Abstract
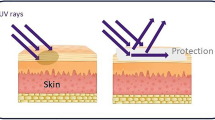
Numerous studies have confirmed the damage caused by excessive exposure to ultraviolet-A rays. Malignant melanoma and skin cancer are two of the most serious health consequences. Thus, the UV-A protectant is intended to protect the skin, especially the two primary layers of skin (epidermis that represents the interface between the body and its surroundings and dermis). Spider silk is the most powerful natural fibre due to its regeneration, biocompatibility, antimicrobial, wound healing, antiseptic, and blood clotting properties. This work targeted to determine the protective effect of spidroin extract against UV-A radiation damage. Earthworms Aporrectodea caliginosa were collected from Assiut University’s farm. Each set of ten earthworms was separated into six groups and placed in a plastic container. Webs of spiders collected from trees and old houses. Spidroin was extracted and utilised in this work to determine the potential effects of topical application on UV-A protection. The experiment is divided into two sections: (1) UV-A exposure and (2) the use of spidroin extract to protect the earthworms from ultraviolet radiation. Two control groups (1،2) of worms were not received UV-A exposure, and four groups (3,4,5,6) were exposed to UVR-A. In contrast, groups (5,6) were received spidroin extract before exposure to UV-A. Each group from the groups (3,4,5,6) was exposed for three consecutive days (¼ hour/day, ½ hour/day, and 1 h/day), using a UV-Lamp with a wavelength of 366 nm. The histopathological changes revealed that after 1⁄4 h of UV exposure, the cuticle was swollen with a slightly detached epithelium. The cuticle was down after 1⁄2 h of exposure, and the epidermis was totally damaged and necrosed. After 1 h, the exposure showed destruction of the epidermis in the circular muscle with a loss of muscle filament integrity, varying size, and altered nucleus form, along with mild disintegration of longitudinal muscle. Spidroin extract is critical for earthworm protection against UV-A radiation damage and able to regeneration. For the first time, morphological and histological analysis was established to detect the Spidroin extract evaluated for topical application on earthworms. Earthworms can be considered as a robust human skin model prior to UV-A exposure. It induces a complete protective effect against UV-A radiation damage in earthworms.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Undoubtedly, solar radiation is the main source of ultraviolet rays. The majority of those rays reach the Earth's surface, causing tanning, skin ageing, eye damage, and even immunosuppression (Clydesdale et al. 2001).
Ultraviolet rays are a form of electromagnetic radiation that falls between visible light and x-rays; their wavelength ranges from 10 to 400 nm. Ultraviolet rays are typically classified into three categories based on their interaction with other biological materials: UV-A (longest wavelengths from 315 to 400 nm), UV-B (290–315 nm), and UV-C (shortest wavelengths from 290 to 315 nm) (Arrigo 1994; Meyer-Rochow et al. 2000; Steeger et al. 2001; Norval et al. 2007). Ultraviolet rays are invisible to the naked eye (Ivanov et al. 2018). They can cause the emission of electromagnetic radiation when they collide with certain aquatic biological materials (Dong et al. 2007).
The decrease in ozone in the stratosphere caused by human activity resulted in an increase in ultraviolet radiation (UVR) at the terrestrial level, which has a detrimental effect on living organisms (Sayed et al. 2007). UVR accounts for about 10% of sunlight (Solano 2020); just one-third of this percentage can penetrate the atmosphere and touch the surface of the Earth. The most harmful rays, both longer and shorter wavelengths (UVA rays have the longest wavelengths, followed by UVB, and UVC rays which have the shortest wavelengths), are absorbed mostly by ozone, oxygen, and water vapour in the atmosphere. Sunburn is the most widespread damage caused by ultraviolet (Durkee 2006).
Melanin pigment can dissipate over 99.9% of absorbed ultraviolet radiation (Meredith and Riesz 2004). Melanin absorbs and scatters these harmful rays as heat, and the accumulated melanin in exposed skin cells leads to pigmentation and tanning. However, melanin is considered a natural sunscreen, prolonged exposure to sunlight results in a malfunction of this mechanism (Agar and Young 2005). These photons can induce apparent damage to cell DNA rather than creating sunburn (Hadi et al. 2009). The constant blood supply aids the healing process to these damaged cells, which causes inflammation and pain. These DNA-containing cells can occasionally turn malignant, resulting in skin cancer (Elwood and Jopson 1997). Increased UV exposure causes direct cellular damage, which is a key factor in the growth of cancer (Ivanov et al. 2018).
