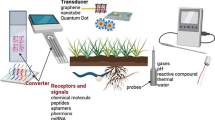
Abstract
Plant diseases significantly impact the global economy, and plant pathogenic microorganisms such as nematodes, viruses, bacteria, fungi, and viroids may be the etiology for most infectious diseases. In agriculture, the development of disease-free plants is an important strategy for the determination of the survival and productivity of plants in the field. This article reviews biosensor methods of disease detection that have been used effectively in other fields, and these methods could possibly transform the production methods of the agricultural industry. The precise identification of plant pathogens assists in the assessment of effective management steps for minimization of production loss. The new plant pathogen detection methods include evaluation of signs of disease, detection of cultured organisms, or direct examination of contaminated tissues through molecular and serological techniques. Laboratory-based approaches are costly and time-consuming and require specialized skills. The conclusions of this review also indicate that there is an urgent need for the establishment of a reliable, fast, accurate, responsive, and cost-effective testing method for the detection of field plants at early stages of growth. We also summarized new emerging biosensor technologies, including isothermal amplification, detection of nanomaterials, paper-based techniques, robotics, and lab-on-a-chip analytical devices. However, these constitute novelty in the research and development of approaches for the early diagnosis of pathogens in sustainable agriculture.






Similar content being viewed by others
Data availability
As this is a review article, data availability is not applicable. However, the resource pool can be provided on request.
References
Adachi T, Kitazumi Y, Shirai O, Kano K (2020) Development perspective of bioelectrocatalysis-based biosensors. Sensors (Basel) 20(17):4826. https://doi.org/10.3390/s20174826
Al-Hiary H, Bani-Ahmad S, Reyalat M et al (2011) Fast and accurate detection and classification of plant diseases. Int J Comput Appl 17:31–38
Ali AA, Altemimi AB, Alhelfi N, Ibrahim SA (2020) Application of biosensors for detection of pathogenic food bacteria: a review. Biosensors 10. https://doi.org/10.3390/BIOS10060058
Altschuh D, Dubs M-C, Weiss E, Zeder-Lutz G, van Regenmortel MHV (1992) Determination of kinetic constants for the interaction between a monoclonal antibody and peptides using surface plasmon resonance. Biochemistry 31:6298–6304. https://doi.org/10.1021/bi00142a019
Arachchillaya B (2018) Development and evaluation of a paper based biochemical sensor for realtime detection of food pathogen. Bachelor Proj. 2018
Ariga K, McShane M, Lvov YM, Ji Q, Hill JP (2011) Layer-by-layer assembly for drug delivery and related applications. Expert Opin Drug Deliv 8:633–644
Arya H, Kaul Z, Wadhwa R, Taira K, Hirano T, Kaul SC (2005) Quantum dots in bio-imaging: revolution by the small. Biochem Biophys Res Commun 329:1173–1177
Babu B, Washburn BK, Miller SH, Poduch K, Sarigul T, Knox GW, Ochoa-Corona FM, Paret ML (2017) A rapid assay for detection of Rose rosette virus using reverse transcription-recombinase polymerase amplification using multiple gene targets. J Virol Methods 240:78–84
Balasubramanian S, Sorokulova IB, Vodyanoy VJ, Simonian AL (2007) Lytic phage as a specific and selective probe for detection of Staphylococcus aureus—a surface plasmon resonance spectroscopic study. Biosens Bioelectron 22:948–955
Balogh B, Jones JB, Iriarte FB, Momol MT (2010) Phage therapy for plant disease control. Curr Pharm Biotechnol 11:48–57
Barth R, IJsselmuiden J, Hemming J, Van Henten EJ (2018) Data synthesis methods for semantic segmentation in agriculture: a Capsicum annuum dataset. Comput Electron Agric 144:284–296
Batchelor-McAuley C, Wildgoose GG, Compton RG (2009) The physicochemical aspects of DNA sensing using electrochemical methods. Biosens Bioelectron 24:3183–3190
Belkin S (2003) Microbial whole-cell sensing systems of environmental pollutants. Curr Opin Microbiol 6:206–212
Bergwerff AA, Van Knapen F (2006) Surface plasmon resonance biosensors for detection of pathogenic microorganisms: strategies to secure food and environmental safety. J AOAC Int 89:826–831
Blackmore BS (2007) A systems view of agricultural robots. In: Proceedings 6th European conference on precision agriculture (ECPA), pp 23–31
Boltovets PM, Boyko VR, Kostikov IY, Dyachenko NS, Snopok BA, Shirshov YM (2002) Simple method for plant virus detection: effect of antibody immobilization technique. J Virol Methods 105:141–146
Boonham N, Glover R, Tomlinson J, Mumford R (2008) Exploiting generic platform technologies for the detection and identification of plant pathogens. Sustainable disease management in a European context. Springer, In, pp 355–363
Boonham N, Tomlinson J, Mumford R (2007) Microarrays for rapid identification of plant viruses. Annu Rev Phytopathol 45:307–328
Bragazzi NL, Amicizia D, Panatto D, et al (2015) Quartz-crystal microbalance (QCM) for public health: an overview of its applications. 1st edn. Elsevier Inc.
