Abstract
Previous studies have suggested that maternal exposure to air pollution might affect term birth weight. However, the conclusions are controversial. Birth data of all term newborns born in Xi’an city of Shaanxi, China, from 2015 to 2018 and whose mother lived in Xi’an during pregnancy were selected form the Birth Registry Database. And the daily air quality data of Xi’an city was collected from Chinese Air Quality Online Monitoring and Analysis Platform. Generalized additive models (GAM) and 2-level binary logistic regression models were used to estimate the effects of air pollution exposure on term birth weight, the risk term low birth weight (TLBW), and macrosomia. Finally, 321521 term newborns were selected, including 4369(1.36%) TLBW infants and 24,960 (7.76%) macrosomia. The average pollution levels of PM2.5, PM10, and NO2 in Xi’an city from 2015 to 2018 were higher than national limits. During the whole pregnancy, maternal exposure to PM2.5, PM10, SO2, and CO all significantly reduced the term birth weight and increased the risk of TLBW. However, NO2 and O3 exposure have significantly increased the term birth weight, and O3 even increased the risk of macrosomia significantly. Those effects were also observed in the first and second trimesters of pregnancy. But during the third trimester, high level of air quality index (AQI) and maternal exposure to PM2.5, PM10, SO2, NO2, and CO increased the term birth weight and the risk of macrosomia, while O3 exposure was contrary to this effect. The findings suggested that prenatal exposure to air pollution might cause adverse impacts on term birth weight, and the effects varied with trimesters and pollutants, which provides further pieces of evidence for the adverse effects of air pollution exposure in heavy polluted-area on term birth weight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the rapid development of global industrialization and urbanization, the problem of ambient air pollution has been getting increasingly prominent, which is the largest health environmental risk affecting all regions, socioeconomic groups, and age groups (Brauer et al. 2016). Some studies have suggested that air pollutants might increase the risk of cardiovascular and respiratory diseases through inducing some abnormal reactions, such as oxidative stress and DNA methylation (Chen et al. 2020a; Chu et al. 2015; Kaufman et al. 2016).
As the most sensitive populations, pregnant women and newborns are more vulnerable to the adverse effects of ambient air pollution. Some epidemiological studies indicated that maternal exposure to air pollution had adverse effects on infant health, such as adverse birth outcome and respiratory and neurodevelopmental effects (Backes et al. 2013; Forns et al. 2018; Korten et al. 2017; Pedersen et al. 2017; Shang et al. 2019; Yorifuji et al. 2015). Among them, birth weight is one of the most important predictors of morbidity and mortality in neonates and childhood and adult morbidity. Abnormal birth weight (including low birth weight (LBW) and macrosomia) is generally associated with several long-term chronic diseases in adults, including cardiovascular disease, type 2 diabetes, and certain cancers (Moraitis et al. 2014; Zhang et al. 2014).
Increasing evidence suggested that ambient air pollution exposure might affect fetal growth and result in abnormal birth weight. A retrospective cohort study based on a large sample suggested that PM2.5 exposure during pregnancy might increase the risk of term low birth weight (TLBW), but not in other air pollutants, and it also suggested that 3% of LBW cases can be directly attributed to residential PM2.5 exposure higher than 13.8 μg/m3 during pregnancy (Smith et al. 2017). And this conclusion was consistent with other similar studies (Janssen et al. 2017; Li et al. 2019). But He et al. found that with the increase of SO2 during pregnancy, birth weight was significantly decreased, while birth weights were significantly increased with NO2 exposure (He et al. 2018). Therefore, studies on the effect of maternal exposure to air pollution on birth weight are still not uniform. And most studies have only estimated the effect of air pollution exposure during the whole pregnancy and lacked of exposure assessment at different trimesters of pregnancy, at which fetus will have different growth and development mechanisms that might lead to inconsistent effects of air pollution. In addition, few studies evaluated the effects of air pollution during pregnancy on birth weight gain and the risk of macrosomia.
Therefore, we conducted a retrospective cohort study with large sample in Xi’an city of Shaanxi province in northwestern China. In this study, the birth data of term newborns born from 2015 to 2018 in Xi’an city was collected from the birth registration system, and their birth weight was recorded. And prenatal exposure levels of air pollution at each trimester of pregnancy were calculated to estimate the effects of air pollution exposure during various trimesters of pregnancy on term birth weight, the risk of TLBW, and macrosomia.
Methods
Study population
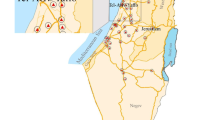
This study including all births in Xi’an city of Shaanxi province in China from January 2015 to December 2018 (n = 536993). The data on newborns were collected from the Birth Registry Database covering all midwifery clinics and hospitals in Xi’an city (Fig. 1).