Earthworms are critical invertebrates in the agroecosystems, as they control the complex structure and organic matter of the soil. In other words, they are the primary component of soil fauna populations in the majority of habitats and account for a sizable proportion of soil fauna biomass. Earthworms increase the organic matter content of the soil and contribute to the humus formation processes, influencing plant growth and productivity (Jouquet et al. 2008; Bottinelli et al. 2010; Blouin et al. 2013; Pelosi et al. 2014; Abd El-Aziz 2018; Le Bayon et al. 2021).
Earthworms have been utilised in Chinese folk medicine since ancient times for various reasons: (a) they are cheap, (b) they are non-controversial animal models (without ethical concerns), (c) provide food, (d) create useful compost, (e) provide medicinal remedies, and (f) have a short life cycle (Pelosi et al. 2014) either the intermediate host of parasites that infected humans nor are they annoying pests (Cooper et al. 2012).
Earthworms can regenerate lost segments, but this ability varies by species and depends on the severity of the harm. Biologists are particularly taken with the earthworm’s capacity for rapid regeneration. However, some aspects of its rehabilitation remain a mystery (Shao et al. 2020). Numerous scientists are intrigued by the earthworms' amazing capacity to regenerate (CHO et al. 2001; Shao et al. 2020). Thus, they have researched their regeneration (Allolobophora caliginosa) (Rosi and Giovannini 1971; Koritsánszky 1974; Koritsánszky and Hartwig 1974; Gałuszka et al. 2015; Abd Ellah et al. 2019).
Following a literature review using earthworms as a model for examining environmental risk factors, only a few studies on earthworms were established (Albro et al. 1997; Misra et al. 2005; Hirano and Tamae 2010; Abd Ellah et al. 2019). Earthworms are used in preclinical studies instead of higher laboratory animals because they are plain, inexpensive, and responsive animal models (Misra et al. 2005).
Spider silk (webs) is a natural product, which is the strongest natural fibre with the greatest elasticity. It was previously utilised for its remarkable biomedical, mechanical, and antibacterial properties, clot formation, neural repair, ligament regeneration, and wound healing (Eisoldt et al. 2011; Mirghani et al. 2012). Spidroin has a wide range of applications; it is tolerated in vitro, cell culture, and in vivo, such as pigs, without eliciting an inflammatory response or adverse body reaction. These findings suggest that they could be utilised in medicine without triggering biocompatibility issues, eventually paving the way for a slew of new opportunities in regenerative medicine and tissue engineering (Scheller et al. 2001). Spiders use their webs to shield their eggs from harmful rays and external stimuli, ensuring their safety and health (Humenik et al. 2011).
The skin is the body’s largest organ, consisting of two distinct layers: epidermis and dermis. The ectodermal epidermis serves as the interface between the body and the environment. Thus, epidermal biological and physical properties play a significant role in the resistance of the epidermis to environmental stressors such as infectious diseases, chemical agents, and UV resistance (Slominski and Wortsman 2000; Lowe 2006; Fuchs 2007; Slominski et al. 2012).
The primary objective of this analysis was to determine the efficacy of spidroin extract in protecting against the harmful effects of UV-A radiation. To the best of our knowledge, this is the first article to investigate the protective effect of spidroin extract against UV-A harm using earthworms as a human skin model.
Materials and methods
Earthworms’ collection
Earthworms were collected by digging and hand sorting soil from Assiut University's farm grassland. Earthworms were kept in perforated plastic boxes to allow ventilation. The collected samples were transferred to the laboratory of the Zoology Department, Faculty of Science, Assiut University. The earthworms were then identified and separated according to their species. According to keys, the earthworms (Aporrectodea caliginosa) were isolated from their substrates and other earthworm species (Brinkhurst 1966; Brinkhurst and Jamieson 1971; Zorn et al. 2008; Yousefi et al. 2009; Plisko 2010). They were kept under laboratory conditions (25–28 °C, 12 h light/12 h darkness) and fed daily cow manure and leaves for two weeks before the studies to acclimate to the laboratory setting after being placed in perforated small plastic boxes with substrate wet filter paper.
Earthworms regeneration
Similarly, earthworms (Aporrectodea caliginosa) were divided into six groups (each of 10 worms); groups (1, 3& 5) were left without any intervention, and groups (2, 4 & 6) were split in the anterior part of the skin (front ten segments) with a disposable scalpel to inflict injury. Simultaneously, group 2 (Control cut) earthworms were left to natural healing (untreated control). Visual examination of wound healing over 12 days was used to determine the presence of residual worms in the control group.
Collection of spider webs and Spidroin extract
Spider (Araneus diadematus and Tegenaria domestica) webs or silks were collected from trees and old buildings using a stick, as previously performed (Barrion and Litsinger 1995). Species were identified by keys and articles that recognised spiders to avoid any trapped plants, insects, dust, and other extraneous items. We washed the gathered webs several times and then allowed them to dry at room temperature. These spider webs have been preserved in tightly sealed containers for further usage.