Candresse T, Svanella-Dumas L, Gentit P, Caglayan K, Çevik B (2007) First report of the presence of Plum pox virus Rec strain in Turkey. Plant Dis 91:331
Cecchini F, Manzano M, Mandabi Y, Perelman E, Marks RS (2012) Chemiluminescent DNA optical fibre sensor for Brettanomyces bruxellensis detection. J Biotechnol 157:25–30
Champoiseau PG, Jones JB, Allen C (2009) Ralstonia solanacearum Race 3 Biovar 2 causes tropical losses and temperate anxieties. Plant Heal Prog 10:35. https://doi.org/10.1094/php-2009-0313-01-rv
Chartuprayoon N, Hangarter CM, Rheem Y, Jung H, Myung NV (2010) Wafer-scale fabrication of single polypyrrole nanoribbon-based ammonia sensor. J Phys Chem C 114:11103–11108
Chartuprayoon N, Rheem Y, Ng JCK, Nam J, Chen W, Myung NV (2013) Polypyrrole nanoribbon based chemiresistive immunosensors for viral plant pathogen detection. Anal Methods 5:3497–3502
Chen H, Heng CK, Puiu PD, Zhou XD, Lee AC, Lim TM, Tan SN (2005) Detection of Saccharomyces cerevisiae immobilized on self-assembled monolayer (SAM) of alkanethiolate using electrochemical impedance spectroscopy. Anal Chim Acta 554:52–59
Chen M, Qin X, Zeng G (2016) Single-walled carbon nanotube release affects the microbial enzyme-catalyzed oxidation processes of organic pollutants and lignin model compounds in nature. Chemosphere 163:217–226. https://doi.org/10.1016/j.chemosphere.2016.08.031
Chen M, Zhou S, Zhu Y, Sun Y, Zeng G, Yang C, Xu P, Yan M, Liu Z, Zhang W (2018a) Toxicity of carbon nanomaterials to plants, animals and microbes: recent progress from 2015-present. Chemosphere 206:255–264. https://doi.org/10.1016/j.chemosphere.2018.05.020
Chen Y, Wang Z, Liu Y, Wang X, Li Y, Ma P, Gu B, Li H (2018b) Recent advances in rapid pathogen detection method based on biosensors. Eur J Clin Microbiol Infect Dis 37:1021–1037. https://doi.org/10.1007/s10096-018-3230-x
Chiriacò MS, Luvisi A, Primiceri E, Sabella E, de Bellis L, Maruccio G (2018) Development of a lab-on-a-chip method for rapid assay of Xylella fastidiosa subsp. pauca strain CoDiRO. Sci Rep 8:1–8. https://doi.org/10.1038/s41598-018-25747-4
Coy MR, Hoffmann M, Kingdom Gibbard HN, Kuhns EH, Pelz-Stelinski KS, Stelinski LL (2014) Nested-quantitative PCR approach with improved sensitivity for the detection of low titer levels of Candidatus Liberibacter asiaticus in the Asian citrus psyllid, Diaphorina citri Kuwayama. J Microbiol Methods 102:15–22. https://doi.org/10.1016/j.mimet.2014.04.007
Cui F, Xu Y, Wang R, Liu H, Chen L, Zhang Q, Mu X (2018a) Label-free impedimetric glycan biosensor for quantitative evaluation interactions between pathogenic bacteria and mannose. Biosens Bioelectron 103:94–98. https://doi.org/10.1016/j.bios.2017.11.068
Cui Z, Luan X, Jiang H, Li Q, Xu G, Sun C, Zheng L, Song Y, Davison PA, Huang WE (2018b) Application of a bacterial whole cell biosensor for the rapid detection of cytotoxicity in heavy metal contaminated seawater. Chemosphere 200:322–329. https://doi.org/10.1016/j.chemosphere.2018.02.097
Daher RK, Stewart G, Boissinot M, Boudreau DK, Bergeron MG (2015) Influence of sequence mismatches on the specificity of recombinase polymerase amplification technology. Mol Cell Probes 29:116–121
Daly P, Collier T, Doyle S (2002) PCR-ELISA detection of Escherichia coli in milk. Lett Appl Microbiol 34:222–226
Dickert FL, Hayden O (2002) Bioimprinting of polymers and sol− gel phases. Selective detection of yeasts with imprinted polymers. Anal Chem 74:1302–1306
Dimov IK, Garcia-Cordero JL, O’grady J et al (2008) Integrated microfluidic tmRNA purification and real-time NASBA device for molecular diagnostics. Lab Chip 8:2071–2078
Ding F, Duan Y, Yuan Q, Shao J, Hartung JS (2016) Serological detection of Candidatus Liberibacter asiaticus’ in citrus, and identification by GeLC-MS/MS of a chaperone protein responding to cellular pathogens. Sci Rep 6. https://doi.org/10.1038/srep29272
Djelouah K, Frasheri D, Valentini F et al (2014) Direct tissue blot immunoassay for detection of Xylella fastidiosa in olive trees. Phytopathol Mediterr 559–564
Drygin YF, Blintsov AN, Grigorenko VG, Andreeva IP, Osipov AP, Varitzev YA, Uskov AI, Kravchenko DV, Atabekov JG (2012) Highly sensitive field test lateral flow immunodiagnostics of PVX infection. Appl Microbiol Biotechnol 93:179–189