In a total of 536993 newborns, we only selected newborns who lived in Xi’an city during pregnancy and recorded the exact residential address to the street as study objects (n = 349069). Among them, only term newborns with a gestational age of ≥ 37 weeks and < 42 weeks were enrolled (n = 332405). And those records in which maternal gestational age was missing or less than 15 years or older than 50 years were excluded (n = 254). In addition, some records with missed information of birth weight (n = 445) and ambiguous or incorrect residence (n = 1548) were also excluded. In addition, since exposure data on air pollution prior to May 13, 2014, were not available, we excluded pregnant women who were pregnant before this date (n = 8637). Finally, 321,521 newborns’ records were included in our study. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2017LSK-106).
Air pollution exposure
Real-time data of ambient air pollution exposure from May 13, 2014, to December 30, 2018, were provided by Chinese Air Quality Online Monitoring and Analysis Platform (https://www.aqistudy.cn/). It covered 13 air pollution monitoring sites in Xi’an city of Shaanxi province and originated mainly from the real-time data recorded by the Ministry of Ecology and Environment of the People’s Republic of China (http://www.mee.gov.cn/). Differential optical absorption spectroscopy method was used to analyze the concentration of NO2, SO2, CO, and O3, and the tapered element oscillating microbalance method was used to analyze the concentration of PM2.5 and PM10 automatically. AQI is an important indicator that presents the overall air pollution level, which is calculated according to the new ambient air quality standards (GB3095-2012). The definition of the used AQI is presented in the Supplementary Material (Bao et al. 2015), and the higher AQI presents more serious air pollution. The proportion of missing values is 0.5% for air pollutants. The past and future 7-day averages were used to impute the missing daily exposure data.
Maternal exposure estimates were calculated by pollutant concentrations measured at the nearest monitoring station to their residence. Prior to the assessment, the 24-h average of AQI, PM2.5, PM10, SO2, NO2, and CO and the 8-h average of O3 over all monitors were calculated, and the residences of the pregnant woman during pregnancy were collected from the birth registration information. Then, the latitudes and longitudes of all monitors and maternal residences were converted by geocoding. After the latitude and longitude distance differences from the residences to the monitoring stations were calculated, the ambient air pollution concentrations of the nearest monitor were assigned as individual exposure estimates. Finally, based on the start and end dates of each trimester of pregnancy, we calculated the average exposure levels of air pollution for each pregnant woman during the whole pregnancy (from pregnancy to delivery), the first trimester (from pregnancy to the 13th week of gestation), the second trimester (from the 14th to 27th week of gestation), and the third trimester (from the 28th week of gestation to delivery).
Outcomes and covariate data
Our main outcomes were term birth weight, TLBW, and macrosomia. Term birth weights for each newborn were collected from birth records directly. Term low birth weight (TLBW) was defined as birth weight less than 2500 g and gestational age of 37 weeks or more. And macrosomia was defined as birth weight equal or more than 4000 g. Meanwhile, maternal age, gestational age, ethnicity, and the date of birth of each newborn were all recorded. Pregnancy date was calculated based on birth date and gestational age to classify the season of beginning pregnancy (including warm season (from April to September) and cold season (from October to March of the next year)). And average temperature exposures during pregnancy were calculated based on the date of birth, gestational age, and hourly temperature distribution in Xi’an city from 2015 to 2018.
Statistical analysis
The average exposure values of AQI, PM2.5, PM10, SO2, NO2 CO, and O3 from 2015 to 2018 in Xi’an city were estimated based on hourly average exposure levels by the weighted average method. Generalized additive model (GAM) fitting in the Gaussian distribution was performed to evaluate the nonlinear relationship of air pollution exposure and birth weight (Shang et al. 2019). After fitting the related confounders, including maternal age, gestational age ethnicity, and pregnancy season, the degrees of freedom of confounders were calculated by using the Akaike information criterion (AIC). Then, the basic model was finally established as follows:
E(Yt) is the expected birth weight for newborn t, Xt represents the exposure level of air pollution for pregnant women t. Based on this model, the effects of per 10 unit increase of AQI, per 10 μg/m3 increase of PM2.5, PM10, SO2, NO2, O3, and per 0.1 mg/m3 increase of CO on term birth weight, were evaluated.
In addition, macrosomia or low birth weight infants were selected as the case group, while those infants with normal birth weight (2500 g ≤ birth weight and < 4000 g, n = 292192) were selected as a reference, and then 2-level binary logistic regression model was performed to investigate the associations between air pollution exposure and the risk of TLBW and macrosomia. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for the effects of per 0.1 mg/m3 increase of CO and per 10 unit increase of AQI, PM2.5, PM10, NO2, SO2, and O3 on the risk of TLBW and macrosomia.
Due to the non-significant relationship between average temperature and birth weight, we only included maternal age, gestational age, ethnic group, and pregnancy season as confounding factors in the final GAM and binary logistic regression model. All analyses were conducted using “mgcv (Mixed GAM Computation Vehicle)” and “glm” package and in R version 3.5.3. Significant results were considered at a P value lower than 0.05 (P < 0.05).
