used for spectrophotometric determination of rare earth elements (REEs), in acid solutions, arsenazo (III) has a purplish-red color while at higher pH values, it is blue-violet. The absorbance of free arsenazo III (λ max = 520–530 nm) at the absorption maxima of the metal complexes (λ = 655–675 nm) is very slight. This large difference (Δλ) between the wavelength of the absorption maxima of the complex and the free reagent is important and suitable for the exact determination of metal ions in their solutions. At pH 1–4, arsenazo (III) reacts with rare earth elements to form colored complexes.
The presence of the arsonic group AsO(OH)2 causes the formation of stable complexes of some metals even in strong acid solutions. The presence of the azo group ensures the color reaction (Marczenko and Freiser 1981).
Results and discussion
Characterization
Zero charges point (pHpzc), XRD, FTIR, DTA, TGA, and SEM were employed to define the produced adsorbent. The pH value that the total of + Ve and − Ve charges on a solid’s surface equals zero is the zero charges point (pHpzc). While the GRA pH falls below pHpzc, it becomes positively charged, and while the pH rises beyond pHpzc, it becomes negatively charged. Figure 1 shows the difference between the initial and final values versus the initial pH values. The same experimental procedure was shown in De Rossi et al. (2020). The GRA adsorbent’s pHpzc value was 5.0, suggesting that the adsorbent generated was neutral during this pH.
The SEM photographs of graphite/alginate (GRA) adsorbent before and after adsorption were evaluated (magnification was X750, and 20 µm scale) and they are shown in Fig. 2. Before adsorption (Fig. 2A), GRA adsorbents have a well-defined interconnected, unique 3D porous network framework. The 3D porous network can enable metal ion-free diffusion and ensure that metal ions are transported to the adsorbents’ interior structure. After the adsorption of Ce(III) and La(III) (Fig. 2B and C), the GRA surface appeared to be cloudy and dull but at the same time, the structure of the prepared adsorbent remains stable in the adsorption process. The structural stability of the adsorbent is an indispensable part of the application of the adsorbent. Cho et al. (2013) described that the nanosized carbons impregnated in the bead were not visible in the SEM analysis, probably due to the encapsulation of nanosized carbons in the interior space (Cho et al. 2013).
The prepared adsorbent’s FT-IR spectra were observed before adsorption of Ce(III) and La(III) and after their adsorption to detect the groups responsible for adsorption and the changes that occurred after adsorption (Fig. 3). The existence of a carbonyl C = O stretching vibration group connected with the COO− groups of alginate loaded on the interface of graphite is evidenced by a strong absorption peak at 1655 cm−1(Verma et al. 2020). It was noted that the C = O peak was changed through shifting or shrinking due to interaction and chemical bond formation with La(III) (GRA/La beak) and Ce(III) (GRA/Ce beak). The broad absorption band at 3290–2800 cm−1 is assigned to O–H stretching vibrations of phenolic or alcoholic functional groups. The absorption at 880–995 cm−1 is attributed to R = C–H and R = CH2 stretching vibrations that are shifted to a lower frequency because of conjunction with lanthanides metal ions. The sharp medium peak at 2230 cm−1 is attributed to a conjugated C≡N (nitrile) stretching vibration.
XRD patterns of GR, alginate, and GRA are in Fig. 4. The GR pattern showed an intense and sharp peak at 30.30. According to the Bragg equation, this peak corresponded to a d-spacing of 296°A, which confirms the synthesis of GR. In the XRD spectrum of alginate, the broad peak at 13.2° demonstrated the amorphous structure of alginate. Alginate was usually semi-crystalline due to the strong interaction between alginate chains through intermolecular hydrogen bonding (Thakur et al. 2016). The GRA pattern was similar to the GR pattern. This may be due to the low alginate content in the GRA. Because of the low content of alginate in GRA, the XRD spectrum of GRA shows a significant GR diffraction peak; moreover, more information was obtained, which indicated that the GRA had a crystalline structure.
The degree of particle size (D) of the prepared material could be calculated from the XRD line extending the curve of the Scherrer formula (Attallah et al. 2019a, b); D = Kλ/βcosθ, where D is the crystalline size; λ is the X-ray wavelength (CuKα-1.541836); k is the Scherrer constant (0.89), and β is the angular width of the full width of the diffracted peak at half maximum (FWHM) in the diffraction angle radii (2θ). The average particle sizes were determined from the most intense peaks at 30.12 (2θ). The calculated particle size of GR and GRA was 80 and 90.28 nm respectively.
Thermal analyses of TGA and DTA of GRA adsorbent are illustrated in Fig. 5. According to TGA analysis, the thermal degradation of the synthesized material is usually accompanied by a few main steps. The 1st step includes physicochemical transformation and occurs at low temperatures. The low-temperature mass loss from 30 to 200 °C corresponds to intact water desorption (evaporation of physically absorbed water). The most weight loss was found for the GRA degradation step (200–600 °C). Three degradation peaks (at 220, 480, and 600 °C) are observed. The loss of moisture-bound water in GRA happens around 220 °C and is represented in the curves by a slight weight loss (− 6.7). By raising the temperatures (up to 480 °C) destruction takes place of the less condensed fractions, such as the alginate (organic component) with weight loss (− 40.55%) corresponding to endothermic beak according to DTA analysis at 440 °C. Finally, at about 600 °C, the thermal actions are related to the pyrolysis (crystal dehydration) of the more condensed materials of graphite with weight loss (− 8.6%), and the total weight loss reaches about (55.9%).
The data of surface area is clarified in Table 1. The bulk density evaluates the sample volume, the open as well as closed pores, whereas the apparent density is interested by closed pores and the sample volume. By comparing the bulk density and apparent density for both the materials, it is evident that the open pores in GRA composite will increase. From Table 1, the value of average pore diameter of GRA composite decreases about twice time as the porosity and densities increase for GR sample. This is confirming the increasing the open pores that will occupy and so enhancement adsorption process of the sample. According to the elemental analysis for GRA, the percent of C, O, S, and Na is 94.2, 1.7, 3.01, and 1.087 respectively.
Batch technique
Effect of pH
Among the environmental parameters that have much influence on the adsorption process is the pH of the solution. This is for the degree of protonation, metal speciation, and adsorbent surface functional groups that are usually affected by the pH change. Experiments were carried out in the pH range 2–8 to study the effect of the maximum removal efficiency of cerium and lanthanum on the value of pH. Figure 6 illustrates cerium (III) and lanthanum (III) adsorption by GR and GRA as a function of pH. As illustrated, Ce and La adsorption increases rashly with pH from 1 to 5, then stabilizes around pH 6 until pH 8.