Dubertret B, Calame M, Libchaber AJ (2001) Single-mismatch detection using gold-quenched fluorescent oligonucleotides. Nat Biotechnol 19:365–370
Dudak FC, Boyacı İH (2009) Rapid and label-free bacteria detection by surface plasmon resonance (SPR) biosensors. Biotechnol J Healthc Nutr Technol 4:1003–1011
Duhan JS, Kumar R, Kumar N, Kaur P, Nehra K, Duhan S (2017) Nanotechnology: the new perspective in precision agriculture. Biotechnol Reports 15:11–23
Etefagh R, Azhir E, Shahtahmasebi N (2013) Synthesis of CuO nanoparticles and fabrication of nanostructural layer biosensors for detecting Aspergillus niger fungi. Sci Iran 20:1055–1058
Eun AJ, Huang L, Chew F (2002) Detection of two orchid viruses using quartz crystal microbalance (QCM) immunosensors. J Virol Methods 99:71–79
Fang Y, Ramasamy RP (2015) Current and prospective methods for plant disease detection. Biosensors 5:537–561. https://doi.org/10.3390/bios5030537
Fang Z, Wu W, Lu X, Zeng L (2014) Lateral flow biosensor for DNA extraction-free detection of salmonella based on aptamer mediated strand displacement amplification. Biosens Bioelectron 56:192–197
Fountas S, Mylonas N, Malounas I, Rodias E, Hellmann Santos C, Pekkeriet E (2020) Agricultural robotics for field operations. Sensors (Switzerland) 20:1–27. https://doi.org/10.3390/s20092672
Fukuta S, Kato S, Yoshida K, Mizukami Y, Ishida A, Ueda J, Kanbe M, Ishimoto Y (2003) Detection of tomato yellow leaf curl virus by loop-mediated isothermal amplification reaction. J Virol Methods 112:35–40
Gaggiotti S, Della Pelle F, Mascini M, Cichelli A, Compagnone D (2020) Peptides, DNA and MIPs in gas sensing. From the realization of the sensors to sample analysis. Sensors (Basel) 20(16):4433. https://doi.org/10.3390/s20164433
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X (2007) Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol 2:577–583
Ghorbanpour M, Hadian J (2015) Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon N Y 94:749–759
Ghosh DK, Motghare M, Gowda S (2018) Citrus greening: overview of the most severe disease of citrus. Adv Agric Res Technol J 2:83–100
Goodridge L, Chen J, Griffiths M (1999) The use of a fluorescent bacteriophage assay for detection of Escherichia coli O157: H7 in inoculated ground beef and raw milk. Int J Food Microbiol 47:43–50
Grabowska I, Malecka K, Jarocka U, Radecki J, Radecka H (2014) Electrochemical biosensors for detection of avian influenza virus--current status and future trends. Acta Biochim Pol 61
Gutiérrez-Aguirre I, Mehle N, Delić D, Gruden K, Mumford R, Ravnikar M (2009) Real-time quantitative PCR based sensitive detection and genotype discrimination of Pepino mosaic virus. J Virol Methods 162:46–55
He P, Liu L, Qiao W, Zhang S (2014) Ultrasensitive detection of thrombin using surface plasmon resonance and quartz crystal microbalance sensors by aptamer-based rolling circle amplification and nanoparticle signal enhancement. Chem Commun 50:1481–1484. https://doi.org/10.1039/c3cc48223e
Jiang B, Zhu D, Song Y, Zhang D, Liu Z, Zhang X, Huang WE, Li G (2015) Use of a whole-cell bioreporter, Acinetobacter baylyi, to estimate the genotoxicity and bioavailability of chromium (VI)-contaminated soils. Biotechnol Lett 37:343–348
Jiang T, Halsall HB, Heineman WR, Giersch T, Hock B (1995) Capillary enzyme immunoassay with electrochemical detection for the determination of atrazine in water. J Agric Food Chem 43:1098–1104
Jiao K, Sun W, Zhang S-S (2000) Sensitive detection of a plant virus by electrochemical enzyme-linked immunoassay. Fresenius J Anal Chem 367:667–671
Julich S, Riedel M, Kielpinski M, Urban M, Kretschmer R, Wagner S, Fritzsche W, Henkel T, Möller R, Werres S (2011) Development of a lab-on-a-chip device for diagnosis of plant pathogens. Biosens Bioelectron 26:4070–4075. https://doi.org/10.1016/j.bios.2011.03.035
Katoh H, Yamazaki S, Fukuda T, Sonoda S, Nishigawa H, Natsuaki T (2020) Detection of Fusarium oxysporum f. sp. fragariae by using loop-mediated isothermal amplification. Plant Dis. doi: 10.1094/PDIS-03-20-0590-RE
Kattke MD, Gao EJ, Sapsford KE, Stephenson LD, Kumar A (2011) FRET-based quantum dot immunoassay for rapid and sensitive detection of Aspergillus amstelodami. Sensors 11:6396–6410