Results
Basic characteristic
A total of 321,521 newborns’ birth records were included in this study. The mean (10th–90th percentile) maternal age was 28.48 (23–34) years. And the Han ethnic group accounted for the majority (96.19%). The mean gestational age of newborns was 275.65 (265–287) days. And the mean birth weight was 3328.74 g. Among them, the incidence of TLBW and macrosomia were 1.36% (4369 cases) and 7.76% (24960 cases), respectively (Table 1).
The distribution of air pollution in Xi’an, Shaanxi province, from 2015 to 2018
The average levels of PM2.5, PM10, and NO2 in Xi’an city of Shaanxi province from 2015 to 2018 were all higher than the National Ambient Air Quality Standard (GB 3095–2012) limits. Among them, the concentration of PM10 exposure was the most severe. In addition, the exposure levels of SO2, CO, and O3 were lower than the National Ambient Air Quality Standard (GB 3095–2012) limits (Table 2).
The effect of maternal exposure to air pollution during whole pregnancy on term birth weight
During the whole pregnancy, per 10 μg/m3 increase of PM2.5, PM10, SO2, and per 0.1 mg/m3 increase of CO exposure all significantly reduced the term birth weight of newborns (β(95%CI) values were − 2.739 (− 3.693, − 1.785), − 2.458 (− 3.116, − 1.800), − 3.982 (− 5.511, − 2.453) and − 1.511 (− 1.970, − 1.053), respectively) and increased the risk of TLBW (OR (95%CI) values were 1.025 (1.005–1.045), 1.035 (1.020–1.049), 1.034 (1.004–1.065), and 1.013 (1.004–1.023), respectively). However, every 10 μg/m3 increase in NO2 (1.734 (0.533, 2.935)) and O3 (4.531 (3.239, 5.823)) significantly increased the birth weight of term newborns, and O3 even increased the risk of macrosomia significantly (1.028 (1.015–1.040)) (Table 3). In the generalized additive model, the exposure levels of air pollutants during the whole pregnancy explained 11.3 to 11.7% of the deviance in birth weight change.
The effect of maternal exposure to air pollution during various periods of pregnancy on term birth weight, TLBW, and macrosomia
The effect of air pollution exposure during the first trimester on term birth weight was consistent with that in the whole pregnancy. It showed that high levels of AQI and maternal exposure to PM2.5, PM10, SO2, and CO during the first trimester were negatively associated with term birth weight (AQI: − 2.100 (− 2.576, − 1.624); PM2.5: − 1.700 (− 2.088, − 1.312); PM10: − 4.258 (− 5.408, − 3.108); − 4.258 (− 5.408, − 3.108); CO: − 1.105 (− 1.394, − 0.816)) and positively associated with the incidence of TLBW (AQI: 1.016 (1.006–1.027); PM2.5: 1.017 (1.006–1.028); PM10: 1.017 (1.009–1.026); SO2: 1.033 (1.010–1.057); CO: 1.007 (1.001–1.014)). But maternal O3 exposure increased the risk of term birth weight (β = 4.150, 95%CI: 3.493–4.807) and macrosomia (OR = 1.023, 95%CI: 1.017–1.030). No significant association was found between maternal NO2 exposure and birth weight during early pregnancy (Fig. 2, Table S2).
The effect of maternal exposure to air pollution during various periods of pregnancy on term birth weight, TLBW, and macrosomia. Note: The above models were all adjusted for maternal age, gestational age, and ethnicity. Infants with normal term birth weight (≤ 2500 g birth weight and < 4000 g, n = 292192) were used as the control group while assessing the impact of air pollution exposure on the risk of TLBW and macrosomia. AQI means air quality index, which is an important indicator that presents the overall air pollution level. And * means P < 0.05
The effect of maternal exposure to air pollution during the second trimester on term birth weight was also consistent with that in the whole pregnancy. It showed that a high level of AQI and maternal exposure to PM2.5, PM10, SO2, and CO during the first trimester reduced the term birth weight and increased the risk of TLBW. But O3 exposure increased the risk of term birth weight and macrosomia (Fig. 2, Table S2).
Particularly, the effect of air pollution exposure during the third trimester on birth weight was opposite to that in the first or second trimester. High levels of AQI and maternal exposure to PM2.5, PM10, SO2, NO2, and CO during the third trimester have significantly increased newborn’s birth weight (AQI: 2.274 (1.865, 2.683); PM2.5: 2.344 (1.914, 2.774); PM10: 1.705 (1.363, 2.047); SO2: 3.270 (2.237, 4.303); NO2: 5.219 (4.331, 6.107); CO: 0.855 (0.580, 1.130)). Conversely, O3 exposure reduced the term birth weight (β = − 3.258, 95%CI: - 3.852, - 2.664) and increases the risk of TLBW (OR = 1.024, 95%CI: 1.011–1.037) significantly for newborns (Fig. 2, Table S2). In the generalized additive model, the exposure levels of air pollutants during each trimester of pregnancy explained 11.3 to 11.9% of the deviance in birth weight changes.
