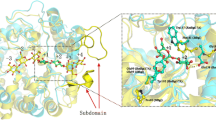
Abstract
N-glycosylation alters the properties of different enzymes in different ways. Rhizopus homothallicus was first described as an environmental isolate from desert soil in Guatemala. A new gene encoding glucanase RhGlu16B was identified in R. homothallicus. It had high specific activity (9673 U/mg) when barley glucan was used as a substrate, and β-glucan is hemicellulose that is abundant in nature. RhGlu16B has only one N-glycosylation site in its Ala55-Gly64 loop. It was found that N-glycosylation increased its Tm value and catalytic efficiency by 5.1 °C and 59%, respectively. Adding N-glycosylation to the same region of GH16 family glucanases TlGlu16A (from Talaromyces leycettanus) increased its thermostability and catalytic efficiency by 6.4 °C and 38%, respectively. In a verification experiment using GH16 family glucanases BisGlu16B (from Bisporus) in which N-glycosylation was removed, N-glycosylation also appeared to promote thermostability and catalytic efficiency. N-glycosylation reduced the overall root mean square deviation of the enzyme structure, creating rigidity and increasing overall thermostability. This study provided a reference for the molecular modification of GH16 family glucanases and guided the utilization of β-glucan in hemicellulose.
Graphical abstract







Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article. The raw data retrieved during the current study are available from the corresponding author on reasonable request.
References
Altschul SF, Madden TL, Schffer AA, Zhang J, Zhang Z, Webb M, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389. https://doi.org/10.1093/nar/25.17.3389
Bernardi RC, Cann I, Schulten K (2014) Molecular dynamics study of enhanced Man5B enzymatic activity. Biotechnol Biofuels 7(1):1–9. https://doi.org/10.1186/1754-6834-7-83
Chang S, Saldivar RK, Liang P, Hsieh, (2021) Structures, biosynthesis, and physiological functions of (1,3;1,4)-β-d-glucans. Cells 10(3):510. https://doi.org/10.3390/cells10030510
Chen X, Meng K, Shi P, Bai Y, Luo H, Huang H, Yuan T, Yang P, Yao B (2012) High-level expression of a novel Penicillium endo-1,3(4)-β-D-glucanase with high specific activity in Pichia pastoris. J Ind Microbiol Biot 39(6):869–876. https://doi.org/10.1007/s10295-012-1087-z
Chen J, Yu H, Liu C, Liu J, Shen Z (2013) Improving stability of nitrile hydratase by bridging the saltbridges in specific thermal-sensitive regions. J Biotech 164(2):354–362. https://doi.org/10.1016/j.jbiotec.2013.01.021
Cheng YM, Hsieh FC, Meng M (2009) Functional analysis of conserved aromatic amino acids in the discoidin domain of Paenibacillus beta-1,3-glucanase. Microb Cell Fact 8:62. https://doi.org/10.1186/1475-2859-8-62
Cherdthong A, Seankamsorn A, Suriyapha C, Chanjula P, Wanapat M (2018) Effect of beta-glucan supplementation on feed intake, digestibility of nutrients and ruminal fermentation in thai native beef-cattle. J Anim Physiol Anim N 102(6):1509–1514. https://doi.org/10.1111/jpn.12989
Conroy LR, Stanback AE, Young LEA, Clarke HA, Sun RC (2021) In situ analysis of N-linked glycans as potential biomarkers of clinical course in human prostate cancer. Molecular Cancer Res 19(10):19. https://doi.org/10.1158/1541-7786.MCR-20-0967
Dotsenko AS, Gusakov AV, Volkov PV, Rozhkova AM, Sinitsyn AP (2016) N-linked glycosylation of recombinant cellobiohydrolase I (Cel7A) from Penicillium verruculosum and its effect on the enzyme activity. Biotechnol Bioeng 113(2):283–291. https://doi.org/10.1002/bit.25812