Keinan A, Clark AG (2012) Recent explosive human population growth has resulted in an excess of rare genetic variants. Science 336:740–743. https://doi.org/10.1126/science.1217283
Keremane ML, Ramadugu C, Rodriguez E, Kubota R, Shibata S, Hall DG, Roose ML, Jenkins D, Lee RF (2015) A rapid fi eld detection system for citrus huanglongbing associated ‘Candidatus Liberibacter asiaticus’ from the psyllid vector, Diaphorina citri Kuwayama and its implications in disease management. Crop Prot 68:41–48. https://doi.org/10.1016/j.cropro.2014.10.026
Khater M, de la Escosura-Muñiz A, Merkoçi A (2017) Biosensors for plant pathogen detection. Biosens Bioelectron 93:72–86. https://doi.org/10.1016/j.bios.2016.09.091
Khedri M, Ramezani M, Rafatpanah H, Abnous K (2018) Detection of food-born allergens with aptamer-based biosensors. TrAC - Trends Anal Chem 103:126–136. https://doi.org/10.1016/j.trac.2018.04.001
Khiyami MA, Almoammar H, Awad YM, Alghuthaymi MA, Abd-Elsalam KA (2014) Plant pathogen nanodiagnostic techniques: forthcoming changes? Biotechnol Biotechnol Equip 28:775–785
Lau HY, Botella JR (2017) Advanced DNA-Based Point-of-Care Diagnostic Methods for Plant Diseases Detection. In: Advanced DNA-based point-of-care diagnostic methods for plant diseases detection. Front, Plant Sci
Lau HY, Wang Y, Wee EJH, Botella JR, Trau M (2016) Field demonstration of a multiplexed point-of-care diagnostic platform for plant pathogens. Anal Chem 88:8074–8081
Lautner G, Balogh Z, Bardóczy V, Mészáros T, Gyurcsányi RE (2010) Aptamer-based biochips for label-free detection of plant virus coat proteins by SPR imaging. Analyst 135:918–926
Lee HY, Jung HS, Fujikawa K, Park JW, Kim JM, Yukimasa T, Sugihara H, Kawai T (2005) New antibody immobilization method via functional liposome layer for specific protein assays. Biosens Bioelectron 21:833–838
Li W, Levy L, Hartung JS (2009) Quantitative distribution of ‘Candidatus Liberibacter asiaticus’ in citrus plants with Citrus Huanglongbing. Phytopathology 99:139–144. https://doi.org/10.1094/phyto-99-2-0139
Lillis B, Hurley E, Berney H et al (2006) Investigation into the effect that probe immobilisation method type has on the analytical signal of an EIS DNA biosensor. Biosens Bioelectron 22:1289–1295. https://doi.org/10.1016/j.bios.2006.05.021
Lin H-Y, Huang C-H, Lu S-H, Kuo IT, Chau LK (2014a) Direct detection of orchid viruses using nanorod-based fiber optic particle plasmon resonance immunosensor. Biosens Bioelectron 51:371–378
Lin H, Zhang W, Jia S, Guan Z, Yang CJ, Zhu Z (2014b) Microfluidic approaches to rapid and efficient aptamer selection. Biomicrofluidics 8:1–15. https://doi.org/10.1063/1.4890542
Lin LH, Ntambo MS, Rott PC, Wang QN, Lin YH, Fu HY, Gao SJ (2018) Molecular detection and prevalence of Xanthomonas albilineans, the causal agent of sugarcane leaf scald, in China. Crop Prot 109:17–23. https://doi.org/10.1016/j.cropro.2018.02.027
López MM, Llop P, Olmos A, Marco-Noales E, Cambra M, Bertolini E (2009) Are molecular tools solving the challenges posed by detection of plant pathogenic bacteria and viruses? Curr Issues Mol Biol 11:13–46
Luka G, Ahmadi A, Najjaran H, Alocilja E, DeRosa M, Wolthers K, Malki A, Aziz H, Althani A, Hoorfar M (2015) Microfluidics integrated biosensors: a leading technology towards lab-on-a-chip and sensing applications. Sensors (Switzerland) 15:30011–30031. https://doi.org/10.3390/s151229783
Madufor NJK, Perold WJ, Opara UL (2018) Detection of plant diseases using biosensors: a review. Acta Hortic 1201:83–90. https://doi.org/10.17660/ActaHortic.2018.1201.12
Mahato K, Srivastava A, Chandra P (2017) Paper based diagnostics for personalized health care: emerging technologies and commercial aspects. Biosens Bioelectron 96:246–259
Mahmud MS, Zaman QU, Esau TJ et al (2019) Development of an artificial cloud lighting condition system using machine vision for strawberry powdery mildew disease detection. Comput Electron Agric 158:219–225
Martinelli F, Scalenghe R, Davino S, Panno S, Scuderi G, Ruisi P, Villa P, Stroppiana D, Boschetti M, Goulart LR, Davis CE, Dandekar AM (2015) Advanced methods of plant disease detection. A review. Agron Sustain Dev 35:1–25