Discussion
Main results
Based on a large sample study, we found that maternal exposure to air pollution might cause significant impacts on birth weight. During the entire pregnancy, as well as the first and second trimesters of pregnancy, maternal exposure to PM2.5, PM10, SO2, and CO significantly reduced the term birth weight of newborns and increased the risk of TLBW, while O3 exposure increased term birth weight and the risk of macrosomia. But during the third trimester, AQI, PM2.5, PM10, SO2, NO2, and CO exposure increased the term birth weight and the risk of macrosomia, while O3 exposure had the opposite effect.
The effect of air pollution exposure on birth weight
Air pollution exposure during the whole pregnancy and the first and second trimester
Our study found that a high level of AQI and maternal exposure to PM2.5, PM10, SO2, and CO during the whole pregnancy and the first and second trimesters significantly reduced birth weight and increased the risk of TLBW. This conclusion had also been observed in other similar studies (Arroyo et al. 2019; Chen et al. 2018; He et al. 2018; Li et al. 2019; Smith et al. 2017; Yorifuji et al. 2015). A retrospective cohort study of 540,365 singleton term live births conducted in London suggested that PM2.5 exposure throughout pregnancy was correlated with increased risk of long-term LBW (Smith et al. 2017). Another study, conducted in Guangdong province of China, also showed an increased risk of LBW associated with PM2.5, PM10, and NO2 in the first trimester (Liu et al. 2019). In addition, similar conclusions have also been found in some meta-analysis and reviews (Guo et al. 2019; Li et al. 2017). And a meta-analysis also suggested that pregnant women who smoked were underweight, overweight/obese, or had lower socioeconomic status had an increased risk of having a child with LBW when exposed to ambient PM2.5, SO2, and NO2 (Westergaard et al. 2017). However, no significant association appeared in studies by Laurent et al. (2013) and Lavigne et al. (2016b) for air pollution exposure, which may be because the concentrations of air pollution in those study areas were relatively cleaner, so that the adverse effects did not show up. However, contrary to our findings, most studies indicated that maternal exposure to O3 might reduce birth weight or increase the risk of LBW (Brauer et al. 2008; Laurent et al. 2013; Li et al. 2017). And nearly none of the studies has found the effect of O3 exposure on birth weight increase. Only one study reported that O3 exposure in the second trimester reduced the risk of low birth weight (Ebisu and Bell 2012), which may indicate its possible increased effect on birth weight. Based on all the results of these studies, we did not find a consistent conclusion of dose-response pattern among studies. In other words, the association between air pollution and term birth weight is still controversial.
Air pollution exposure during the third trimester
Conversely, we found that maternal exposure to air pollutants other than O3 during the third trimester might increase birth weight and the risk of macrosomia. Only few studies drew consistent conclusions with our study (Chen et al. 2020b; Li et al. 2019; Zhao et al. 2018). A nationwide prospective cohort study in China has suggested that per 10 μg/m3 increase of PM2.5 concentration over the third trimesters obviously increased the risk of macrosomia (OR: 1.033; 95%CI: 1.026–1.039) (Chen et al. 2020b). In addition, Li et al. found that exposure to NO2 in the third trimester significantly increased birth weight, while O3 exposure decreased it, which was consistent with our conclusion (Li et al. 2019). However, contrary to our findings, most previous related studies still suggested that prenatal exposure to PM2.5, PM10, and CO in the third trimester might cause birth weight reduction significantly (Chen et al. 2018; Guo et al. 2017; He et al. 2018; Santos Vde et al. 2016; Ye et al. 2018). Several reasons, such as the concentration difference and misclassification of air pollution, and co-linearity questions might explain the inconsistent results among these studies. It suggests that the effects of air pollution exposure in the third trimester on birth weight, especially macrosomia, remain to be determined further.
Potential mechanism
The mechanism by which maternal air pollution exposure affects birth weight is still unclear, but some population studies have supported that some abnormal reactions, such as the gene methylation level in cord blood (He et al. 2018) and mitochondrial DNA (mtDNA) content in placenta (Clemente et al. 2016), might mediate the effect of air pollution exposure on birth weight reduction. Based on previous pieces of evidence, we concluded that air pollution enters the human body through the respiratory tract, causing some abnormal reactions such as oxidative stress (Nagiah et al. 2015), inflammatory response (Pope et al. 2016), and DNA methylation (Plusquin et al. 2017). Mitochondria, the organelles that regulate energy production, lack protection and repair mechanisms, which are easily damaged by reactive oxygen species produced by oxidative stress (Janssen et al. 2012). Such damage might eventually lead to the reduced numbers of mitochondria and the damage of energy flow, thus resulting in the reduction of material energy supply and even the reduction of birth weight (van den Hooven et al. 2012). In addition, it is reported that air pollutants also caused histopathological changes and vascularization injuries of the placenta, which might further lead to nutrient and waste transfer obstruction and abnormal cellular growth, thus reducing the birth weight of newborns (Yue et al. 2019).