The reduction in H+ ion interaction with the GR and GRA surfaces can be attributed to the quick increase in uptake quantity with a rise in pH. Because there are more hydrogen ions in the liquid phase vying for sorption sites with cerium and lanthanum ions at low pH, this can result in hydrogen ions preferentially adsorbing onto the adsorbent surface over Ce and La ions, resulting in a reduction in uptake at low pH.
When the pH was raised above pH 5 (low hydrogen ion accumulation), the hydroxyl (OH), carboxyl (COOH), and nitrile (CN) present on the surface, as shown in FTIR analyses, became negatively charged and interfered with the strength of the combination between functional groups of GRA and ions in adsorbate, interfering with the ionic exchange of ions in the bio-adsorbent. These results are confirmed with the result of PZC Fig. 1. Beyond pH = 5, the surface becomes negatively charged which increases the opportunity for chelating reaction (via formation of an ionic and covalent bond with metal ions) on GRA.
According to Mahmood et al. (2017), alginate is crucial in the removal of toxic metals in water treatment. Biodegradability, biocompatibility, cost-effectiveness, and environmental friendliness are among its benefits. During the adsorption phase, ion exchange appears to exchange toxic metal ions with sodium or calcium ions. From Fig. 6, it was noted that GRA has a higher R% (98 and 68% for Ce and La respectively) than GR (75 and 63% for Ce and La respectively) so that further batch experiments including kinetic and isotherm were performed using GRA and GR was neglected.
In particular, from Fig. 7, it has been shown that Ce(III) is present in its stable trivalent form at pH ˂ 5 and under this condition, precipitation of Ce(OH)3 is negligible (hydroxyl form starts gradually at pH = 5). However, as pH increases, precipitation of Ce(OH)2 starts at about pH 5.1, and Ce(OH)2 starts at about pH 7.9 (Olatunji et al. 2016) Ce(OH) at pH 10.
The lanthanum ion may easily be linked to many water molecules because of its low charge to radius ratio. Bouyer et al. discussed that, during lanthanum ion hydrolysis, hydroxy-ligands replace coordinated water molecules, resulting in the constitution of different hydrolysis species (Bouyer et al. 2006). Figure 7 shows the hydrolysis species as a proportion of pH. The hydrolysis of lanthanum ions is insignificant at acidic conditions (pH < 5) as may be shown. Above that critical pH value, hydroxyl form increases gradually, and several hydrolysis products coexist until pH 12 where [La(OH)4]− is the only hydrolyzed species in solution. At around pH 6.1, La(OH)2 is starting to precipitate and at pH 7.5, La(OH)3 is the dominant hydrolytic.
Effect of contact time
All experiments were executed in triplicate and the obtained data were scrutinized by variance and stander deviation test using Excel 2010 software and is ranged from 0.37 to 0.51 and 0.19–0.21 for La(III) and Ce(III), correspondingly. The sorption of La(III) and Ce(III) on GRA and GR was investigated by shaking time and temperature at pH 5 and 25, 35, and 55 °C (Fig. 8). At first, the ions selectively adsorbed and occupied the active functional groups on GRA and GR. As the contact time increased, the adsorption sites on the adsorbents were filled. The rate of adsorption slowed slightly before increasing (this is attributed to the availability of large surface areas of the adsorbent) (Mustapha et al. 2019) reached a plateau which indicates that the sorption process is highly time-dependent (Hojati and Landi 2015). The maximum sorption was obtained at pH 5 as a consequence of the experiments and equilibrium was attained after 20 min. All subsequent studies in our work were conducted with a time limit of 20 min.
In the case of GRA (Fig. 8), the removal percent increase with increased temperature. At 25, 35, and 55 °C, the R percent of La(III) reaches around 43.3, 46.5, and 49.2 during the first 5 min, respectively, then increase gradually up to 67.6, 70.2, and 72.7 at 25, 35, and 55 °C respectively at equilibrium time (20 min). While in the case of Ce(III), the R% reach about 56.1, 59.5, and 63.2 at 25, 35, and 55 °C, respectively, at the first (5 min) then increases gradually up to 95.5, 96.5, and 98.2 at 25, 35, and 55 °C, respectively, at equilibrium time (20 min).
While in the case of GR, the R% for La increase gradually up to 62.6, 69.2, and 72.7 at 25, 35, and 55 °C respectively at equilibrium time (20 min). While in the case of Ce(III), the R% reach about 75.5, 76.5, and 83.2 at 25, 35, and 55 °C, respectively, at equilibrium time (20 min). It is clear that GRA shows a super separation between La(III) and Ce(III) more than GR. So GRA was chosen to complete the isotherm experiments.
Effect of initial cerium and lanthanum concentration
Adsorption assays with starting ion concentrations ranging from 50 to 250 mg/L 1 were carried out to see how the amount of Ce(III) and La(III) uptake was affected by the initial ion concentration. For each experiment, 0.01 g of GRA adsorbent was used at pH 5≈6, 200 rpm shaking speed, 10 ml solution volume, and shaking time of 20 min at ambient temperature of 25 °C. The uptake percent by the adsorbent decreased (from 98 to 60.6% and from 84 to 34% in the state of La and Ce respectively) as the started concentration go up from 50 to 250 mg/L (Fig. 9). This is for the increased competition of the ions for small available sites (Sert et al. 2008). When all the unoccupied sites in the solution–adsorbent interphase have been used, leaving the adsorbate ions in the solution, Ucun et al. (2003) described the drop in efficiency at higher concentrations to adsorbate saturation in the solution–adsorbent interphase. In the current study, similar interpretations can be given to the reduction in uptake amount as initial cerium and lanthanum concentrations varied from 50 to 250 mg/L.
Kinetic study
Kinetic modeling for La(III) and Ce(III) adsorption on GRA was investigated using three common models: pseudo-first-order, pseudo-second-order, and intraparticle diffusion kinetic equations. The pseudo-first-order Eq. 3 (Lagergren 1898), pseudo-second-order Eq. 4 (Ho and Mckay 1999), and intraparticle diffusion kinetic model Eq. 5 (Weber and Morris 1963) were shown as follows (Mozaffari et al. 2020a, b, c):
The amounts of metal adsorbed per gram of GRA (mg/g) at equilibrium and at any moment are qe and qt, respectively, and k1 = rate, constant of pseudo first order sorption (h−1), and k2 (g/mg hour) are the rate determining step for pseudo 2nd order adsorption. The intraparticle diffusion rate constant (mg (g min1/2)−1) is denoted by kp. C is constantly proportional to the boundary layer thickness.
