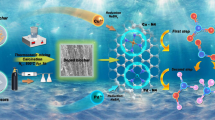
Abstract
Catalytic hydrogen reduction appears to be a promising strategy for nitrate removal. However, the danger and low utilization of H2 are the disadvantages of catalytic hydrogen reduction. Sodium borohydride (NaBH4), considered a potential candidate for hydrogen storage, has been investigated as an electron source for the catalytic reduction of contaminants. However, extensive use of NaBH4 makes commercialization costly and causes environmental pollution. In this study, we prepared supported Cu/Pd bimetallic nanoparticles that could prestore hydrogen. No additional reducing agent was required during the nitrate reduction process. The performance and mechanism of Cu/Pd bimetallic nanoparticles for nitrate reduction are discussed. Good performance was obtained with high reactivity (99.04% nitrate removal efficiency) and high selectivity for N2 (94.71%). The Cu/Pd bimetallic catalyst could be recovered by NaBH4 for 5 cycles. Moreover, a 97.49% nitrate removal efficiency was obtained for actual wastewater, indicating good prospects for nitrate reduction applications.







Similar content being viewed by others
References
Abdel-Aziz HM, Farag RS, Abdel-Gawad SA (2019) Carbamazepine removal from aqueous solution by green synthesis zero-valent iron/Cu nanoparticles with Ficus benjamina leaves’ extract. Int J Environ Res 13:843–852
Belkada FD, Kitous O, Drouiche N, Aoudj S, Bouchelaghem O, Abdi N, Grib H, Mameri N (2018) Electrodialysis for fluoride and nitrate removal from synthesized photovoltaic industry wastewater. Sep Purif Technol 204:108–115
Bhat IUH, Anwar MNK, Appaturi JN (2019) Polymer based palladium nanocatalyst for the degradation of nitrate and Congo red. J Polym Environ 27:1475–1487
Bradu C, Capat C, Papa F, Frunza L, Olaru E-A, Crini G, Morin-Crini N, Euvrard E, Balint I, Zgura I, Munteanu C (2019) Pd-Cu catalysts supported on anion exchange resin for the simultaneous catalytic reduction of nitrate ions and reductive dehalogenation of organochlorinated pollutants from water. Appl Catal A Gen 570:120–129
Du R, Cao S, Li B, Niu M, Wang S, Peng Y (2017) Performance and microbial community analysis of a novel DEAMOX based on partial-denitrification and anammox treating ammonia and nitrate wastewaters. Water Res 108:46–56
Garcia-Segura S, Lanzarini-Lopes M, Hristovski K, Westerhoff P (2018) Electrocatalytic reduction of nitrate: fundamentals to full-scale water treatment applications. Appl Catal B Environ 236:546–568
Gasparovicova D, Kralik M, Hronec M, Vallusova Z, Vinek H, Corain B (2007) Supported Pd-Cu catalysts in the water phase reduction of nitrates: functional resin versus alumina. J Mol Catal A Chem 264:93–102
Gomez-Velez JD, Harvey J, Cardenas MB, Kiel B (2015) Denitrification in the Mississippi River network controlled by flow through river bedforms. Nat Geosci 8:941–U975
Gong X, Liu Y, Wang B, Yang W, Fan L, Liu Y (2019) Nitrate reduction via micro-electrolysis on Zn-Ag bimetal combined with photo-assistance. Sci Total Environ 683:89–97
Guo S, Powell CD, Villagran D, Wong MS (2020) Magnetic In-Pd catalysts for nitrate degradation. Environ Sci-Nano 7:2681–2690
Haroon MF, Hu S, Shi Y, Imelfort M, Keller J, Hugenholtz P, Yuan Z, Tyson GW (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500:567–570