Martinez AW (2011) Microfluidic paper-based analytical devices: from POCKET to paper-based ELISA. Bioanalysis 3:2589–2592
Masdor NA, Altintas Z, Shukor MY, Tothill IE (2019) Subtractive inhibition assay for the detection of Campylobacter jejuni in chicken samples using surface plasmon resonance. Sci Rep 9:1–10
Mendes RK, Carvalhal RF, Stach-Machado DR, Kubota LT (2009) Surface plasmon resonance immunosensor for early diagnosis of Asian rust on soybean leaves. Biosens Bioelectron 24:2483–2487
Miles GP, Stover E, Ramadugu C et al (2017) Apparent tolerance to Huanglongbing in citrus and citrus-related germplasm. HortScience 52:31–39. https://doi.org/10.21273/hortsci11374-16
Miller DAB (2009) Device requirements for optical interconnects to silicon chips. Proc IEEE 97:1166–1185
Miranda BS, Linares EM, Thalhammer S, Kubota LT (2013) Development of a disposable and highly sensitive paper-based immunosensor for early diagnosis of Asian soybean rust. Biosens Bioelectron 45:123–128
Mudgal N, Yupapin P, Ali J, Singh G (2020) BaTiO 3-graphene-affinity layer–based surface plasmon resonance (SPR) biosensor for Pseudomonas bacterial detection. Plasmonics 1–9
Noi K, Iijima M, Kuroda S, Ogi H (2019) Ultrahigh-sensitive wireless QCM with bio-nanocapsules. Sensors Actuators B Chem 293:59–62
Noori JS, Mortensen J, Geto A (2020) Recent Development on the Electrochemical Detection of Selected Pesticides: A Focused Review. Recent development on the electrochemical detection of selected pesticides: a focused review. Sensors (Switzerland) 20:20. https://doi.org/10.3390/s20082221
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:e63
Oh SY, Heo NS, Shukla S et al (2017) Development of gold nanoparticle-aptamer-based LSPR sensing chips for the rapid detection of Salmonella typhimurium in pork meat. Sci Rep 7:1–10
Ozalp VC, Bayramoglu G, Erdem Z, Arica MY (2015) Pathogen detection in complex samples by quartz crystal microbalance sensor coupled to aptamer functionalized core–shell type magnetic separation. Anal Chim Acta 853:533–540
Papadakis G, Skandalis N, Dimopoulou A, Glynos P, Gizeli E (2015) Bacteria murmur: application of an acoustic biosensor for plant pathogen detection. PLoS One 10(7):e0132773. https://doi.org/10.1371/journal.pone.0132773
Park M-K, Li S, Chin BA (2013a) Detection of Salmonella typhimurium grown directly on tomato surface using phage-based magnetoelastic biosensors. Food Bioprocess Technol 6:682–689
Park M-K, Park JW, Wikle HC III, Chin BA (2013b) Evaluation of phage-based magnetoelastic biosensors for direct detection of Salmonella typhimurium on spinach leaves. Sensors Actuators B Chem 176:1134–1140
Paternolli C, Antonini M, Ghisellini P, Nicolini C (2004) Recombinant cytochrome P450 immobilization for biosensor applications. Langmuir 20:11706–11712. https://doi.org/10.1021/la048081q
Patil KN, Singh P, Muniyappa K (2011) DNA binding, coprotease, and strand exchange activities of mycobacterial RecA proteins: implications for functional diversity among RecA nucleoprotein filaments. Biochemistry 50:300–311
Pedersen SM, Fountas S, Have H, Blackmore BS (2006) Agricultural robots—system analysis and economic feasibility. Precis Agric 7:295–308
Piepenburg O, Williams CH, Stemple DL, Armes NA (2006) DNA detection using recombination proteins. PLoS Biol 4:e204
Pilli SK, Nallathambi B, George SJ, Diwanji V (2015) eAGROBOT—a robot for early crop disease detection using image processing. In: 2015 2nd International Conference on Electronics and Communication Systems (ICECS). IEEE, pp 1684–1689
Pohanka M, Jun D, Kuca K (2007) Mycotoxin assays using biosensor technology: a review. Drug Chem Toxicol 30:253–261
Poitras C, Tufenkji N (2009) A QCM-D-based biosensor for E. coli O157: H7 highlighting the relevance of the dissipation slope as a transduction signal. Biosens Bioelectron 24:2137–2142
Priyanka B, Patil RK, Dwarakanath S (2016) A review on detection methods used for foodborne pathogens. Indian J Med Res 144:327–338
Qian W, Lu Y, Meng Y, Ye Z, Wang L, Wang R, Zheng Q, Wu H, Wu J (2018) Field detection of citrus Huanglongbing associated with ‘Candidatus Liberibacter Asiaticus’ by recombinese polymerase amplification within 15 min. J Agric Food Chem 66:5473–5480