The kinetic characteristics of La(III) and Ce(III) adsorption onto prepared adsorbents are presented in Table 2 and Fig. 10. As seen from the magnitude of correlation coefficients (R2), for the sorption of La(III) and Ce (III) ions, the pseudo 2nd order exhibited a significant and comparable correlation. The suitability of the proposed kinetic model is confirmed by the comparison of the evaluated adsorption capacity considering the pseudo-second-order equation (qe) and that found experimentally (qexp). It proposes that the rate-determining step might be a chemical process and the adsorption involves the valance forces throughout sharing of electrons between the La(III), Ce(III) ions, and prepared GRA (Gad and Youssef 2018). The nonlinear relationship between the plotted data of the intra-particle diffusion model and the computed value of “C” (C ≠ 0) implies that this model does not play a significant role in the adsorption of La(III) and Ce(III) ions, and so is not valid for adsorption kinetics.
Adsorption isotherms
Isotherm is a crucial criterion because they define how the adsorbate interacts with the adsorbent. Three linear isotherm models were studied to describe the experimental data of La(III) and Ce(III) adsorption on GRA, namely Langmuir types I, II, III, and IV (Eqs. 6, 7, 8, and 9 respectively) (Hamzaoui et al. 2018), Freundlich (Eq. 9) (Moradi et al. 2009), and Dubinin–Radushkevich (D–R) (Robati et al. 2016) (Eq. 10).
where Ce (mg/L) and qe (mg/g) denote the leftover concentrations of metals in solution and, correspondingly, the quantity of metal immobilized on the adsorbent at equilibrium. The maximum number of cations per unit mass of bio-adsorbent is qmax (mg/g), and b is the equilibrium constant related to the adsorbent-metal ion affinity. Kf denotes the adsorbent’s sorption capacity, with n indicating the sorption process’s favorability. n represents the sorption strength (mg L−1). (a) If 1/n = 1, the adsorption operation is linear and the distribution of metal (M+) between the two phases is unaffected by their concentration. (b) If 1/n < 1, the process is chemical, and (c) if 1/n > 1, the adsorption operation is physical. The 1/n number approaches zero as the adsorbent turned to be more diverse (Torab-Mostaedi et al. 2011). If n lies between 1 and 0, this indicates a favorable sorption process (Goldberg 2005). β is the Polanyi potential, the average adsorption energy per mole of the adsorbate as it is moved from an infinite to the solid phase.
The quantity of mean sorption energy E (kJ/mol) can be determined from the D–R parameter β (Eq. 13), where T is the solution temperature (Kel.) and R is the gas constant, which is equal to 8.314 J/mol:
The mean sorption energy value provides data on chemical and physical sorption. E magnitude can be connected to the reaction mechanism. When E is between 8 and 16 kJ/mol, ion exchange regulates sorption. Physical forces may alter the sorption mechanism when E is less than 8.0 kJ/mol. Chemisorptions, on the other hand, governs the sorption process when E > 16 kJ/mol (Hamed et al. 2016; Attallah et al. 2019a, b). The Langmuir adsorption isotherm model was tested using RL to see if the sorption operation was favorable or unfavorable.
where C0 (mg/L) and b (L/mg) are initial metal concentration and Langmuir constant, respectively. The RL cleared the shape of the isotherm to be unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), or irreversible (RL = 0). If RL from 0 to 1, this cleared favorable adsorption.
The data is given in Table 3 and Fig. 11. The experimental data for the sorption of La(III) and Ce(III) on GRA can be suited to the Langmuir model (especially the type I model), which assumes that the binding of the adsorbate onto the adsorbent occurs primarily through a chemical reaction and all sites have equal affinity for the metal, based on the calculated correlation coefficients (R2) for the D–R, Langmuir, and Freundlich isotherms.
According to Langmuir model (type 1), the GRA has great affinity and selectivity for Ce3+ (qm = 200 mg/g at all temperature range) more than La3+ (qm = 83.3, 100, 111.1 mg/g at 25, 35, and 55 °C respectively).
As shown in Table 3, the value of RL was found to be 0.08, 0.01, and 0.14 at 25, 35, and 55 °C respectively for La(III) and 0.09, 0.08, and 0.06 at 25, 35, and 55 °C respectively for Ce(III). The calculated RL values indicated that the adsorption of M+ on GRA was favorable.
According to the D–R model, the energy value (E) in the case of La(III) adsorption was found to be 12.9 kJ mol−1 at 25 °C and increases gradually with increasing temperature, and this gives an indication that at low temperature, the reaction mechanism was carried out via ion exchange reaction of metal ions with divalent calcium and monovalent sodium ions including in the crystal lattice of GRA, while the E reach to about 22.4 kJ/mol at high temperature 55 °C may be due to the low qm value 1.98 mg/g comparing with a huge amount of qm at 51.5 mg/g at 25 °C.
In the state of Ce(III), E was constant values (22.4 kJ/mol) at all temperature range from 25 to 55 °C which give an indication for chemical reaction via complexation or chelating with different functions groups (COO−, OH−) of alginate on the graphite surface. The energy (E) differential between La(III) (cation exchange reaction) and Ce(III) (chemical reaction) could explain why Ce(III) has a higher R percent than La(III) (III). According to the Freundlich isotherm model, the value of 1/n ˂ 1 which approves the adsorption process is chemically controlled.
A comparison of the qmax values obtained for the La(III) and Ce(III) adsorption on GRA with those data collected in the literature about the adsorption of La and Ce on different inorganic materials is shown in Table 4.
Thermodynamic study
Numerous thermodynamic parameters for the present systems were calculated to understand the thermodynamic nature of the sorption process. The temperature-dependent enthalpy (ΔH°), entropy (ΔS°), and free energy (ΔG°) can all be calculated. The Van’t Hoff Eq. (15) allows the analysis of data by plotting ln Kd versus 1/T (Rajabi et al. 2019):
where Kd is the sorption equilibrium constant that can be calculated using Eq. (16).