Fernandes VO, Costa M, Ribeiro T, Serrano L, Cardoso V, Santos H, Lordelo M, Ferreira LMA, Fontes, (2016) 1,3–1,4-β-glucanases and not 1,4-β-glucanases improve the nutritive value of barley-based diets for broilers. Anim Feed Sci Tech 211:153–163. https://doi.org/10.1016/j.anifeedsci.2015.11.007
Han C, Liu YF, Liu MY, Wang SQ, Wang QQ (2020) Improving the thermostability of a thermostable endoglucanase from Chaetomium thermophilum by engineering the conserved noncatalytic residue and N-glycosylation site. Int J Biol Macromol 164:3361–3368. https://doi.org/10.1016/j.ijbiomac.2020.08.225
Han C, Wang QQ, Sun YX, Yang RR, Liu MY, Wang SQ, Liu YF, Zhou LF, Li DC (2020) Improvement of the catalytic activity and thermostability of a hyperthermostable endoglucanase by optimizing N-glycosylation sites. Biotechnol Biofuels 13(1):30. https://doi.org/10.1186/s13068-020-1668-4
Henrissat B, Bairoch A (1993) New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 293(3):781–788. https://doi.org/10.1042/bj2930781
Hoshida H, Fujita T, Chaaim K, Akada R (2013) N-glycosylation deficiency enhanced heterologous production of a Bacillus licheniformis thermostable α-amylase in Saccharomyces cerevisiae. Appl Microbiol Biot 97(12):5473–5482. https://doi.org/10.1007/s00253-012-4582-2
Hua C, Yan Q, Jiang Z, Li Y, Katrolia P (2010) High-level expression of a specific β-1,3–1,4-glucanase from the thermophilic fungus Paecilomyces thermophila in Pichia pastoris. Appl Biochem Biotechnol 88(2):509. https://doi.org/10.1007/s00253-010-2759-0
Huang H, Yang P, Luo H, Tang H, Shao N, Yuan T, Wang Y, Bai Y, Yao B (2008) High-level expression of a truncated 1,3–1,4-β-d-glucanase from Fibrobacter succinogenes in Pichia pastoris by opti-mization of codons and fermentation. Appl Microbiol Biot 78:95–103. https://doi.org/10.1007/s00253-007-1290-4
Jing Z, Itaru W, Amanda P, Matthew K, Barbara G, Chaowen Y, Esperanza R (2003) Allowed N-glycosylation sites on the Kv1.2 potassium channel S1-S2 linker implications for linker secondary structure and the glycosylation effect on channel function. Biochem J 375(3):769–775. https://doi.org/10.1042/BJ20030517
Kajiwara S, Yamada R, Matsumoto T, Ogino H (2020) N-linked glycosylation of thermostable lipase from Bacillus thermocatenulatus to improve organic solvent stability. Enzyme Microb Tech 132:109–416. https://doi.org/10.1016/j.enzmictec.2019.109416
Kar B, Verma P, Den H, Riaan S, Ashwani K (2018) Effect of N-linked glycosylation on the activity and stability of a beta-glucosidase from Putranjiva roxburghii. Int J Biol Macromol 112:490–498. https://doi.org/10.1016/j.ijbiomac.2018.01.201
Kashyap A, Gupta R (2021) Disrupting putative N -glycosylation site N17 in lipase Lip11 of Yarrowia lipolytica yielded a catalytically efficient and thermostable variant accompanying conformational changes. Enzyme Microb Tech 151:109922. https://doi.org/10.1016/j.enzmictec.2021.109922
Kebede A, Kebede M (2021) In silico analysis of promoter region and regulatory elements of glucan endo-1,3-beta-glucosidase encoding genes in Solanum tuberosum: cultivar DM 1–3 516 R44. J Gene Engine Biotech 19(1):145. https://doi.org/10.1186/s43141-021-00240-0
Khan SI, Zada NS, Sahinkaya M, Nigar CD, Ahmed S, Hasan F, Belduz AO, Çanakçi S, Khan S, Badshah M, Shah AA (2021) Cloning, expression and biochemical characterization of lignin-degrading DyP-type peroxidase from Bacillus sp. Strain BL5. Enzyme Microb Technol 36:1–10. https://doi.org/10.1016/j.enzmictec.2021.109917
Kilgore HR, Latham AP, Ressler VT, Zhang B, Raines RT (2020) Structure and dynamics of N-glycosylated human ribonuclease 1. Biochem 59(34):3148–3156. https://doi.org/10.1021/acs.biochem.0c00191
Li J, Tang C, Shi H, Wu M (2011) Cloning and optimized expression of a neutral endoglucanase gene (ncel5A) from Volvariella volvacea WX32 in Pichia pastoris. J Biosci Bioeng 111(5):537–540. https://doi.org/10.1016/j.jbiosc.2011.01.002