On the contrary, we also found that some air pollutants at certain periods increased term birth weight, which might be related to the increase of leptin and adiponectin in cord blood. Several studies have reported that prenatal PM2.5 and traffic-related air pollution exposure increased the levels of umbilical blood leptin and high-molecular-weight adiponectin in cord blood (Alderete et al. 2018; Bass et al. 2013; Lavigne et al. 2016a), which were found to be positively associated with increased birth weight (Mantzoros et al. 2009; Tsai et al. 2004). And the levels of leptin in the third trimester are higher (Stefaniak et al. 2019), which might cause its level to be more sensitive to air pollution exposure in the third trimester. Therefore, it provided some molecular basis for the effect of air pollution exposure in the third trimester on birth weight increase. And air pollution exposure in the third trimester also might contribute to neonatal weight gain by mediating the high risk of gestational diabetes mellitus (Kc et al. 2015). In addition, the hypothesis of “thrifty phenotype” also can explain our findings that the effect of air pollution exposure in the first and second trimester is opposite to that in the third trimester to some extent (Hales and Barker 2001). However, further researches are still needed to confirm the above hypothesis and clarify the mechanism of air pollution on birth weight.
Advantage and limitation
Our study had some advantages compared with other studies. Firstly, it is a large sample study based on 321,521 pregnant women and their infants, and very strict inclusion and exclusion criteria were adopted, which helps to control information bias. Secondly, our research was based on the birth registration data of Xi’an city in China from 2015 to 2018, and the exposure level of air pollution was very high and the exposure range is wide. Therefore, compared with other studies conducted in cleaner areas, our research can better reflect the adverse effects of high exposure levels of air pollution on birth weight. Thirdly, through the individual exposure assessment based on exact residence (down to street) during pregnancy, we comprehensively estimated the impact of air pollution exposure during four different periods of pregnancy on the term birth weight and the risk of TLBW and macrosomia and drawn comprehensive and significant conclusions.
But several limitations should be considered in interpreting the results of this study. Due to the limited information collected by the birth registration system, some confounding factors were not considered in our study, such as diet, maternal BMI, sex of newborns, and the history of diseases during pregnancy. However, previous studies have found the effect value changed little whether or not the above risk factors were adjusted (Brauer et al. 2008; Kashima et al. 2011; Kim et al. 2007). And we excluded premature infants most of them accompanied by disease history during pregnancy, and only estimated the effect for air pollution exposure on term infants, so as to control the bias caused by disease to a certain extent. In addition, exposure misclassification might exist due to the lack of information on maternal activity and residential mobility during pregnancy. However, exposure misclassification is more likely to be non-differential (Wang et al. 2018).
Conclusion
Our research has suggested that the effects of air pollution on birth weight varied with exposure periods and pollutants. During the entire pregnancy, as well as the first and second trimesters of pregnancy, maternal exposure to high AQI, PM2.5, PM10, SO2, and CO significantly reduced the term birth weight of newborn and increased the risk of TLBW, while O3 exposure increased term birth weight and the risk of macrosomia. However, during the third trimester, AQI, PM2.5, PM10, SO2, NO2, and CO exposure increased the term birth weight and the risk of macrosomia, while O3 exposure had the opposite effect.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available since we are still conducting other major analyses based on this database, but they are available from the corresponding author on reasonable request.
References
Alderete TL, Song AY, Bastain T, Habre R, Toledo-Corral CM, Salam MT, Lurmann F, Gilliland FD, Breton CV (2018) Prenatal traffic-related air pollution exposures, cord blood adipokines and infant weight. Pediatric Obesity 13:348–356. https://doi.org/10.1111/ijpo.12248
Arroyo V, Díaz J, Salvador P, Linares C (2019) Impact of air pollution on low birth weight in Spain: An approach to a National Level Study. Environ Res 171:69–79. https://doi.org/10.1016/j.envres.2019.01.030