Calculating enthalpy (ΔH°) and entropy (ΔS°) is made easier with this analysis. Equation (17) cleared how to determine (ΔG°) in the following way:
The negative (ΔG°) value shows that adsorption occurs spontaneously, and ΔG° decreases as temperature rises. As shown in Table 4, indicated that the adsorption of La(III) and Ce (III) onto GRA is more favorable at a higher temperature. This is for a rise in the numeral of active surface sites available for metal ions, as well as a shortage in the thickness of the boundary layer around the adsorbent, both of which will speed up the mass transfer process (Ma et al. 2010).
The enthalpy change (ΔH°) is a measurement of the energy barrier that the metal ion must exceed during adsorption onto an adsorbent. As shown in Table 5, the positive values of ΔH° (259.4 and 643.2 KJmol−1 for Ce(III) and La(III) respectively) give an indication of the endothermic nature of the Eu(III) adsorption process onto GRA (Dolatyari et al. 2016).
The positive standard entropy change ΔS° (kJ/mol K) may be due to the release of water molecules produced by the ion-exchange reaction between the metal ions and the surface functional groups of GRA, reflects the affinity of the GRA surfaces for the Ce(III) (which has a large entropy value 112.2 kJ/mol K) than La(III) (2.78 kJ/mol K), and this leads to the increase of randomness at the solid/liquid interface during the adsorption process (Moradi and Zare 2011). Also, Ce(III) has a small ionic radius than La(II) so the diffusion of Ce(III) is higher than La(III) and so the degree of disorder of Ce(III) is higher than La(III).
Reaction mechanism
In this study, there are two types of the mechanism included, first preparation of GRA adsorbent and the other includes the selectivity of the adsorbent for Ce(III) more than La(III).
Sodium alginate as a natural anionic polysaccharide mainly possesses abundant free carboxyl groups (-COOH) and hydroxyl (-OH) groups. The carboxyl groups can conjugate with multivalent metal ions, forming egg-box structures (Scheme 1), which have gotten a lot of attention. Firstly, sodium alginate was cross-linked with CaCl2 for bead formation and for La(III) and Ce(III) removal from aqueous solution in different conditions. Moradi and Zare reported that the existence of a functional group (COOH) on this adsorbent causes a rise in the negative charge on the carbon surface. The oxygen atoms in the functional groups donate single pair of electrons to metal ions and consequently increase their cation exchange capacity. It can be related also that the carboxylic group can form a complex with the metal ion, leading to an increase in the amount of ion adsorption (Moradi and Zare 2011; Moradi et al. 2010; Moradi 2013).
The graphite material has a 3D layered structure of graphene sheets stacked together by weak (van der Waals) forces after the inclusion of Ca-alginate beads. Graphite has excellent chemical, thermal, and mechanical properties. So, graphite is appropriate material for enhancing the stability of hydrogel composites (alginate) (Verma et al. 2020), the alginate bead was encapsulated with graphite through electrostatic interaction or covalent bond, and GRA adsorbent was formed.
The prepared adsorbent graphite/alginate (GRA) has a great affinity for cerium (98%) more than lanthanum (68%) (although both metal ions have approximately the same characteristics of the lanthanides group) at the optimum condition of temperature (25 °C) and pH = 6. This may be related to several reasons:
-
1-
Electron negativity: metals with high electron negativity display a strong affinity for the electron clouds of oxygen atoms in COO− and OH− group at the surface of graphite/alginate adsorbent (Fan et al. 2021). According to Pauling, the electron negativity of Ce(III) (1.12) ˃ La(III) (1.10). Also, electron negativity is significant in deciding if a link is ionic or covalent and if it is polarized or non-polarized. The distinction between these two bound atoms in electronegativity. When two M+ with a difference in electronegativity ˃ 2 units are bound together, they form an ionic connection. The much more electron-withdrawing element has a negative surface charge, while the weaker electron-withdrawing element has a positive electrical charge. The oxygen of COO− in alginate adsorbent has electronegativity = 3.44. So the bond formation is ionic in nature because the difference between electron negativity between oxygen and Ce (O-Ce) and La (O-La) is 2.32 and 2.30 respectively.
-
2-
Hydration energy (HE): the lower the (HE) the easier for the metal ion to dehydrate, because dehydration depends on (HE) of the metal ion, CeIII (− 3340 kJ/mol) ˂ LaIII (− 3285 kJ/mol) (Moldoveanu and Papangelakis 2013). Accordingly, Ce(III) may lose all its hydration water to diffuse through the graphite/alginate surface more easily than La(IIII). So La(III) may undergo a partial ion exchange reaction or chemical complexation reaction but at the same time, GRA displayed more adsorption capacity for Ce(III) (200 mg/g) than La(III) (83.3 mg/g) at optimum conditions. The calculated enthalpy from Table 4 has the same trend of Ce (250.4 kJ/mol) ˂ La(III) (643.2 kJ/mol). The same behavior was observed for Pd, Cd, Cu, and Zn adsorption on the synthetic NaX zeolite (Fan et al. 2021).
-
3-
Hydrated radius: the elimination percent increases as the hydrated radius decreases (Fanou et al. 2007). La(III) has reduced mobility in aqueous solution and penetration through GRA adsorbent because of its greater hydration radius (4.52 A°). Ce(III), on the other hand, has higher mobility and permeability due to its lower hydration radius (1.85 A°) (Kennedy and Tezel 2018).
Effect of interfering ions
The effect of competing ions such as Na(I), Ca(II), and Fe(III) on the uptake of La(III) and Ce(III) ions on GRA material was studied as demonstrated in Fig. 12. A mixture of the counterions and metal under investigation La(III) or Ce(III) was prepared. Constant concentration La or Ce (100 ppm) and different concentrations of interfering ions mixture (10, 20, and 30 ppm) were added to 0.01 g GRA at optimum conditions of temp. and pH. It is clear that the percent removal of La(III) and Ce(III) decreased with an increased concentration of competing ions. The significant negative effect of interfering ion (Na, Ca, and Fe) concentrations may be due to either the competition of electrostatic interaction of these ions with the metal under investigation in this study (Kotp 2008) or it may be related to the stereochemical factor. It could possibly be due to Na1+ (0.95°A), Ca2+ (1.00°A), and Fe3+ (0.55°A) that have smaller ionic radius than La (1.15°A) and Ce (1.01°A). It is reflected that the smallest ionic radius implies more molecules could be adsorbed onto the pores of GRA material.