Hirayama J, Kamiya Y (2018) Tin-palladium supported on alumina as a highly active and selective catalyst for hydrogenation of nitrate in actual groundwater polluted with nitrate. Catal Sci Technol 8:4985–4993
Jaroszek H, Dydo P (2018) Potassium nitrate synthesis by electrodialysis-metathesis: The effect of membrane type. J Membr Sci 549:28–37
Jaworski MA, Bertolini GR, Cabello CI, Siri GJ, Casella ML (2018) Use of Rh (III)-heteropolymolybdate as potential catalysts for the removal of nitrates in human drinking water: synthesis, characterisation and catalytic performance. Water Air Soil Pollut 229:309
Koga H, Kitaoka T (2011) One-step synthesis of gold nanocatalysts on a microstructured paper matrix for the reduction of 4-nitrophenol. Chem Eng J 168:420–425
Li E, Wang R, Jin X, Lu S, Qiu Z, Zhang X (2018) Investigation into the nitrate removal efficiency and microbial communities in a sequencing batch reactor treating reverse osmosis concentrate produced by a coking wastewater treatment plant. Environ Technol 39:2203–2214
Lin F-H, Doong R-A (2011) Bifunctional Au-Fe3O4 Heterostructures for magnetically recyclable catalysis of nitrophenol reduction. J Phys Chem C 115:6591–6598
Lin F-H, Doong R-A (2014) Highly efficient reduction of 4-nitrophenol by heterostructured gold-magnetite nanocatalysts. Appl Catal A Gen 486:32–41
Liu BH, Li ZP (2009) A review: Hydrogen generation from borohydride hydrolysis reaction. J Power Sources 187:527–534
Lundberg JO, Weitzberg E, Cole JA, Benjamin N (2004) Nitrate, bacteria and human health. Nat Rev Microbiol 2:593–602
Marchesini FA, Mendow G, Picard NP, Zoppas FM, Aghemo VS, Gutierrez LB, Querini CA, Miro EE (2019) PdIn catalysts in a continuous fixed bed reactor for the nitrate removal from groundwater. Int J Chem React Eng 17:17
Martinez J, Ortiz A, Ortiz I (2017) State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates. Appl Catal B Environ 207:42–59
Panic S, Srdic V, Varga T, Konya Z, Kukovecz A, Boskovic G (2018) Diversity of Pd-Cu active sites supported on pristine carbon nanotubes in terms of water denitration structure sensitivity. Appl Catal A Gen 559:187–194
Park J, Hwang Y, Bae S (2019) Nitrate reduction on surface of Pd/Sn catalysts supported by coal fly ash-derived zeolites. J Hazard Mater 374(15):309–318
Pirsaheb M, Khosravi T, Sharafi K, Mouradi M (2016) Comparing operational cost and performance evaluation of electrodialysis and reverse osmosis systems in nitrate removal from drinking water in Golshahr, Mashhad. Desalin Water Treat 57:5391–5397
Rezvani F, Sarrafzadeh M-H, Ebrahimi S, Oh H-M (2019) Nitrate removal from drinking water with a focus on biological methods: a review. Environ Sci Pollut Res 26:1124–1141
Ruprich J (2013) Nitrite and nitrate in human health and disease. Cent Eur J Public Health 21:58–58
Song W, Gao B, Xu X, Wang F, Xue N, Sun S, Song W, Jia R (2016) Adsorption of nitrate from aqueous solution by magnetic amine-crosslinked biopolymer based corn stalk and its chemical regeneration property. J Hazard Mater 304:280–290
Su JF, Kuan W-F, Liu H, Huang CP (2019) Mode of electrochemical deposition on the structure and morphology of bimetallic electrodes and its effect on nitrate reduction toward nitrogen selectivity. Appl Catal B Environ 257(15):117909
Su Z, Zhang Y, Jia X, Xiang X, Zhou J (2020) Research on enhancement of zero-valent iron on dissimilatory nitrate/nitrite reduction to ammonium of Desulfovibrio sp. CMX. Sci Total Environ 746:141126