Rad F, Mohsenifar A, Tabatabaei M et al (2012) Detection of Candidatus Phytoplasma aurantifolia with a quantum dots fret-based biosensor. J Plant Pathol 94:525–534
Ramnani P, Saucedo NM, Mulchandani A (2016) Carbon nanomaterial-based electrochemical biosensors for label-free sensing of environmental pollutants. Chemosphere 143:85–98. https://doi.org/10.1016/j.chemosphere.2015.04.063
Rani A, Donovan N, Mantri N (2019) Review: The future of plant pathogen diagnostics in a nursery production system. Biosens Bioelectron 111631:111631. https://doi.org/10.1016/j.bios.2019.111631
Ray M, Ray A, Dash S, Mishra A, Achary KG, Nayak S, Singh S (2017) Fungal disease detection in plants: traditional assays, novel diagnostic techniques and biosensors. Biosens Bioelectron 87:708–723. https://doi.org/10.1016/j.bios.2016.09.032
Rey B, Aleixos N, Cubero S, Blasco J (2019) XF-ROVIM. A field robot to detect olive trees infected by Xylella fastidiosa using proximal sensing. Remote Sens 11:221
Roberts MJ (2006) The value of plant disease early-warning systems: a case study of USDA's soybean rust coordinated framework (No. 18). USDA Economic Research Service
Rohrman BA, Richards-Kortum RR (2012) A paper and plastic device for performing recombinase polymerase amplification of HIV DNA. Lab Chip 12(17):3082–3088. https://doi.org/10.1039/c2lc40423k
Rosset P (2008) Food sovereignty and the contemporary food crisis. Development 51:460–463. https://doi.org/10.1057/dev.2008.48
Rossier JS, Girault HH (2001) Enzyme linked immunosorbent assay on a microchip with electrochemical detection. Lab Chip 1:153–157
Ruiz-Ruiz S, Moreno P, Guerri J, Ambrós S (2009) Discrimination between mild and severe Citrus tristeza virus isolates with a rapid and highly specific real-time reverse transcription-polymerase chain reaction method using TaqMan LNA probes. Phytopathology 99:307–315
Sadani K, Nag P, Mukherji S (2019) LSPR based optical fiber sensor with chitosan capped gold nanoparticles on BSA for trace detection of Hg (II) in water, soil and food samples. Biosens Bioelectron 134:90–96. https://doi.org/10.1016/j.bios.2019.03.046
Safarpour H, Safarnejad MR, Tabatabaei M, Mohsenifar A, Rad F, Basirat M, Shahryari F, Hasanzadeh F (2012) Development of a quantum dots FRET-based biosensor for efficient detection of Polymyxa betae. Can J plant Pathol 34:507–515
Sakakibara M, Ohmori Y, Ha NTH, Sano S, Sera K (2011) Phytoremediation of heavy metal-contaminated water and sediment by Eleocharis acicularis. CLEAN–Soil, Air, Water 39:735–741
Savary S, Ficke A, Aubertot JN, Hollier C (2012) Crop losses due to diseases and their implications for global food production losses and food security. Food Secur 4:519–537. https://doi.org/10.1007/s12571-012-0200-5
Scala A, Allmann S, Mirabella R, et al (2013) Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. 17781–17811. https://doi.org/10.3390/ijms140917781
Schor N, Bechar A, Ignat T, Dombrovsky A, Elad Y, Berman S (2016) Robotic disease detection in greenhouses: combined detection of powdery mildew and tomato spotted wilt virus. IEEE Robot Autom Lett 1:354–360
Schor N, Berman S, Dombrovsky A, Elad Y, Ignat T, Bechar A (2017) Development of a robotic detection system for greenhouse pepper plant diseases. Precis Agric 18:394–409
Senauer B, Sur M (2001) Ending global hunger in the 21st century: projections of the number of food insecure people. Rev Agric Econ 23:68–81. https://doi.org/10.1111/1058-7195.00046
Seok Kim Y, Ahmad Raston NH, Bock Gu M (2016) Aptamer-based nanobiosensors. Biosens Bioelectron 76:2–19. https://doi.org/10.1016/j.bios.2015.06.040
Shen F, Davydova EK, Du W et al (2011) Digital isothermal quantification of nucleic acids via simultaneous chemical initiation of recombinase polymerase amplification reactions on SlipChip. Anal Chem 83:3533–3540
Siddiquee S, Rovina K, Yusof NA, Rodrigues KF, Suryani S (2014) Nanoparticle-enhanced electrochemical biosensor with DNA immobilization and hybridization of Trichoderma harzianum gene. Sens Bio-Sensing Res 2:16–22
Singh A, Poshtibtiban S, Evoy S (2013) Recent advances in bacteriophage based biosensors for food-borne pathogen detection. Sensors (Basel) 13(2):1763–1786. https://doi.org/10.3390/s130201763