Conclusion
Graphite was prepared and its surface was chemically modified using alginate to prepare GRA adsorbent. The prepared adsorbent was used for selective separation and removal of La(III) and Ce(III) from an aqueous solution. The adsorption isotherms were well described by the Langmuir and the maximum adsorption capacity reaches 200 and 83.3 mg/g for Ce(III) and La(III), respectively.
The difference between two cations in the affinity towards GRA Adsorbent is strongly related to electron negativity, hydration energy, and hydration radius. The uptake of both Ce(III) and La(III) onto the prepared GRA followed the pseudo second order kinetic model. Ce(III) and La(III) adsorption on the produced GRA followed a pseudo second order model. The sorption operation was spontaneous and endothermic, and they became more active as the temperature rose. This research reveals that GRA can be utilized as an adsorbent to remove lanthanides from a liquid phase.
Novelty statement
Radioactive waste is radionuclides produced from uranium fission and can be easily discharged into the environment during an accident at a nuclear reactor, such as those that occurred at Chernobyl in 1986, at Three Mile Island in Pennsylvania in 1979, and in 2011 at Fukushima, Japan. In 2009, also discharging for radioactive waste elements such as cesium radionuclide occurred during minor accidents at nuclear power stations in Britain and Germany as well as the USA. So, there have been attempts in developing efficient adsorbents for the removal of the radioactive waste elements from aqueous environments and immobilizing them for safe disposal.
The removal of waste elements (cerium (III) and lanthanum (III)) from the waste stream using synthesized graphite as an inorganic exchanger has never been reported in the literature. Therefore, this study aims to synthesize modified graphite and estimate its potential application in the removal of cerium (III) and lanthanum (III) from the waste stream. The adsorbent was synthesized by thermal annealing technique from easily obtainable and inexpensive raw materials.
The synthesized modified graphite was successfully applied to the removal of cerium (III) and lanthanum (III) from different samples. The modified graphite is shown capacity equal 200 and 83.3 mg/g for Ce(III) and La(III) at 25 °C respectively, from waste water samples, indicating that modified could have wide application prospects in adsorption Ce(III) and La(III) from environmental and waste water samples.
Data availability
All authors sure that all data and materials as well as software application or custom code support the published claims and comply with field standards.
Change history
27 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11356-022-21676-y
References
Abdel-Maksoud MIA, Sami NM, Hassan HS, Bekhit M, Ashour AH (2022) Novel adsorbent based on carbon-modified zirconia/spinel ferrite nanostructures: evaluation for the removal of cobalt and europium radionuclides from aqueous solutions. J Colloid Interface Sci 607:111–124
Ahmadi SAR, Kalaee MR, Moradi O, Nosratinia F, Abdouss M (2021) Core–shell activated carbon-ZIF-8 nanomaterials for the removal of tetracycline from polluted aqueous solution. Adv Compos Hybrid Mater 4(6):1384–1397. https://doi.org/10.1007/s42114-021-00357-3
Ahmadi SAR, Kalaee MR, Moradi O, Nosratinia F, Abdouss M (2022) Synthesis of novel zeolitic imidazolate framework (ZIF-67) – zinc oxide (ZnO) nanocomposite (ZnO@ZIF-67) and potential adsorption of pharmaceutical (tetracycline (TCC)) from water. J Mol Struct 1251:132013. https://doi.org/10.1016/j.molstruc.2021.132013
Alguacil FJ, García-Díaz I, Baquero EE, Largo OR, Lopez FA (2020) Oxidized and non-oxidized multiwalled carbon nanotubes as materials for adsorption of lanthanum(III) aqueous solutions. Metals 10:765
Allahyari SA, Saberi R, Sepanloo K, Lashkari A (2021) Adsorptive separation of La(III) from aqueous solution via the synthesized [Zn(bim)2(bdc)]n metal-organic framework. J Rare Earths 39(6):742–748. https://doi.org/10.1016/j.jre.2020.07.027
Allam EA, El-Sharkawy RM, Gizawy MA, Mahmoud ME (2021) Assembly of CeO2–MoO3–SiO2 (CH2)3-(alginate)2 as a novel nanocomposite for removal of MnII/CrVI and 56Mn/51Cr radionuclides from water. Mater Chem Phys 26:124–278
Anisha GS, Prema P (2008) Cell immobilization technique for the enhanced production of α-galactosidase by Streptomyces griseoloalbus. Bioresource Technol 99(9):3325–3330
Attallah MF, Hassan HS, Youssef MA (2019a) Synthesis and sorption potential study of Al2O3-ZrO2-CeO2 composite material for removal of some radionuclides from radioactive waste effluent. Appl Rad Isotope 147:40–47
Attallah MF, Youssef MA, Imam DM (2019b) Preparation of novel nano composite materials from biomass waste and their sorptive characteristics for certain radionuclides. Radiochim Acta 108(2):137–149. https://doi.org/10.1515/ract-2019-3108
Babaei M, Azar PA, Tehrani MS, Farjaminezhad M, Hussain SW (2021) Green and simple synthesized graphene/MnO2 quantum dot nanocomposite: characterization and application as an efficient adsorbent for solid-phase extraction of heavy metals. J Nanostruct Chem 12:249–261. https://doi.org/10.1007/s40097-021-00410-z
Babudurai M, Nwakanma O, Romero-Nuñez A, Manisekaran R, Subramaniam V, Castaneda H, Jantrania A (2021) Mechanical activation of TiO2/Fe2O3 nanocomposite for arsenic adsorption: effect of ball-to-powder ratio and milling time. J Nanostruct Chem 11:619–632. https://doi.org/10.1007/s40097-021-00388-8
Bonal C, Morel JP, Morel-Desrosiers N (1998) Interactions between lanthanide cations and nitrate anions in water part 2 microcalorimetric determination of the Gibbs energies, enthalpies and entropies of complexation of Y3+ and trivalent lanthanide cations. J Chem Soc Faraday Trans 94(10):1431–1436