Marion M, Bradford (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Academic Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Meldgaard M, Svendsen L (1994) Different effects of N-glycosylation on the thermostability of highly homologous bacterial (1,3–1,4)-β-glucanases secreted from yeast. Microbiology 140:159–166. https://doi.org/10.1099/13500872-140-1-159
Miller GL (1959) Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal Biochem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Mohanty S, Chaudhary BP, Zoetewey D (2020) Structural insight into the mechanism of N-linked glycosylation by oligosaccharyltransferase. Biomolecules 10(4):624. https://doi.org/10.3390/biom10040624
Murphy EJ, Rezoagli E, Major I, Rowan NJ, Laffey JG (2020) β-glucan metabolic and immunomodulatory properties and potential for clinical application. J Fungi 6(4):356. https://doi.org/10.3390/jof6040356
Nguyen N, Le N, Tran T, Pham DM (2021) Incorporating a transfer learning technique with amino acid embeddings to efficiently predict N-linked glycosylation sites in ion channels. Comput Biol Med 130(5):104212. https://doi.org/10.1016/j.compbiomed.2021.104212
Niu C, Zhu L, Hill A, Alex Speers R, Li Q (2017) Construction of a highly thermostable 1,3–1,4-β-glucanase by combinational mutagenesis and its potential application in the brewing industry. Biotechnol Lett 39(1):113–122. https://doi.org/10.1007/s10529-016-2212-2
Pastor RW, Brooks BR, Szabo A (1988) An analysis of the accuracy of Langevin and molecular dynamics algorithms. Mol Phys 65(6):1409–1419. https://doi.org/10.1080/00268978800101881
Qin Y, Qu Y (2014) Asn124 of Cel5A from Hypocrea jecorina not only provides the N-glycosylation site but also is essential in maintaining enzymatic activity. BMB Rep 47(5):256–261. https://doi.org/10.5483/BMBRep.2014.47.5.166
Sakonwat K, Yi Z, Hideki K, Yuya K, Soottawat B, Srinivasan D, Sappasith K (2021) Enzymological characteristics of pepsinogens and pepsins purified from lizardfish (Saurida micropectoralis) stomach. Food Chem 366:130532. https://doi.org/10.1016/j.foodchem.2021.130532
Shental-Bechor D, Levy Y (2009) Folding of glycoproteins: toward understanding the biophysics of the glycosylation code. Curr Opin Struct Biol 19(5):524–533. https://doi.org/10.1016/j.sbi.2009.07.002
Silva D, Yu S, Ulge UY, Spangler JB, Jude KM, Labão-Almeida C, Ali LR, Quijano-Rubio A, Ruterbusch M, Leung I, Biary T, Crowley SJ, Marcos E, Walkey CD, Weitzner BD, Pardo-Avila F, Castella-nos J, Carter L, Stewart L, Riddell SR, Pepper M, Bernardes GJL, Dougan M, Garcia KC, Baker D (2019) De novo design of potent and selective mimics of IL-2 and IL-15. Nature 565:186–191. https://doi.org/10.1038/s41586-018-0830-7
Sue MP, Mariana L, Fazenda B, Linda MH (2005) Heterologous protein production using the Pichia pastoris expression system. Yeast 22(4):249–270. https://doi.org/10.1002/yea.1208
Teh A, Fazli NH, Furusawa G (2020) Crystal structure of a neoagarobiose-producing GH16 family β-agarase from Persicobacter sp CCB-QB2. Appl Microbiol Biot 104(1):633–641. https://doi.org/10.1007/s00253-019-10237-y
Tian M, Fu JY, Wang ZY, Miao CL, Lv PM, He D, Li ZD, Liu T, Li M, Luo W (2021) Enhanced activity and stability of Rhizomucor miehei lipase by mutating N-linked glycosylation site and its application in biodiesel production. Fuel 304:121514. https://doi.org/10.1016/j.fuel.2021.121514
Tian M, Fu JY, Wang ZY, Miao CL, Lv PM, He D, Li ZD, Liu T, Li M, Luo W (2021) Improved methanol tolerance of Rhizomucor miehei lipase based on N-glycosylation within the α-helix region and its application in biodiesel production. Biotechnol Biofuels 14(1):237. https://doi.org/10.1186/s13068-021-02087-6