Backes CH, Nelin T, Gorr MW, Wold LE (2013) Early life exposure to air pollution: how bad is it? Toxicol Lett 216:47–53. https://doi.org/10.1016/j.toxlet.2012.11.007
Bao J, Yang X, Zhao Z, Wang Z, Yu C, Li X (2015) The spatial-temporal characteristics of air pollution in China from 2001-2014. Int J Environ Res Public Health 12:15875–15887. https://doi.org/10.3390/ijerph121215029
Bass V, Gordon CJ, Jarema KA, MacPhail RC, Cascio WE, Phillips PM, Ledbetter AD, Schladweiler MC, Andrews D, Miller D, Doerfler DL, Kodavanti UP (2013) Ozone induces glucose intolerance and systemic metabolic effects in young and aged Brown Norway rats. Toxicol Appl Pharmacol 273:551–560. https://doi.org/10.1016/j.taap.2013.09.029
Brauer M, Freedman G, Frostad J, van Donkelaar A, Martin RV, Dentener F, Dingenen R, Estep K, Amini H, Apte JS, Balakrishnan K, Barregard L, Broday D, Feigin V, Ghosh S, Hopke PK, Knibbs LD, Kokubo Y, Liu Y, Ma S, Morawska L, Sangrador JLT, Shaddick G, Anderson HR, Vos T, Forouzanfar MH, Burnett RT, Cohen A (2016) Ambient air pollution exposure estimation for the global burden of disease 2013. Environ Sci Technol 50:79–88. https://doi.org/10.1021/acs.est.5b03709
Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C (2008) A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect 116:680–686. https://doi.org/10.1289/ehp.10952
Chen C, Liu X, Wang X, Qu W, Li W, Dong L (2020a) Effect of air pollution on hospitalization for acute exacerbation of chronic obstructive pulmonary disease, stroke, and myocardial infarction. Environ Sci Pollut Res 27:3384–3400. https://doi.org/10.1007/s11356-019-07236-x
Chen G, Guo Y, Abramson MJ, Williams G, Li S (2018) Exposure to low concentrations of air pollutants and adverse birth outcomes in Brisbane, Australia, 2003-2013. Sci Total Environ 622-623:721–726. https://doi.org/10.1016/j.scitotenv.2017.12.050
Chen S et al (2020b) Effect of PM2.5 on macrosomia in China: a nationwide prospective cohort study. 15:e12584. https://doi.org/10.1111/ijpo.12584
Chu M, Sun C, Chen W, Jin G, Gong J, Zhu M, Yuan J, Dai J, Wang M, Pan Y, Song Y, Ding X, Guo X, du M, Xia Y, Kan H, Zhang Z, Hu Z, Wu T, Shen H (2015) Personal exposure to PM2.5, genetic variants and DNA damage: a multi-center population-based study in Chinese. Toxicol Lett 235:172–178. https://doi.org/10.1016/j.toxlet.2015.04.007
Clemente DBP, Casas M, Vilahur N, Begiristain H, Bustamante M, Carsin AE, Fernández MF, Fierens F, Gyselaers W, Iñiguez C, Janssen BG, Lefebvre W, Llop S, Olea N, Pedersen M, Pieters N, Santa Marina L, Souto A, Tardón A, Vanpoucke C, Vrijheid M, Sunyer J, Nawrot TS (2016) Prenatal ambient air pollution, placental mitochondrial dna content, and birth weight in the INMA (Spain) and ENVIRONAGE (Belgium) birth cohorts. Environ Health Perspect 124:659–665. https://doi.org/10.1289/ehp.1408981
Ebisu K, Bell ML (2012) Airborne PM2.5 chemical components and low birth weight in the northeastern and mid-Atlantic regions of the United States. Environ Health Perspect 120:1746–1752. https://doi.org/10.1289/ehp.1104763
Forns J et al (2018) Air pollution exposure during pregnancy and symptoms of attention deficit and hyperactivity disorder in children in Europe. Epidemiology (Cambridge, Mass) 29:618–626. https://doi.org/10.1097/ede.0000000000000874
Guo L-Q, Chen Y, Mi BB, Dang SN, Zhao DD, Liu R, Wang HL, Yan H (2019) Ambient air pollution and adverse birth outcomes: a systematic review and meta-analysis. J Zhejiang Univ Sci B 20:238–252. https://doi.org/10.1631/jzus.B1800122
Guo LQ et al. (2017) [Relationship between air pollution exposure during pregnancy and birth weight of term singleton live-birth newborns]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 38:1399-1403 https://doi.org/10.3760/cma.j.issn.0254-6450.2017.10.021
Hales CN, Barker DJ (2001) The thrifty phenotype hypothesis. Br Med Bull 60:5–20. https://doi.org/10.1093/bmb/60.1.5
He T, Zhu J, Wang J, Ren X, Cheng G, Liu X, Ma Q, Zhang Y, Li Z, Ba Y (2018) Ambient air pollution, H19/DMR methylation in cord blood and newborn size: a pilot study in Zhengzhou City, China. Chemosphere 212:863–871. https://doi.org/10.1016/j.chemosphere.2018.08.140
Janssen BG, Munters E, Pieters N, Smeets K, Cox B, Cuypers A, Fierens F, Penders J, Vangronsveld J, Gyselaers W, Nawrot TS (2012) Placental mitochondrial DNA content and particulate air pollution during in utero life. Environ Health Perspect 120:1346–1352. https://doi.org/10.1289/ehp.1104458
Janssen BG, Saenen ND, Roels HA, Madhloum N, Gyselaers W, Lefebvre W, Penders J, Vanpoucke C, Vrijens K, Nawrot TS (2017) Fetal thyroid function, birth weight, and in utero exposure to fine particle air pollution: a birth cohort study. Environ Health Perspect 125:699–705. https://doi.org/10.1289/ehp508