Bouyer F, Sanson N, Destaracb M, Gérardin C (2006) Hydrophilic block copolymer-directed growth of lanthanum hydroxidenanoparticles. New J Chem 30(3):399–408
Cajnko MM, Novak U, Likozar B (2019) Cascade valorization process of brown algaseaweed Laminariahyperborea by isolation of polyphenols and alginate. J Appl Phycol 31:3915–3924
Ceren K, Senol S, Berkan C, Ezgi Y, Acar BM (2012) Biosorption of Ce(III) onto modified Pinus brutialeaf powder using central composite design. Wood Sci Technol 07(46):4
Chegouche S, Mellaha A, Telmoune S (1997) Removal of lanthanum from aqueous solution by natural bentonite. Wat Res 31(7):1733–1737
Cho D-W, Wung W, Sigdel A, Kwon O-H, Lee S-H, Kabra AN, Jeon B-H (2013) Adsorption of Pb(II) and Ni(II) from aqueous solution by nanosized graphite carbon-impregnated calcium alginate bead. Geosyst Eng 16(3):200–208. https://doi.org/10.1080/12269328.2013.832402
De Rossi A, Rigueto CVT, Dettmer A, Colla LM, Piccin JS (2020) Synthesis, characterization, and application of Saccharomyces cerevisiae/ alginate composites beads for adsorption of heavy metals. J Environ Chem Eng 8(4):104009
Diniz V, Volesky B (2005) Effect of counter ions on lanthanum biosorption by Sargassum polycystum. Water Res 39:2229–2236
Dolatyari L, Yaftian MR, Rostamnia S (2016) Adsorption characteristics of Eu(III) and Th(IV) ions onto modified mesoporous silica SBA-15 materials, J Taiwan Inst Chem. Eng 60:174–184
El-Din AMS, Sayed MA, Monir TM, Sami NM, Aly AM (2021) Sponge-like Ca-alginate/Lix-84 beads for selective separation of Mo (VI) from some rare earth elements. Int J Biol Macromol 184:689–700
Fan X, Liu H, Anang E, D. Ren D, (2021) Effects of electronegativity and hydration energy on the selective adsorption of heavy metal ions by synthetic NaX zeolite. Materials 14(15):4066
Fanou D, Yao B, Siaka S, Ado G (2007) Heavy metals removal in aqueous solution by two delta-diketones. J Appl Sci 7:310–313
Gad HMH, Youssef MA (2018) Sorption behavior of Eu(III) from an aqueous solution onto modified hydroxyapatite: kinetics, modeling and thermodynamics. Environ Technol 39:2583–2596
Goldberg S (2005) Equations and models describing adsorption processes in soils. Chem Proc Soils 8:489–517
Gupta CK, Krishnamurthy N (1992) Extractive metallurgy of rare earths. Int Mater Rev 37(5):197–248
Gupta VK, Moradi O, Tyagi I, Agarwal S, Sadegh H, Shahryari-Ghoshekandi R, Garshasbi A (2015) Study on the removal of heavy metal ions from industry waste by carbon nanotubes: effect of the surface modification: a review. Crit Rev Environ Sci Technol 46(2):93–118. https://doi.org/10.1080/10643389.2015.1061874
Hamed MM, Holiel M, Ismail ZH (2016) Removal of 134Cs and 152+154Eu from liquid radioactive waste using Dowex HCR-S/S, Radiochim. Acta 104:399–413
Hamzaoui M, Bestani B, Benderdouche N (2018) The use of linear and nonlinear methods for adsorption isotherm optimization of basic green 4-dye onto sawdust-based activated carbon. J Mater Environ Sci 9(4):1110–1118
Hassan HS, Elmaghraby EK (2012) Preparation of graphite by thermal annealing of polyacrylamide precursor for adsorption of Cs(I) and Co(II) ions from aqueous solutions. Can J Chem 90(10):843–850
Hassan HS, Elmaghraby EK (2019) Retention behavior of cesium radioisotope on poly (acrylamido-sulfonicacid) synthesized by chain polymerization. Appl Rad Isotope 146:40–47
Hassan HS, El-Kamash AM, Ibrahim HA (2019) Evaluation of hydroxyapatite/poly(acrylamide-acrylic acid) for sorptive removal of strontium ions from aqueous solution. Environ Sci Pollut Res 26:25641–25655
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Hojati S, Landi A (2015) Removal of zinc from a metal plating wastewater using an Iranian sepiolite: determination of optimum conditions. Desalin Water Treat 53(8):2117–2124
Huixue R, Zhimin G, Daoji W, Jiahui J, Youmin S, Congwei L (2016) Efficient Pb(II)removal using sodium alginate–carboxymethyl cellulose gel beads: preparation, characterization, and adsorption mechanism. Carbohydr Polym 137:402–409
Hussain T, Iqbal M (2011) Pyrolysis of methane by catalytic properties exhibited by ceramics. J Anal Appl Pyrolysis 90(2):106–111
Iuima S (1991) Helical microtubules of graphic carbon. Nature 354(7):56–58
Junior AB, Pinheiro EF, Romano Espinosa DC, Soares Tenório JA, Galluzzi Baltazar MDP (2021) Adsorption of lanthanum and cerium on chelating ion exchange resins: kinetic and thermodynamic studies, Separ Sci Technol 1–10.https://doi.org/10.1080/01496395.2021.1884720
Kazemi J, Javambakht V (2019) Alginate beads impregnated with magnetic Chitosan@Zeolite nanocomposite for cationic methylene blue dye removal from aqueous solution. Int J Biol Macromol 154:1426–1437
Kennedy DA, Tezel FH (2018) Cation exchange modification of clinoptilolite –screening analysis for potential equilibrium and kinetic adsorption separations involving methane, nitrogen, and carbon dioxide. Microporous Mesoporous Mater 262:235–250
Kim JA, Dodbiba G, Tanimura Y, Mitsuhashi K, Fukuda N, Okaya K, Matsuo S, Fujita T (2011) Leaching of rare-earth elementsand their adsorption by using blue-green algae. Mater Trans 52(9):1799–1806
Kotp YH (2008) Removal of some heavy elements using new synthesized inorganic ion exchange materials [M. Sc. thesis], Chem. Depart., Faculty of Science, Al-Azhar University (Girls)
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Lankapati HM, Dankhara PM, Lathiya DR, Shah B, Chudasama UV, Choudhary L, Maheria KC (2021) Removal of lanthanum, cerium and thorium metal ions from aqueous solution using ZrT hybrid ion exchanger. Sustain Energy Technol Assess 47:101–415