According to an updated procedure, spidroin was extracted using Sodium hydroxide solution (Lateef et al. 2016). In a nutshell, 1 g of spider silk was spread across 10 mL of 0.1 M NaOH. The dispersion was heated at 90 °C with continuous stirring for 1 h and then cooled (Abd Ellah et al. 2019). The resulting dispersion was centrifuged at 5000 rpm for 30 min to clear any residual spider silk. The supernatant was collected and centrifuged at 5000 rpm for an additional 30 min. The final supernatant containing the spider silk extract (more precisely, spidroin protein) was refrigerated for later use.
Spidroin protein quantitation
Bradford protein assay was used to quantify the total protein content of the extract (Bradford 1976). The unknown protein samples were combined with Bradford reagent (Coomassie Brilliant Blue 0.01% in 4.7% ethanol and 8.5% phosphoric acid, 2.5 ml) and incubated for 15 min at room temperature. The blue-coloured solutions were spectrophotometrically compared to a similarly treated blank at 595 nm. Triplicates of the samples were tested. The unknown samples' content was measured using standard BSA solutions (5–50 g/100 l) to create a calibration curve.
Experimental setup and exposure to UV-A radiation
Earthworms were divided into six groups (10 Earthworms per set): Group 1 (control) and groups 3 and 5 were left without intervention, while the other three groups (2, 4, and 6) were subjected to a sterile scalpel cut or amputation in the skin of the frontal part (the first ten body segments). Worms from group 2 (control cut) were left to regenerate naturally. Worms from Groups 3 and 4 were exposed to UV-A using a 366 nm peak transmission UV-Lamp (ULTRA-VIOLET Products, Inc., San Gabriel, California, USA model UVL-56). The typical intensity is 750 µW per cm2. Dose ( J ̸ cm2) = intensity(mW ̸ cm2) × times(s)% 1000 = 750 × 60 × 60%1000 = 2700 J ̸ cm2.
The UVR-A supply was installed 30 cm above the experimental area. Worms from Groups 5 and 6 were treated with spidroin extract prior to UV-A exposure. Spidroin extract was used for three consecutive days once a day. The remaining worms in group 2 had their wound healing assessed visually over 12 days. We applied the spidroin extract using a spray bottle by covering the whole worm body. Each group received irradiation for 15 min per day, 30 min per day, and 1 h per each group for three days and three container replicates per group (Kligman et al. 1983; Häkkinen and Oikari 2004). UVR-A effects were observed in plastic containers (20 cm * 20 cm) containing 2 mm buffered saline (0.01 M, pH 7.4) or moist filter paper with buffered saline.
Histological investigations
After three days, tissues were collected from three worms per group for histological examination, while wound healing was visually assessed for the remaining worms in Group 2 (control cut) over 12 days. Overnight fixation in 10% neutral buffer formalin was performed on the samples (pH 7.2). Following a thorough cleaning with water, the samples are dehydrated in increasing concentrations of ethanol, then embedded in methyl benzoate and finally in melted paraffin wax. Tissue samples were carved into paraffin blocks using a microtome. The tissue section was 5 μm. A 5–6 μm.thick paraffin piece was cut and put on glass slides before being dried in an incubator. Sections were stained with Eosin and Hematoxylin for general histological inspection by light microscope after deparaffinisation and rehydration (Drury and Wallington 1980).
Statistical analysis
The experiment of six groups (control and exposed groups) was repeated on three occasions. One-way ANOVA examined the significance of differences between the control groups and the treated groups. A P < 0.05 or 0.01 degree of significance was recognised as essential or highly relevant. Statistical prisms were used.
Results
Macroscopic observation of earthworms changes during regeneration
Regeneration was observed in the anterior earthworm (Aporrectodea caliginosa). Group 1 was left without any interference. Group 2 on zero h after haemorrhage, amputation, redness, oedema, and exudation around the wound region took more than 12 days for regeneration; these findings suggest that the worm's ability to survive and regenerate is harmed when a part of it is cut. Cutworms fared better than worms exposed to UV-A in terms of survival and regeneration in group 2 (Fig. 1). Groups 5 and 6 were received the spidroin extract before exposure to UV-A. Group (5) was not damaged. Group (6) coagulated the fastest and demonstrated complete wound closure in 5 days (Fig. 2B &C). Earthworms were divided into 6 groups. Group 1(control), group 2 (regenerated control), groups 3 and 4 were exposed to UV-A (Fig. 2A). While groups 5 and 6 were sprayed with spidroin extract before being exposed to UV-A (Figs. 3, 4, 5).