Skottrup P, Hearty S, Frøkiær H, Leonard P, Hejgaard J, O’Kennedy R, Nicolaisen M, Justesen AF (2007) Detection of fungal spores using a generic surface plasmon resonance immunoassay. Biosens Bioelectron 22:2724–2729
Skottrup PD, Nicolaisen M, Justesen AF (2008) Towards on-site pathogen detection using antibody-based sensors. Biosens Bioelectron 24:339–348. https://doi.org/10.1016/j.bios.2008.06.045
Strange RN, Scott PR (2005) Plant disease: a threat to global food security. Annu Rev Phytopathol 43:83–116. https://doi.org/10.1146/annurev.phyto.43.113004.133839
Sun K, Xing W, Yu X et al (2016) Recombinase polymerase amplification combined with a lateral flow dipstick for rapid and visual detection of Schistosoma japonicum. Parasit Vectors 9:1–9
Syedmoradi L, Gomez FA (2017) Paper-based point-of-care testing in disease diagnostics. Bioanalysis 9(11):841–843. https://doi.org/10.4155/bio-2017-0080
Syedmoradi L, Daneshpour M, Alvandipour M, Gomez FA, Hajghassem H, Omidfar K (2017) Point of care testing: the impact of nanotechnology. Biosens Bioelectron 87:373–387
Tang Y, Xing D, Zhu D, Liu J (2007) An improved electrochemiluminescence polymerase chain reaction method for highly sensitive detection of plant viruses. Anal Chim Acta 582:275–280
Tao X, He Y, Fortner JD, Chen Y, Hughes JB (2013) Effects of aqueous stable fullerene nanocrystal (nC60) on copper (trace necessary nutrient metal): enhanced toxicity and accumulation of copper in Daphnia magna. Chemosphere 92:1245–1252. https://doi.org/10.1016/j.chemosphere.2013.04.056
Thompson RQ, Barone GC III, Halsall HB, Heineman WR (1991) Comparison of methods for following alkaline phosphatase catalysis: spectrophotometric versus amperometric detection. Anal Biochem 192:90–95
Tomita N, Mori Y, Kanda H, Notomi T (2008) Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat Protoc 3:877–882
Vaseghi A, Safaie N, Bakhshinejad B et al (2017) Advances in biosensors: principle, architecture and applications. Biosens Bioelectron 12:1–15. https://doi.org/10.1016/j.jab.2013.02.001
Vidal E, Torre-Cisneros J, Blanes M, Montejo M, Cervera C, Aguado JM, Len O, Carratalá J, Cordero E, Bou G, Muñoz P, Ramos A, Gurguí M, Borrell N, Fortún J, on behalf of the Spanish Network for Research in Infectious Diseases (REIPI) (2012a) Bacterial urinary tract infection after solid organ transplantation in the RESITRA cohort. Transpl Infect Dis 14:595–603
Vidal E, Yokomi RK, Moreno A, Bertolini E, Cambra M (2012b) Calculation of diagnostic parameters of advanced serological and molecular tissue-print methods for detection of Citrus tristeza virus: a model for other plant pathogens. Phytopathology. 102:114–121. https://doi.org/10.1094/PHYTO-05-11-0139
Vincelli P, Tisserat N (2008) Nucleic acid-based pathogen detection in applied plant pathology. Plant Dis 92:660–669. https://doi.org/10.1094/PDIS-92-5-0660
Wang C, Yao Z, Bai L, Wang C, Jiang H (2019) Application of a microbial fuel cell-based biosensor for the energy-saving operation of macrophyte residues bioreactor with low concentration of dissolved organic carbon in effluents. Chemosphere 220:1075–1082. https://doi.org/10.1016/j.chemosphere.2018.12.209
Wang L, Li PCH (2010) Optimization of a microfluidic microarray device for the fast discrimination of fungal pathogenic DNA. Anal Biochem 400:282–288
Wang YX, Ye ZZ, Si CY, Bin Ying Y (2012) Application of aptamer based biosensors for detection of pathogenic microorganisms. Fenxi Huaxue/ Chinese J Anal Chem 40:634–642. https://doi.org/10.1016/S1872-2040(11)60542-2
Wang Z, Yin Y, Hu H, Yuan Q, Peng G, Xia Y (2006) Development and application of molecular-based diagnosis for “Candidatus Liberibacter asiaticus”, the causal pathogen of citrus huanglongbing. Plant Pathol 55:630–638. https://doi.org/10.1111/j.1365-3059.2006.01438.x
Wee EJH, Lau HY, Botella JR, Trau M (2015) Re-purposing bridging flocculation for on-site, rapid, qualitative DNA detection in resource-poor settings. Chem Commun 51:5828–5831
Wilson JS, Mann CL, Otsuki T (2005) Assessing the benefits of trade facilitation: a global perspective. World Econ 28:841–871
Wongkaew P, Poosittisak S (2014) Diagnosis of sugarcane white leaf disease using the highly sensitive DNA based voltammetric electrochemical determination. Am J Plant Sci 5(5):2256–2268