Kashima S, Naruse H, Yorifuji T, Ohki S, Murakoshi T, Takao S, Tsuda T, Doi H (2011) Residential proximity to heavy traffic and birth weight in Shizuoka, Japan. Environ Res 111:377–387. https://doi.org/10.1016/j.envres.2011.02.005
Kaufman JD et al (2016) Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the multi-ethnic study of atherosclerosis and air pollution): a longitudinal cohort study. Lancet (London, England) 388:696–704. https://doi.org/10.1016/s0140-6736(16)00378-0
Kc K, Shakya S, Zhang H (2015) Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab 66(Suppl 2):14–20. https://doi.org/10.1159/000371628
Kim OJ, Ha EH, Kim BM, Seo JH, Park HS, Jung WJ, Lee BE, Suh YJ, Kim YJ, Lee JT, Kim H, Hong YC (2007) PM10 and pregnancy outcomes: a hospital-based cohort study of pregnant women in Seoul. J Occup Environ Med 49:1394–1402. https://doi.org/10.1097/JOM.0b013e3181594859
Korten I, Ramsey K, Latzin P (2017) Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev 21:38–46. https://doi.org/10.1016/j.prrv.2016.08.008
Laurent O, Wu J, Li L, Chung J, Bartell S (2013) Investigating the association between birth weight and complementary air pollution metrics: a cohort study. Environmental Health : a global access science source 12:18. https://doi.org/10.1186/1476-069x-12-18
Lavigne E, Ashley-Martin J, Dodds L, Arbuckle TE, Hystad P, Johnson M, Crouse DL, Ettinger AS, Shapiro GD, Fisher M, Morisset AS, Taback S, Bouchard MF, Sun L, Monnier P, Dallaire R, Fraser WD (2016a) Air pollution exposure during pregnancy and fetal markers of metabolic function: the MIREC study. Am J Epidemiol 183:842–851. https://doi.org/10.1093/aje/kwv256
Lavigne E, Yasseen AS III, Stieb DM, Hystad P, van Donkelaar A, Martin RV, Brook JR, Crouse DL, Burnett RT, Chen H, Weichenthal S, Johnson M, Villeneuve PJ, Walker M (2016b) Ambient air pollution and adverse birth outcomes: differences by maternal comorbidities. Environ Res 148:457–466. https://doi.org/10.1016/j.envres.2016.04.026
Li X et al (2017) Association between ambient fine particulate matter and preterm birth or term low birth weight: an updated systematic review and meta-analysis. Environ Pollut (Barking, Essex : 1987) 227:596–605. https://doi.org/10.1016/j.envpol.2017.03.055
Li Z, Yuan X, Fu J, Zhang L, Hong L, Hu L, Liu L (2019) Association of ambient air pollutants and birth weight in Ningbo, 2015-2017. Environ Pollut (Barking, Essex : 1987) 249:629–637. https://doi.org/10.1016/j.envpol.2019.03.076
Liu Y, Xu J, Chen D, Sun P, Ma X (2019) The association between air pollution and preterm birth and low birth weight in Guangdong, China. BMC Public Health 19:3–3. https://doi.org/10.1186/s12889-018-6307-7
Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW (2009) Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics 123:682–689. https://doi.org/10.1542/peds.2008-0343
Moraitis AA, Wood AM, Fleming M, Smith GC (2014) Birth weight percentile and the risk of term perinatal death. Obstet Gynecol 124:274–283. https://doi.org/10.1097/aog.0000000000000388
Nagiah S, Phulukdaree A, Naidoo D, Ramcharan K, Naidoo RN, Moodley D, Chuturgoon A (2015) Oxidative stress and air pollution exposure during pregnancy: a molecular assessment. Hum Exp Toxicol 34:838–847. https://doi.org/10.1177/0960327114559992
Pedersen M, Garne E, Hansen-Nord N, Hjortebjerg D, Ketzel M, Raaschou-Nielsen O, Nybo Andersen AM, Sørensen M (2017) Exposure to air pollution and noise from road traffic and risk of congenital anomalies in the Danish National Birth Cohort. Environ Res 159:39–45. https://doi.org/10.1016/j.envres.2017.07.031
Plusquin M, Guida F, Polidoro S, Vermeulen R, Raaschou-Nielsen O, Campanella G, Hoek G, Kyrtopoulos SA, Georgiadis P, Naccarati A, Sacerdote C, Krogh V, Bas Bueno-de-Mesquita H, Monique Verschuren WM, Sayols-Baixeras S, Panni T, Peters A, Hebels DGAJ, Kleinjans J, Vineis P, Chadeau-Hyam M (2017) DNA methylation and exposure to ambient air pollution in two prospective cohorts. Environ Int 108:127–136. https://doi.org/10.1016/j.envint.2017.08.006
Pope CA 3rd, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O'Toole T (2016) Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res 119:1204–1214. https://doi.org/10.1161/circresaha.116.309279
Santos Vde P, Medeiros AP, Lima TA, Nascimento LF (2016). Air pollutants associated with insufficient birth weight. Revista brasileira de epidemiologia = Brazilian journal of epidemiology 19:89-99 https://doi.org/10.1590/1980-5497201600010008
Shang L et al (2019) Maternal exposure to PM(2.5) may increase the risk of congenital hypothyroidism in the offspring: a national database based study in China. BMC Public Health 19:1412. https://doi.org/10.1186/s12889-019-7790-1