Macroscopic observation of earthworms (Aporrectodeacaliginosa) (A) the control group (1) and(B,C,D,E and F) control regenerated (group, 2) regeneration of earthworms (A.caliginosa) after induction of surgical wounds and examination of wound healing and regeneration; worms were left untreated to the mercy of natural healing and cut in the first six segments (circle).per.(peristomium), pro.(prostomium)
Tissue structure of (Aporrectodea caliginosa) (A) group (1) (control group) showed normal structure of earthworm: cuticle (C), epidermis (Ep) arrow, circular muscle (C.M.) and longitudinal muscle (L.M.), Histopathological changes in group (3) (B) After ¼ hr exposure, the cuticle (Cu) swelled with slightdetached epithelium (arrow), (C) After ½ hr exposure showed Slight desquamation of epithelial layer, (D) After 1 h exposure showed complete necrosis and desquamation of epithelium. H&E. bar = 50 µm
Tissue structure of (Aporrectodea caliginosa) (A) (control group 2) showed normal structure of earthworm: wall cuticle (C), epidermis (Ep) arrow, circular muscle (C.M.) and longitudinal muscle (L.M.), Histopathological changes in group (4) (B) After ¼ hr exposure showed cloudy swelling and progressivehypertrophy of the epidermis(arrow), (C) After ½ hr exposure, the cuticle shows breakdown, and the epidermis completely damaged and necrosed, (D) After 1 h exposure showed complete destruction of the epidermis accompanied with of necrosis circular muscleand mild disintegration of longitudinal muscle (H&E. bar = 50)
Macroscopic observation of (A): the earthworms of group(4) after UV-A exposure,(B), the earthworms of group(5) after UV-A exposure,(C): the earthworms of group(6) after UV-A exposure. Photomicrograph of: Tissue structure of (Aporrectodea caliginosa) (D,E),group(4)(E,H),group(5)and (F,I),group(6): cuticle (C), epidermis (Ep) arrow, circular muscle (C.M.) and longitudinal muscle (L.M.). H&E. bar = 50 µm
Histological study of earthworms
On the 12th day of worm regeneration, histological examination was performed at the amputation site without obtaining the spidroin extract. Histological observation of the cross-section of the body wall of earthworms in the control group demonstrated the normal earthworm architecture of the cuticle (C), the epidermis (Ep), circular muscle (CM), and longitudinal muscle (LM), as shown by hematoxylin and Eosin staining (Fig. 6A). For group (3), the histopathological changes after 1⁄4 h of UV exposure, the cuticle was swollen with a slightly detached epithelium. Further, the epidermis displayed cloudy swelling and progressive epidermis hypertrophy (Fig. 6B). Light epithelial layer desquamation was observed after 1/2 h of exposure. The cuticle was down after 1⁄2 h of exposure, and the epidermis was totally damaged and necrosed (Fig. 6C). Total epithelial necrosis and desquamation were detected after 1 h of exposure. After 1 h, the exposure showed destruction of the epidermis in the circular muscle with a loss of muscle filament integrity, varying size, and altered nucleus form, along with mild disintegration of longitudinal muscle (Fig. 6D).
Tissue structure of Aporrectodea caliginosa in the control group (2) showed the normal structure of earthworm: wall cuticle (C), the epidermis (Ep) arrow, circular muscle (CM), and longitudinal muscle (LM), after regeneration and wound healing (Fig. 7A). Histopathological changes in the group (4) after ¼ hour of exposure revealed cloudy swelling and progressive hypertrophy of the epidermis(arrow) (Fig. 7B). After ½ hour of exposure, the cuticle showed a breakdown, and the epidermis was completely damaged and necrosed (Fig. 7C). The epidermis was destroyed, with necrosis circular muscle and mild disintegration of longitudinal muscle after 1 h of exposure (Figs. 2A, D&G and 7D).
In the group (5) (Figs. 2B, E, and H) and group (6) (Figs. 2C, F & I), the body wall revealed the intact structure of epidermal, circular, and longitudinal muscles, indicating that spidroin extract did promote the protective effect against UV-A radiation damage in earthworms. It was thoroughly covered in spidroin extract before being exposed to UV-A. The skin of aporrectodea caliginosa earthworms receiving the spidroin extract did not show any considerable differences from that of control groups(1&2).
Mortality of earthworms after exposed to UV-A
Findings confirm that exposure to UV-A radiation negatively affects worms and their ability to regenerate. Earthworms exposed to UV-A were about 83.34, 93.33, 56.67, 53.34% in groups (3, 4, 5 & 6) (Fig. 5); suggesting that the UV-A dosage exposure and the significant mortality of earthworms were significant (P < 0.01) and extremely significant (P < 0.0001).