Lee M-S, Lee J-Y, Kim J-S, Lee G-S (2005) Solvent extraction of neodymium ions from hydrochloric acid solution using PC88Aand saponified PC88A. Sep Purif Technol 46(1–2):72–78
Li XZ, Sun YP (2005) Progress in solid–liquid extraction resin for separation of rare earth elements. J Rare Earths 23:581–592
Ma B, Shin WS, Oh S, Park YJ, Choi SJ (2010) Adsorptive removal of Co and Sr ions from aqueous solution by synthetic hydroxyapatite nanoparticles. Sep Sci Technol 45(4):453–462
Mahmood Z, Amin A, Zafar U, Raza MA, Hafeez I, Akram A (2017) Adsorption studies of cadmium ions on alginate–calcium carbonate composite beads. Appl Water Sci 7(2):915–921
Malinga NN, Jarvis ALL (2020) Synthesis, characterization and magnetic properties of Ni, Co and FeCo nanoparticles on reduced graphene oxide for removal of Cr(VI). J Nanostruct Chem 10:55–68. https://doi.org/10.1007/s40097-019-00328-7
Marczenko Z, Freiser H (1981) Spectrophotometric determination of trace elements. CRC Crit Rev Anal Chem 11:3. https://doi.org/10.1080/10408348108085509
Mirzaeian MM, Eskandari L, Rashidi AM (2019) Synthesis of graphene oxide-supported meso-tetrakis (4-carboxyphenyl) porphyrinatoiron (III) chloride as a heterogeneous nanocatalyst for the mercaptan removal from the gas stream. J Nanostruct Chem 9:19–28. https://doi.org/10.1007/s40097-019-0294-9
Moldoveanu GA, Papangelakis VG (2013) Leaching of lanthanides from various weathered elution deposited ores. Can Metall Q 52(3):257–264
Moradi O (2013) Adsorption behavior of basic red 46 by single-walled carbon nanotubes surfaces. Fullerenes, Nanotubes, Carbon Nanostruct 21(4):286–301. https://doi.org/10.1080/1536383X.2011.572317
Moradi O, Sharma G (2021) Emerging novel polymeric adsorbents for removing dyes from wastewater: a comprehensive review and comparison with other adsorbents. Environ Res 201:111534. https://doi.org/10.1016/j.envres.2021.111534
Moradi O, Zare K (2011) Adsorption of Pb(II), Cd(II) and Cu(II) ions in aqueous solution on SWCNTs and SWCNT –COOH surfaces: kinetics studies. Fullerenes, Nanotubes Carbon Nanostruct 19(7):628–652. https://doi.org/10.1080/1536383X.2010.504955
Moradi O, Aghaie M, Zare K, Monajjemi M, Aghaie H (2009) The study of adsorption characteristics Cu2+ and Pb2+ ions onto PHEMA and P(MMA-HEMA) surfaces from aqueous single solution. J Hazard Mater 170(2–3):673–679. https://doi.org/10.1016/j.jhazmat.2009.05.01
Moradi O, Zare K, Monajjemi M, Yari M, Aghaie H (2010) The studies of equilibrium and thermodynamic adsorption of Pb(II), Cd(II) and Cu(II) ions from aqueous solution onto SWCNTs and SWCNT–COOH surfaces. Fullerenes, Nanotubes, Carbon Nanostruct 18(3):285–302. https://doi.org/10.1080/15363831003783005
Moradi O, Madanpisheh MA, Moghaddas M (2021) Synthesis of GO/HEMA, GO/HEMA/TiO2, and GO/Fe3O4/HEMA as novel nanocomposites and their dye removal ability. Adv Compos Hybrid Mater 4:1185–1204. https://doi.org/10.1007/s42114-021-00353-7
Mozaffari N, Solaymani S, Achour A, Kulesza S, Bramowicz M, Nezafat NB, Ţălu S, Mozaffari N, Rezaee S (2020b) New insights into SnO2/Al2O3, Ni/Al2O3, and SnO2/Ni/Al2O3 composite films for CO adsorption: building a bridge between microstructures and adsorption properties. J Phys Chem C 124(6):3692–3701
Mozaffari N, Haji SMA, Sari AH, Fekri AL (2020c) Investigation of carbon monoxide gas adsorption on the Al2O3/Pd(NO3)2/zeolite composite film. J Theor Appl Phys 14:65–74. https://doi.org/10.1007/s40094-019-00360-6
Mozaffari N, Mozaffari N, Elahi SM, Vambol S, Vambol V, Khan NA, Khan N (2020a) Kinetics study of CO molecules adsorption on Al2O3/zeolite composite films prepared by roll-coating method. Surf Eng J 1–10.https://doi.org/10.1080/02670844.2020.1768628
Mustapha S, Shuaib DT, Ndamitso MM, Etsuyankpa MB, Sumaila A, Mohammed UM, Nasirudeen MB (2019) Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions using Albizia lebbeck pods. Appl Water Sci 9(6):1–11
Naeini AH, Kalaee MR, Moradi O, Khajavi R, Abdouss M (2022) Synthesis, characterization and application of carboxylmethyl cellulose, guar gam, and graphene oxide as novel composite adsorbents for removal of malachite green from aqueous solution. Adv Compos Hybrid Mater 5:335–349. https://doi.org/10.1007/s42114-021-00388-w
Ngah WSW, Hanafiah MAKA (2008) Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour Technol 99(10):3935–3948
Olatunji MA, Khandaker MU, Amin YM, Mahmud HNME (2016) Cadmium–109 radioisotope adsorption onto polypyrrole coated sawdust of dryobalanops aromatic: kinetics and adsorption isotherms modeling. PLoS ONE 11(10):164–119
Olatunji MA, Khandaker MU, Mahmud HNME (2018) Investigation of cerium-139 radioisotope adsorption by conducting polymer composite. Polym Bull 75:2491–2509
Pattanayak J, Mondal K, Mathew S, Lalvani S (2000) A parametric evaluation of the removal of As(V) and As(III) by carbon-based adsorbents. Carbon 38(4):589–596. https://doi.org/10.1016/S0008-6223(99)00144-X
Pelekani C, Snoeyink V (1999) Competitive adsorption in natural water: role of activated carbon pore size. Water Res 33(5):1209–1219. https://doi.org/10.1016/S0043-1354(98)00329-7
Rajabi M, Mahanpoor K, Moradi O (2017) Removal of dye molecules from aqueous solution by carbon nanotubes and carbon nanotube functional groups: critical review. RSC Adv 7(74):47083–47090. https://doi.org/10.1039/c7ra09377b
Rajabi M, Mahanpoor K, Moradi O (2019) Preparation of PMMA/GO and PMMA/GO-Fe3O4 nanocomposites for malachite green dye adsorption: kinetic and thermodynamic studies. Compos Part B: Eng 167:544–555, S1359836818345347–. https://doi.org/10.1016/j.compositesb.2019.03.030