Smith RB, Fecht D, Gulliver J, Beevers SD, Dajnak D, Blangiardo M, Ghosh RE, Hansell AL, Kelly FJ, Anderson HR, Toledano MB (2017) Impact of London's road traffic air and noise pollution on birth weight: retrospective population based cohort study. BMJ (Clinical research ed) 359:j5299. https://doi.org/10.1136/bmj.j5299
Stefaniak M, Dmoch-Gajzlerska E, Mazurkiewicz B, Gajzlerska-Majewska W (2019) Maternal serum and cord blood leptin concentrations at delivery. PLoS One 14:e0224863. https://doi.org/10.1371/journal.pone.0224863
Tsai PJ, Yu CH, Hsu SP, Lee YH, Chiou CH, Hsu YW, So SC, Chu CH (2004) Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin Endocrinol 61:88–93. https://doi.org/10.1111/j.1365-2265.2004.02057.x
van den Hooven EH, Pierik FH, de Kluizenaar Y, Hofman A, van Ratingen SW, Zandveld PYJ, Russcher H, Lindemans J, Miedema HME, Steegers EAP, Jaddoe VWV (2012) Air pollution exposure and markers of placental growth and function: the generation R study. Environ Health Perspect 120:1753–1759. https://doi.org/10.1289/ehp.1204918
Wang Q et al (2018) Effects of prenatal exposure to air pollution on preeclampsia in Shenzhen, China. Environmental pollution (Barking, Essex : 1987) 237:18-27. https://doi.org/10.1016/j.envpol.2018.02.010
Westergaard N, Gehring U, Slama R, Pedersen M (2017) Ambient air pollution and low birth weight - are some women more vulnerable than others? Environ Int 104:146–154. https://doi.org/10.1016/j.envint.2017.03.026
Ye L, Ji Y, Lv W, Zhu Y, Lu C, Xu B, Xia Y (2018) Associations between maternal exposure to air pollution and birth outcomes: a retrospective cohort study in Taizhou, China. Environ Sci Pollut Res 25:21927–21936. https://doi.org/10.1007/s11356-018-1944-z
Yorifuji T, Kashima S, Doi H (2015) Outdoor air pollution and term low birth weight in Japan. Environ Int 74:106–111. https://doi.org/10.1016/j.envint.2014.09.003
Yue H, Ji X, Zhang Y, Li G, Sang N (2019) Gestational exposure to PM(2.5) impairs vascularization of the placenta. Sci Total Environ 665:153–161. https://doi.org/10.1016/j.scitotenv.2019.02.101
Zhang Z, Kris-Etherton PM, Hartman TJ (2014) Birth weight and risk factors for cardiovascular disease and type 2 diabetes in US children and adolescents: 10 year results from NHANES. Matern Child Health J 18:1423–1432. https://doi.org/10.1007/s10995-013-1382-y
Zhao N, Qiu J, Ma S, Zhang Y, Lin X, Tang Z, Zhang H, Huang H, Ma N, Huang Y, Bell ML, Liu Q, Zhang Y (2018) Effects of prenatal exposure to ambient air pollutant PM10 on ultrasound-measured fetal growth. Int J Epidemiol 47:1072–1081. https://doi.org/10.1093/ije/dyy019
Acknowledgments
We would like to thank all the participants in the birth registration system and ambient air quality continuous automated monitoring system for providing the data for the study.
Funding
The present study was supported by the Key Research and Development Program of Shaanxi (grant number 2019SF-100); the First Affiliated Hospital of Xi’an Jiaotong University (grant number XJTU1AF-CRF-2019-023); and the Bureau of Xi’an Science and Technology (grant number 201805098YX6SF32(1)).
Author information
Authors and Affiliations
Contributions
All authors (LS, LYH, LRY, LTL, CFQ, GLX, RQW, LQG, WFY, MCC) made substantial contributions to the conception and design of the study, acquisition of data, and analysis and interpretation of data. WFY, LS, and LYH mainly conceived the idea and designed the research; LS and LYH mainly performed the statistical analysis and wrote the manuscript. LQG, RQW, and MCC also mainly performed the statistical analysis. LRY, LTL, CFQ, and GLX mainly participated in the data collection. All authors have been involved in drafting the manuscript or revising it critically for important intellectual content; they have given final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Verbal informed consent was obtained prior to birth registration, and the study was ethically approved by the Medical Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2017LSK-106) based on the institution’s ethical standards and the ethical standards of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Disclaimer
The funders did not participate in any part of the study from design to the writing of the manuscript, except for supporting this project.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shang, L., Huang, L., Yang, L. et al. Impact of air pollution exposure during various periods of pregnancy on term birth weight: a large-sample, retrospective population-based cohort study. Environ Sci Pollut Res 28, 3296–3306 (2021). https://doi.org/10.1007/s11356-020-10705-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10705-3