Discussion
The outcomes of this research demonstrated that: (a) UV-A exposure can have detrimental effects on earthworms, and (b) spidroin extract protects against UV-A damage, but it is used in promoting wound healing.
Regarding the effects of phototoxicity, the contribution of different groups in the present study showed that exposed earthworms to UV-A caused the damage; this was the product of the work on earthworm Metaphire Posthuma (Chuang et al. 2004; Misra et al. 2005), who worked on three species of earthworms (Amynthas gracilis, Pontoscolex corethrurus, and Metaphire Posthuma) which, was explained the destructive impact of ultraviolet radiation on earthworms. In addition, some researchers addressed the health effects of solar UV exposure, which is complex and dependent on different sociodemographic variables, including skin colour (Rendell et al. 2020). Earthworms (Lumbricus castaneus) were exposed to UV-C for 15 min each day showed little alterations (Abd El-Aziz and Ali 2021). These changes are at their peak after 30 min each day. To determine the impact of UV-C radiation on earthworms, the complete absence of cell connections in earthworms with sloughed tissue was demonstrated using SEM examination. It was discovered that Long-term exposure to UV-C radiation resulted in serious damage and an increase in cancer risk. The death rate of both earthworms has risen.
Histological examination of the control group revealed normal earthworm skin composed of the cuticle, epidermis, circular and longitudinal muscles for groups that were left untreated with spidroin extract following wound induction (Groups 3 and 4). The effect of various doses of ultraviolet radiation on earthworms, resulting in pathogenesis, was investigated in this research. After 15 min of exposure to UV-A radiation, the cuticle swelled with a slightly detached epithelium. Additionally, they demonstrated cloudy swelling and progressive hypertrophy of the epidermis in this group; these findings corroborate previous evidence regarding the influence of UVR-A. This evidence is similar to (Albro et al. 1997; Chuang et al. 2004; Misra et al. 2005; Herndon et al. 2018). ( Herndon et al. 2018) stated that UVR hardens the skin and causes it to lose its elasticity. After 30 min of exposure, we observed mild desquamation of the epithelial layer.
The cuticle was completely destroyed, and the epidermis was fully necrosed. After 60 min of exposure, complete necrosis and desquamation with epithelium separation were observed. The epidermis degraded, followed by necrosis in the circular muscle, characterised by irregular loss of muscular filament integrity and altered nucleic form with mild longitudinal disintegration. According to these findings, exposure to ultraviolet rays causes chronic ageing, damage to the immune system, and skin cancer (Matsumura and Ananthaswamy 2004). (Lan 2019) confirmed that UVB chronic exposure (3 times a week for 8 weeks) has significant effects on the skin at high temperatures (i.e., dermal thickness). (Coelho et al. 2009) noted an improvement in human skin pigmentation following one or more UV exposures. UVB exposure has been shown to retard development and decrease survival rates in both the short and long terms (Alves et al. 2020). (Chen 2011) discovered that earthworms could regenerate immediately after a portion of the worm is cut off.
Nobody can deny that direct sunlight exposure is one of the most harmful causes for humans and living organisms since a variety of electromagnetic spectrums like ultraviolet rays are included in the sunlight. Since the skin is the largest organ in the human body and is most vulnerable to ultraviolet rays, it provides critical defence against environmental damage to the human body, leading to skin ageing, dryness, and carcinogenic changes in the dermal layer (Solano 2020). Consequently, sunscreens must be used to guard against ultraviolet damage. In this research, we used the earthworm as a human skin model due to the existence of various triene and tetraene sterols in its skin that are similar to those found in humans, resulting in earthworm sensitivity to UVR- A (Albro et al. 1997; Misra et al. 2005; Abd Ellah et al. 2019; Abd El-Aziz and Ali 2021). In the present work, the mortality rate was 83.34, 93.33, 56.67, and 53.34% in groups 3, 4, and 6, respectively.
According to the findings, spidroin extract protects against UV-A damage and spider silk proteins, which exhibit substantial antimicrobial activity, can contribute to rapid wound healing. (Abd Ellah et al. 2019) worked on spidroin gel (0.6% CP934), which can wound healing and effectively inhibit the growth of isolated methicillin-sensitive clinical bacteria. It is worth noting that the ultraviolet defence was shown to prevent high levels of UV radiation from the skin and other organisms (Lucas et al. 2006). In contrast, vitamin D production essential for bone health requires a moderate degree of UV exposure, but not for a long time.