Tang T-T, Xing Q-J, Zhang S-H, Mu Y, Jiang X-H, Zhou Z-G, Xiao X, Zou J-P (2019) High selective reduction of nitrate into nitrogen by novel Fe-Cu/D407 composite with excellent stability and activity. Environ Pollut 252:888–896
Valiyeva GG, Bavasso I, Di Palma L, Hajiyeva SR, Ramazanov MA, Hajiyeva FV (2019) Synthesis of Fe/Ni bimetallic nanoparticles and application to the catalytic removal of nitrates from water. Nanomaterials 9:13
Wang Z, Guo H, Shen F, Yang G, Zhang Y, Zeng Y, Wang L, Xiao H, Deng S (2015) Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3-), and phosphate (PO43-). Chemosphere 119:646–653
Wang Q, Wang W, Yan B, Shi W, Cui F, Wang C (2017) Well-dispersed Pd-Cu bimetals in TiO2 nanofiber matrix with enhanced activity and selectivity for nitrate catalytic reduction. Chem Eng J 326:182–191
Wang F, Wang Y, Zhang Y, Luo Y, Zhu H (2018) Highly dispersed RuCo bimetallic nanoparticles supported on carbon black: enhanced catalytic activity for hydrogen generation from NaBH4 methanolysis. J Mater Sci 53:6831–6841
Wang R, Tang T, Lu G, Zheng Z, Huang K, Li H, Tao X, Yin H, Shi Z, Lin Z, Wu F, Dang Z (2019) Mechanisms and pathways of debromination of polybrominated diphenyl ethers (PBDEs) in various nano-zerovalent iron-based bimetallic systems. Sci Total Environ 661:18–26
Wu Z, Su X, Lin Z, Owens G, Chen Z (2019) Mechanism of As(V) removal by green synthesized iron nanoparticles. J Hazard Mater 379:120811
Xu C, Pan X, Fang L, Li J, Li F (2019) Enhanced reduction of organic pollutants by Fe/Cu@Pd ternary metallic nanoparticles under aerobic conditions: batch and membrane reactor studies. Chem Eng J 360:180–189
Ye W, Zhang W, Hu X, Yang S, Liang W (2020) Efficient electrochemical-catalytic reduction of nitrate using Co/AC0.9-AB0.1 particle electrode. Sci Total Environ 732:139245
Yi Y, Wei Y, Tsang PE, Fang Z (2019) Aging effects on the stabilisation and reactivity of iron-based nanoparticles green synthesised using aqueous extracts of Eichhornia crassipes. Environ Sci Pollut Res 26:28361–28371
Zhang F, Pi Y, Cui J, Yang Y, Zhang X, Guan N (2007) Unexpected selective photocatalytic reduction of nitrite to nitrogen on silver-doped titanium dioxide. J Phys Chem C 111:3756–3761
Zhang Q, Ding L, Cui H, Zhai J, Wei Z, Li Q (2014) Electrodeposition of Cu-Pd alloys onto electrophoretic deposited carbon nanotubes for nitrate electroreduction. Appl Surf Sci 308:113–120
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Funding
This research was financially funded by the Postdoctoral Science Foundation of China (Nos. 2018M642758 and 2018M632783), the Science Foundation of Henan Normal University (No. 20200179), and the National Natural Science Foundation of China (No. 41702269 and No. 51808485).
Author information
Authors and Affiliations
Contributions
Zhanhui Shen made a great contribution to the writing of the manuscript. Gege Peng analyzed the experiment data. Jialu Shi was the major writer of the manuscript. The elementary experiments were operated by Gao Ya. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 3015 kb)
Rights and permissions
About this article
Cite this article
Shen, Z., Peng, G., Shi, J. et al. A new supported Cu/Pd bimetallic nanoparticles composites prestoring reductant for nitrate removal: high reactivity and N2 selectivity. Environ Sci Pollut Res 28, 51786–51794 (2021). https://doi.org/10.1007/s11356-021-14372-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14372-w