Wu X, Meng C, Wang G, Liu Y, Zhang X, Yi K, Peng J (2016) Rapid and quantitative detection of citrus Huanglongbing bacterium ‘Candidatus Liberibacter asiaticus’ by real-time fluorescent loop-mediated isothermal amplification assay in China. Physiol Mol Plant Pathol 94:1–7
Xu L, Yu W, Graham N, Zhao Y (2021) Chemosphere revisiting the bioelectrochemical system based biosensor for organic sensing and the prospect on constructed wetland-microbial fuel cell. Chemosphere 264:128532. https://doi.org/10.1016/j.chemosphere.2020.128532
Yaman F (2011) The costs of adjusting labor: evidence from temporally disaggregated data
Yao KS, Li SJ, Tzeng KC, Cheng TC, Chang CY, Chiu CY, Liao Cy, Hsu JJ, Lin ZP (2009) Fluorescence silica nanoprobe as a biomarker for rapid detection of plant pathogens. In: Advanced materials research, vol 79. Trans Tech Publications Ltd., pp 513–516
Yue H, He Y, Fan E, Wang L, Lu S, Fu Z (2017) Label-free electrochemiluminescent biosensor for rapid and sensitive detection of Pseudomonas aeruginosa using phage as highly specific recognition agent. Biosens Bioelectron 94:429–432
Zhan F, Wang T, Iradukunda L, Zhan J (2018) A gold nanoparticle-based lateral flow biosensor for sensitive visual detection of the potato late blight pathogen, Phytophthora infestans. Anal Chim Acta 1036:153–161. https://doi.org/10.1016/j.aca.2018.06.083
Zhang S, Ravelonandro M, Russell P, McOwen N, Briard P, Bohannon S, Vrient A (2014) Rapid diagnostic detection of plum pox virus in Prunus plants by isothermal AmplifyRP® using reverse transcription-recombinase polymerase amplification. J Virol Methods 207:114–120
Zhang X, Zhang H, Pu J et al (2013) Development of a real-time fluorescence loop-mediated isothermal amplification assay for rapid and quantitative detection of Fusarium oxysporum f. sp. cubense tropical race 4 in soil. PLoS One 8:e82841
Zhao W, Lu J, Ma W, Xu C, Kuang H, Zhu S (2011) Biosensors and bioelectronics rapid on-site detection of Acidovorax avenae subsp. citrulli by gold-labeled DNA strip sensor. Biosens Bioelectron 26:4241–4244. https://doi.org/10.1016/j.bios.2011.04.004
Zhao Y, Chen F, Li Q, Wang L, Fan C (2015) Isothermal amplification of nucleic acids. Chem Rev 115:12491–12545
Zhao Y, Liu L, Kong D, Kuang H, Wang L, Xu C (2014) Dual amplified electrochemical immunosensor for highly sensitive detection of Pantoea stewartii sbusp. stewartii. ACS Appl Mater Interfaces 6:21178–21183. https://doi.org/10.1021/am506104r
Zheng Y-Y, Kong J-L, Jin X-B, Wang XY, Zuo M (2019) CropDeep: The crop vision dataset for deep-learning-based classification and detection in precision agriculture. Sensors 19:1058
Zou Y, Mason MG, Wang Y, Wee E, Turni C, Blackall PJ, Trau M, Botella JR (2017) Nucleic acid purification from plants, animals and microbes in under 30 seconds. PLoS Biol 15:e2003916
Zuo P, Li X, Dominguez DC, Ye BC (2013) A PDMS/paper/glass hybrid microfluidic biochip integrated with aptamer-functionalized graphene oxide nano-biosensors for one-step multiplexed pathogen detection. Lab Chip 13:3921–3928. https://doi.org/10.1039/c3lc50654a
Acknowledgments
The authors highly acknowledge the efforts of Dr. Muhammad Rizwan, Dr. Freddy Mora-Poblete, and Mr. Muhammad Kamran for their contribution to improve the quality of this review paper.
Funding
This study was funded by the Chilean National Fund for Scientific and Technological Development (FONDECYT) under grant number-1201973.
Author information
Authors and Affiliations
Contributions
We thank Q.A. for the conceptualization and writing the original draft of this review article; S.A, M.A.S, M.K, M.A, M.H.S, M.M, and M.R. for reviewing and editing the first draft; A.M.A. and M.R. for the validation of review contents; and F.M.P, M.K, A.T.A.J., and S.A. for the supervision and funding resources.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, Q., Ahmar, S., Sohail, M.A. et al. Research advances and applications of biosensing technology for the diagnosis of pathogens in sustainable agriculture. Environ Sci Pollut Res 28, 9002–9019 (2021). https://doi.org/10.1007/s11356-021-12419-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-12419-6