Conclusion
Regarding the results of the present investigation, it can be concluded that spidroin extract can be used for topical application on earthworms A. caliginosa before exposure to UV-A. The spidroin extract is a complete protective impact against UV-A radiation damage in earthworms. Furthermore, the histological study showed that the inflammatory cells vanished. Exposure to UV –A caused the mortality to be about 83.34%, 93.33%, 56.67%, and 53.34% in groups (3, 4, 5, and 6), respectively. These findings documented the efficacy of spidroin extract in protecting against UV-A exposure and promoting wound healing; however, future research using mammalian experimental models will be required to use these findings.
Significance statement
In the present investigation, the results corroborate previous evidence on the influence of UV-A radiation damage that can be beneficial for a modifiable risk factor for ageing, immune system damage, and melanoma. This study helps the researcher uncover the critical role of UV-A exposure in causing previously unknown health risks, and the protective impact of spidroin extract against UV-A radiation damage.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
Change history
17 June 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11356-024-33994-4
References
Abd El-Aziz A (2018) Light and scanning electron microscopic studies of the developmental stages of rhabditis sp. (nematoda: rhabditidae) naturally infecting the earthworm. Egypt J Zool 70(70):165–180
Abd Ellah NH, Abd El‐Aziz FEZA, Abouelmagd SA, Abd El‐Hamid BN, Hetta HF (2019). Spidroin in carbopol‐based gel promotes wound healing in earthworm's skin model. Drug Develop Res 80(8):1051–1061
Agar N, Young AR (2005) Melanogenesis: a photoprotective response to DNA damage? Mutat Res Fundam Mol Mech Mutagen 571(1–2):121–132
Albro PW, Bilski P, Corbett JT, Schroeder JL, Chignell CF (1997) Photochemical reactions and phototoxicity of sterols: novel self‐perpetuating mechanism for lipid photooxidation. Photochem Photobiol 66(3):316–325
Alves RN, Mahamed AH, Alarcon JF, Al Suwailem A, Agustí S (2020) Adverse Effects of Ultraviolet Radiation on Growth, Behavior, Skin Condition, Physiology, and Immune Function in Gilthead Seabream (Sparus aurata). Front Mar Sci 7:306
Arrigo KR (1994) Impact of ozone depletion on phytoplankton growth in the Southern Ocean: large-scale spatial and temporal variability. Mar Ecol-Prog Ser 114:1–1
Barrion AT, Litsinger JA (1995) Riceland spiders of south and Southeast Asia. Int Rice Res Inst
Blouin M, Hodson ME, Delgado EA, Baker G, Brussaard L, Butt KR, Dai J, Dendooven L, Pérès G, Tondoh J (2013) A review of earthworm impact on soil function and ecosystem services. Eur J Soil Sci 64(2):161–182
Bottinelli N, Henry-des-Tureaux T, Hallaire V, Mathieu J, Benard Y, Tran TD, Jouquet P (2010) Earthworms accelerate soil porosity turnover under watering conditions. Geoderma 156(1–2):43–47
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Brinkhurst RO (1966) A contribution towards a revision of the aquatic Oligochaeta of Africa. Afr Zool 2(2)
Brinkhurst RO, Jamieson BGM (1971) Aquatic Oligochaeta of the world
Chen C (2011) Function of Lin28: an advance. Acad J Second Mil Med Univ 206–208
Cho SJ, Cho PY, Lee MS, Na Y, Lee JH, Koh KS, Choo JK, Park SC (2001) Up-regulation of multiple serine proteinases during earthworm tail regeneration. Invertebr Reprod Develop 40(2–3):103–108
Chuang SC, Lee H, Chen JH (2004) Diurnal rhythm and effect of temperature on oxygen consumption in earthworms, Amynthas gracilis and Pontoscolex corethrurus. J Exp Zool A Comp Exp Biol 301(9):737–744
Clydesdale GJ, Dandie GW, Muller HK (2001) Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol 79(6):547–568
Coelho SG, Choi W, Brenner M, Miyamura Y, Yamaguchi Y, Wolber R, Smuda C, Batzer J, Kolbe L, Ito S (2009) Short-and long-term effects of UV radiation on the pigmentation of human skin. J Investig Dermatol Symp Proc, Elsevier
Cooper EL, Balamurugan M, Huang C-Y, Tsao CR, Heredia J, Tommaseo-Ponzetta M, Paoletti MG (2012) Earthworms dilong: ancient, inexpensive, non-controversial models may help clarify approaches to integrated medicine emphasising emphasising neuroimmune systems. Evid Based Complement Alternat Med 2012