Tony C, Charles G, Georges F (2005) Xylanases, xylanase families and extremophilic xylanases. Fems Microbiol Re 29(1):3–23. https://doi.org/10.1016/j.femsre.2004.06.005
Virginia DS, Rohitesh G, Yusen Z, Gabrielle PLMK, Sriram N (2020) Robustness in glycosylation systems: effect of modified monosaccharides, acceptor decoys and azido sugars on cellular nucleotidesugar levels and pattern of N-linked glycosylation. Mol Omics 16:377–386. https://doi.org/10.1039/D0MO00023J
Waldron R, McGowan J, Gordon N, Mitchell EB, Fitzpatrick DA, Doyle S (2019) Characterisation of three novel β-1,3 glucanases from the medically important house dust mite Dermatophagoides pteronyssinus (airmid). Insect Biochem Molec 115:103242. https://doi.org/10.1016/j.ibmb.2019.103242
Wang T, Nakagawa S, Iyake TM, Setsu G, Doi M (2020) Identification and functional characterisation of N-linked glycosylation of the orphan G protein-coupled receptor Gpr176. Sci Rep 10(1):4429. https://doi.org/10.1038/s41598-020-61370-y
Wentao C, Leopold K, Stephen C, Juila M, Dendle Y (2016) Stabilizing the CH2 domain of an antibody by engineering in an enhanced aromatic sequon. ACS Chem Biol 11(7):1852–1861. https://doi.org/10.1021/acschembio.5b01035
Xia C, Qiao Y, Dong DC, Chen X, Wang YX, Li D, Ye XF, Jian H, Yan H, Cui ZL, Li ZK (2021) Enzymatic properties of an efficient glucan branching enzyme and its potential application in starch modification. Protein Expres Purif 178:105779. https://doi.org/10.1016/j.pep.2020.105779
You S, Tu T, Zhang L, Wang Y, Huang H, Ma R, Shi P, Bai Y, Su X, Lin Z, Luo H, Yao B (2016) Improvement of the thermostability and catalytic efficiency of a highly active β-glucanase from Tala-romyces leycettanus JCM12802 by optimizing residual charge–charge interactions. Biotechnol Biofuels 9(1):124. https://doi.org/10.1186/s13068-016-0544-8
You S, Tu T, Ma R, Huang H, Wang Y, Bai Y, Su X, Cai H, Yao B, Luo H (2018) Functional analysis of a highly active β-glucanase from Bispora sp. MEY-1 using its C-terminally truncated mutant. J Agr Food Chem 66(37):9728–9737. https://doi.org/10.1021/acs.jafc.8b01928
You S, Li J, Zhang F, Bai Z, Saidi S, Richard H, Zhang W, Wang J (2021) Loop engineering of a thermostable GH10 xylanase to improve low-temperature catalytic performance for better synergistic bio-mass-degrading abilities. Bioresour Technol 342:125962. https://doi.org/10.1016/j.biortech.2021.125962
Zhu J, Liu H, Zhang J, Wang P, Liu S, Liu G, Wu L (2014) Effects of Asn33 glycosylation on the thermostability of Thermomyces lanuginosus lipase. J Appl Microbiol 117(1):151–159. https://doi.org/10.1111/jam.12519
Acknowledgements
The authors thank the Biotechnology Laboratory of Jiangsu University of Science and Technology for providing experimental equipment.
Funding
This work was supported by the National Natural Science Foundation of China (21978121), and the Natural Science Foundation of Jiangsu Province (BK20190957).
Author information
Authors and Affiliations
Contributions
Conceptualization, JW and SY; methodology, ZZ and FZ; formal analysis, ZZ and YC; investigation, ZZ and HH; writing—original draft preparation, ZZ and FZ; writing—review and editing, ZZ, FZ, and SY; visualization, ZZ; supervision, JW. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zha, ZQ., You, S., Hu, YH. et al. Asn57 N-glycosylation promotes the degradation of hemicellulose by β-1,3–1,4-glucanase from Rhizopus homothallicus. Environ Sci Pollut Res 30, 8707–8721 (2023). https://doi.org/10.1007/s11356-022-19959-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19959-5