Rajendran K, Shanmugasundaram M, Arulanandhu DM, Gopu G, Kalaignan GP, Jeon BH, Subbaiah MP (2021) Oxalic acid-induced assembly of CoxNi1−x-bimetallic polyaniline nanocomposite: a bifunctional material for supercapacitor and chromium removal applications. J Nanostruct Chem. https://doi.org/10.1007/s40097-021-00425-6
Robati D, Mirza B, Rajabi M, Moradi O, Tyagi I, Agarwal S, Gupta VK (2016) Removal of hazardous dyes-BR 12 and methyl orange using graphene oxide as an adsorbent from aqueous phase. Chem Eng J 284:687–697. https://doi.org/10.1016/j.cej.2015.08.131
Sert S, Kutahyali C, Inan S, Talip Z, Cetinkaya B, Eral M (2008) Biosorption of lanthanum and cerium from aqueous solutions by platanus orientalis leaf powder. Hydrometallurgy 90:13–18
Sheshdeh RK, Abbasizadeh S, Nikou MRK, Badii K, Sharafi MS (2014) Liquid phase adsorption kinetics and equilibrium of toluene by novel modified-diatomite. J Environ Health Sci Eng 12(1):148
Thakur S, Pandey S, Arotiba OA (2016) Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr Polym 153:34–46
Torab-Mostaedi M (2013) Biosorption of lanthanum and cerium from aqueous solutions using tangerine (Citrus reticulata) peel: equilibrium, kinetic, and thermodynamic studies. Chem Ind Chem Eng Q 19(1):79–88
Torab-Mostaedi M, Ghaemi A, Ghassabzadeh H, Ghannadi-Maragheh M (2011) Removal of strontium and barium from aqeous solutions by adsorption onto expanded perlite. Can J Chem Eng 89(5):1247–1254
Ucun H, Bayhana YK, Kaya Y, Cakici A, Algur OF (2003) Biosorption of lead(II) from aqueous solution by cone biomass of pinus sylvestris. Desalination 154(3):233–238
Vergas SJ, Schaeffer N, Souza JC, da Silva LH, Hespanhol MC (2021) Green separation of lanthanum, cerium and nickel from waste nickel metal hydride battery. Waste Manage 125:154–162
Verma A, Thakur S, Mamba G, Prateek GRK, Thakur P, Thakur VK (2020) Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int J Biol Macromol 148:1130–1139
Vijayaraghavan K, Sathishkumar M, Balasubramanian R (2010) Biosorption of lanthanum, cerium, europium, and ytterbium by a brown marine alga, Turbinaria Conoides Ind. Eng Chem Res 49:4405–4411
Volokh AA, Gorbunov AV, Gundorina SF, Revich BA, Frontasyeva MV, Pal CS (1992) Phosphorus-fertilizer production as a source of rare-earth elements pollution of the environment. Sci Total Environ 95:141–148
Wang F, Zhao J, Li W, Zhou H, Yang X, Sui N, Liu H (2013) Preparation of several alginate matrix gel beads and their adsorption properties towards rare earths (III). Waste Biomass Valoriz 4(3):665–674
Weber WJ, Morris JC (1963) Kinetics of adsorption of carbon from solutions. J Sanit Eng Div 89:31–59
Wu D, Zhao J, Zhang L, Wu Q, Yang Y (2010) Lanthanum adsorption using iron oxide loaded calcium alginate beads. Hydrometallurgy 101:76–83
Wu Y, Pang H, Liu Y, Wang X, Yu S, Fu D, Wang X (2019) Environmental remediation of heavy metal ions by novel-nanomaterials: a review. Environ Pollut 246:608–620
Xiong Y, Chen C, Gu X, Biswas BK, Shan W, Lou Z, Fang D, Zang S (2011) Investigation on the removal of Mo(VI) from Mo–Re containing wastewater by chemically modified persimmon residua. Bioresour Technol 102(13):6857–6862
You Y, Qu K, Huang Z, Ma R, Shi C, Li X, Liu D, Dong M, Guo Z (2019) Sodium alginate templated hydroxyapatite/calcium silicate composite adsorbents for efficient dye removal from polluted water. Int J Biol Macromol 141:1035–1043
Zhang W, Li H, Kan X, Dong L, Yan H, Jiang Z, Yang H, Li A, Cheng R (2012) Adsorption of anionic dyes from aqueous solutions using chemically modified straw. Bioresour Technol 117:40–47
Zhang W, Zhang D, Liang Y (2019) Nanotechnology in remediation of water contaminated by poly-and perfluoroalkyl substances: a review. Environ Pollut 247:266–276
Zhu Y, Zheng Y, Wang A (2015) Preparation of granular hydrogel composite by the redox couple for efficient and fast adsorption of La (III) and Ce (III). J Environ Chem Eng 3(2):1416–1425
Zj S, H. Xi H, Y. F, Wf Z, Xz, Z Cs Z (2018) Engineering sodium alginate-based cross-linked beads with high removal ability of toxic metal ions and cationic dyes. Carbohydr Polym 187:85–93
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
NMS (second author) was responsible for the measurement of the kinetic sorption study and the analysis of its data. These measurements and its analyses were conducted in Hot Laboratories and Waste Management Center, Atomic Energy Authority, Cairo, Egypt. HSH (third author) contributed to the preparation and synthesis of the modified graphite material and the written part of experimental data. He works for Hot Laboratories and Waste Management Center, Atomic Energy Authority, Cairo, Egypt. MAY (first author) contributed to the written the characterizations made for the modified graphite material and part of experimental work. This work was conducted in the Hot Laboratories and Waste Management Center, Atomic Energy Authority, Cairo, Egypt.
Corresponding author
Ethics declarations
Ethics approval
The submitted manuscript is original and have not been published elsewhere in any form or language.
Consent to participate
Done.
Consent to publish
All authors agreed with the content and that all gave explicit consent to submit and we obtained consent from the responsible authorities (Egyptian atomic energy authority where the work has been carried out, before the work is submitted.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The correct institution name of the affiliation is revised in the original published proof.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Youssef, M.A., Sami, N.M. & Hassan, H.S. Extraction and separation feasibility of cerium (III) and lanthanum (III) from aqueous solution using modified graphite adsorbent. Environ Sci Pollut Res 29, 79649–79666 (2022). https://doi.org/10.1007/s11356-022-20823-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20823-9