Dong Q, Svoboda K, Tiersch TR, Monroe WT (2007) Photobiological effects of UVA and UVB light in zebrafish embryos: evidence for a competent photorepair system. J Photochem Photobiol, B 88(2–3):137–146
Drury R, Wallington E (1980) Carleton's histological technique 5th ed. New York: Churchill Livingstone
Durkee J (2006) Management of industrial cleaning technology and processes. Elsevier
Eisoldt L, Smith A, Scheibel T (2011) Decoding the secrets of spider silk. Mater Today 14(3):80–86
Abd El-Aziz A, Ali MF (2021) Towards Study of UV-C Radiation Effect on Earthworms and Isopods Via Electron Microscopy. Egypt Acad J Biol Sci B. Zool 13(2):33–46
Elwood JM, Jopson J (1997) Melanoma and sun exposure: an overview of published studies. Int J Cancer 73(2):198–203
Fuchs E (2007) Scratching the surface of skin development. Nature 445(7130):834–842
Gałuszka A, Klimek B, Bigaj J, Płytycz B (2015) Endogeic earthworm Aporrectodea caliginosa as a model species for studies of modulation of regeneration by environmental stressors. Acta Biologica Cracoviensia Series Zool 57
Hadi A, Shokr A, Alwan S (2009) Effects of aluminum on the biochemical parameters of fresh waterfish Tilapia zillii. J Sci Appl 3(1):33–41
Häkkinen J, Oikari A (2004) A field methodology to study effects of UV radiation on fish larvae. Water Res 38(12):2891–2897
Herndon JM, Hoisington RD, Whiteside M (2018) Deadly ultraviolet UV-C and UV-B penetration to Earth’s surface: Human and environmental health implications. J Geog Environ Earth Sci Intn 14(2):1–11
Hirano T, Tamae K (2010) Heavy metal-induced oxidative DNA damage in earthworms: a review. Appl Environ Soil Sci 2010
Humenik M, Smith AM, Scheibel T (2011) Recombinant spider silks—biopolymers with potential for future applications. Polymers 3(1):640–661
Ivanov IV, Mappes T, Schaupp P, Lappe C, Wahl S (2018) Ultraviolet radiation oxidative stress affects eye health. J Biophotonics 11(7):e201700377
Jouquet P, Podwojewski P, Bottinelli N, Mathieu J, Ricoy M, Orange D, Tran TD, Valentin C (2008) Above-ground earthworm casts affect water runoff and soil erosion in Northern Vietnam. CATENA 74(1):13–21
Kligman LH, Akin FJ, Kligman AM (1983) Sunscreens promote repair of ultraviolet radiation-induced dermal damage. J Invest Dermatol 81(2):98–102
Koritsánszky S (1974) Regeneration monoaminhaltiger nervenzellen und fasersysteme im oberschlundganglion des regenswurms allolobophora caliginosa
Koritsánszky S, Hartwig H (1974) The regeneration of the monoaminergic system in the cerebral ganglion of the earthworm, Allolobophora caliginosa. Cell Tissue Res 151(2):171–186
Lan CCE (2019) Effects and interactions of increased environmental temperature and UV radiation on photoageing and photocarcinogenesis of the skin. Exp Dermatol 28:23–27
Lateef A, Ojo S, Azeez M, Asafa T, Yekeen T, Akinboro A, Oladipo I, Gueguim-Kana E, Beukes L (2016) Cobweb as novel biomaterial for the green and eco-friendly synthesis of silver nanoparticles. Appl Nanosci 6(6):863–874
Le Bayon RC, Bullinger G, Schomburg A, Turberg P, Brunner P, Schlaepfer R, Guenat C (2021) Earthworms, plants, and soils. Hydrogeol Chem Weather Soil Form 81–103
Lowe N (2006) An overview of ultraviolet radiation, sunscreens, and photo-induced dermatoses. Dermatol Clinics 24(1):9–17
Lucas R, McMichael T, Smith W, Armstrong BK, Prüss-Üstün A, Organization WH (2006) Solar ultraviolet radiation: global burden of disease from solar ultraviolet radiation. World Health Organization
Maeda K (2018) Skin-Moisturising Effect of Collagen Peptides Taking Orally. J Nutr Food Sci 8(682):2
Matsumura Y, Ananthaswamy HN (2004) Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol 195(3):298–308
Meredith P, Riesz J (2004) Radiative Relaxation Quantum Yields for Synthetic Eumelanin¶. Photochem Photobiol 79(2):211–216
Meyer-Rochow V, Kashiwagi T, Eguchi E (2000) Effects of photic and thermal stress on distal and proximal rhabdomeres in the crayfish eye: why are the visual membranes of the 8th retinula cell more resilient than the others? Protoplasma 210(3):156–163
Mirghani ME, Kabbashi N, Elfaki F, Zulkifli MFB (2012) Bt-201: Investigation of the spider web for antibacterial activity. Malaysian International Conference on Trends in Bioprocess Engineering (MICOTriBE)
Misra R, Lal K, Farooq M, Hans R (2005) Effect of solar UV radiation on earthworm (Metaphire posthuma). Ecotoxicol Environ Safety 62(3):391–396
Norval M, Cullen A, De Gruijl F, Longstreth J, Takizawa Y, Lucas R, Noonan F, Van der Leun J (2007) The effects on human health from stratospheric ozone depletion and its interactions with climate change. Photochem Photobiol Sci 6(3):232–251
Pelosi C, Barot S, Capowiez Y, Hedde M, Vandenbulcke F (2014) Pesticides and earthworms. A review. Agron Sustain Dev 34(1):199–228
Plisko JD (2010) Megadrile earthworm taxa introduced to South African soils (Oligochaeta: Acanthodrilidae, Eudrilidae, Glossoscolecidae, Lumbricidae, Megascolecidae, Ocnerodrilidae). Afr Invertebr 51(2):289–312
Rendell R, Higlett M, Khazova M, O’Hagan J (2020) Public Health Implications of Solar UV Exposure during Extreme Cold and Hot Weather Episodes in 2018 in Chilton, South East England. J Environ Public Health 2020
Rosi G, Giovannini E (1971) Relationship between caudal regeneration and neuro-secretory activity in Allolobophora caliginosa (Sav.). Rivista di biologia 64(3):355–372
Sayed AE-DH, Ibrahim AT, Mekkawy IA, Mahmoud UM (2007) Acute effects of ultraviolet-A radiation on African catfish Clarias gariepinus (Burchell, 1822). J Photochem Photobiol, B 89(2–3):170–174
Scheller J, Gührs K-H, Grosse F, Conrad U (2001) Production of spider silk proteins in tobacco and potato." Nature Biotechnol 19(6):573–577
Shao Y, Wang X-B, Zhang J-J, Li M-L, Wu S-S, Ma X-Y, Wang X, Zhao H-F, Li Y, Zhu HH (2020) Genome and single-cell RNA-sequencing of the earthworm Eisenia andrei identifies cellular mechanisms underlying regeneration. Nat Commun 11(1):1–15
Slominski A, Wortsman J (2000) Neuroendocrinology of the skin. Endocr Rev 21(5):457–487
Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD (2012) Sensing the environment: Regulation of local and global homeostasis by the skin neuroendocrine system. Adv Anat Embryol Cell Biol 212:v
Solano F (2020) Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 25(7):1537
Steeger H-U, Freitag J, Michl S, Wiemer M, Paul R (2001) Effects of UV-B radiation on embryonic, larval and juvenile stages of North Sea plaice (Pleuronectes platessa) under simulated ozone-hole conditions. Helgol Mar Res 55(1):56–66
Yousefi Z, Ramezani M, Mohamadi S, Mohammadpour R, Nemati A (2009) Identification of earthworms species in Sari Township in Northern Iran, 2007–2008. J Appl Sci 9(20):3746–3751
Zorn MI, Van Gestel C, Morrien E, Wagenaar M, Eijsackers H (2008) Flooding responses of three earthworm species, Allolobophora chlorotica, Aporrectodea caliginosa and Lumbricus rubellus, in a laboratory-controlled environment. Soil Biol Biochem 40(3):587–593
Acknowledgements
This work was supported by Taif University Researchers Supporting Project number (TURSP-2020/82), Taif University, Taif, Saudi Arabia. The authors acknowledge the Deanship of Scientific Research at King Khalid University, Abha, KSA, for funding this work under grant numbers, R.G.P.2/35/43.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was supported by Taif University Researchers Supporting Project number (TURSP-2020/82), Taif University, Taif, Saudi Arabia. The authors acknowledge the Deanship of Scientific Research at King Khalid University, Abha, KSA, for funding this work under grant numbers, R.G.P.2/35/43.
Author information
Authors and Affiliations
Contributions
FA and MS conceived and designed research. AE1 and AE2 revised the manuscript. FA, MS, and AT wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This chapter does not contain any experiments involving human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Informed consent
This study does not include any studies that involve informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Contributions
1. Spidroin extract has a complete protective impact against UV-A radiation damage.
2. The earthworm is considered a robust human skin model.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s11356-024-33994-4
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Aziz, F.EZ.A.A., Ismail, M.S., Askary, A.E. et al. RETRACTED ARTICLE: The assessment of the protective impact of spidroin extract against UV-A radiation damage by using earthworms (Aporrectodea caliginosa) as a robust human skin model via macroscopic and histological observations. Environ Sci Pollut Res 29, 44906–44916 (2022). https://doi.org/10.1007/s11356-022-18861-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18861-4