Abstract
Background
The majority of studies examining long-term exposure to ambient ozone have utilized averages as the exposure parameter. However, averaging ozone exposures may underestimate the impact of ozone peaks and seasonality. The current study aimed to examine the association between ozone exposure evaluated by different exposure metrics and lung function in healthy adolescents.
Methods
We conducted a cross-sectional study among 665 healthy adolescent males living within a 2 km radius of an ozone monitoring station. Multiple ozone exposure metrics were evaluated, including two-year and peak-season averages, peaks, peak intensity, and the total excess of peak level. Lung function was measured using FEV1, FVC, and FEV1/FVC ratio.
Results
The peak intensity during the ozone peak-season was associated with the largest decrease in the FEV1/FVC ratio, -1.52% (95%CI: -2.55%, -0.49%) (p < 0.01). Concurrently, we did not observe a significant association between ozone exposure, assessed by different metrics, and either FEV1 or FVC.
Conclusions
The study findings suggest that when evaluating ambient ozone exposures, ozone peak intensity during peak-season should be considered, as it may predict greater adverse health effects than averages alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ozone (O3) is an oxidant gas formed in the troposphere as the product of photochemical reactions between oxides of nitrogen (NOX) and carbon-containing compounds, including volatile organic compounds (VOCs), carbon monoxide (CO), and methane (CH4) (WHO 2006, 2021; US EPA 2020). As well established in the epidemiological literature reviewed by the World Health Organization (WHO) and the United States Environmental Protection Agency (US EPA), exposure to ozone is associated with increased mortality, respiratory morbidity, hospital admissions, asthma exacerbations, and decreased lung function (LF) (WHO 2006, 2013; US EPA 2020). According to several estimates (Bell et al. 2007; Zhang et al. 2019; HEI 2022), ozone concentrations are expected to increase with global warming. Therefore, understanding ozone-related health effects is a major public health concern.
The acute harmful effects of short-term ozone exposures on LF are well established and attributed to cellular damage, inflammation, airways remodeling, and neuronally-mediated responses in the bronchial airways (Watson et al. 1988; Mudway and Kelly 2000; WHO 2006; Mumby et al. 2019; US EPA 2020). However, the long-term effects of ozone exposure on LF are less clear (WHO 2013; Nuvolone et al. 2018; US EPA 2021). While several studies have demonstrated an association between long-term ozone exposures and decreased LF (Urman et al. 2014; Chen et al. 2015; Xing et al. 2020), others did not find such an association (Barone-Adesi et al. 2015; Gauderman et al. 2015; Fuertes et al. 2015).
Holm and Balmes (2022) recently reviewed 53 studies that analyzed the association between exposure to ozone and LF. The studies reviewed assessed the exposure to ozone by using arithmetic means or by averaging the ozone maximum daily 8-hour running mean concentrations over at least one-day duration. However, the reviewed studies did not consider the frequency or intensity of ozone peaks exceeding a certain threshold over an extended exposure period. Nevertheless, accounting for peak levels as part of the exposure assessment could reveal health phenomena that might otherwise be overlooked (US EPA 1992; Berhane et al. 2004; Greenberg et al. 2017; Virji and Kurth 2021). Furthermore, as ozone formation depends on temperatures and sunlight (Finlayson-Pitts and Pitts 1993; WHO 2006; Pusede et al. 2015; US EPA 2020), averaging long-term ozone exposures over a prolonged period may miss significant seasonal effect during the peak-season - the six consecutive months of the year with the highest six-month running-average ozone concentrations (WHO 2021). Therefore, alternative exposure assessment metrics that consider the exposure profile and seasonality might be important in estimating ozone’s potential health effects.
The present study aims to estimate the long-term effect of exposure to ozone on LF by comparing different exposure assessment metrics including average exposures during two years and the peak-season, the number of peaks and peak intensity during the peak-season, as well as the total excess of peak level over the peak-season, across a healthy adolescent cohort.
Methods
Study design and population
This cross-sectional study analyzed the association between different ambient ozone exposure metrics and LF indices among a cohort of adolescent males aged 16–18 years. Participants were examined at the Israeli Naval Medical Institute (INMI) between 2012 and 2019 as part of their medical screening for recruitment to an elite military unit of the Israel Defense Forces (IDF).
The inclusion criteria were as follows: fully-completed screening medical questionnaires; no current diagnosis of asthma by a physician; availability of at least 75% of daily ozone measurements during two years before the spirometry test and during the six months peak-season (occurring in Israel between April to September) prior to the medical examination; residence within 2 km from an ozone background local air quality monitoring station. The 2 km proximity distance was selected based on published studies demonstrating significant effects within this range (Chang et al. 2012; Dimakopoulou et al. 2020; Xing et al. 2020). Figure 1 shows the locations of background local monitoring stations from which ozone concentrations were measured.
Medical and socio-demographic information
Data on current or childhood asthma were obtained from primary care physician’s statements presented to the INMI and from screening medical evaluation before enlistment to the army, as documented in the electronic medical record of each recruit. Participant’s current or recent smoking status were extracted from questionnaires filled in by the participant and his parents. Recent smokers were defined as subjects who reported regularly smoking in the year before the spirometry date. Participants’ socio-economic level was determined based on their residence, as classified by the Israeli Central Bureau of Statistics (ICBS) on a scale from 1 to 10. Low socio-economic level was categorized as 1–4, medium as 5–7, and high as 8–10. The classification is based on the financial resources of the local population (from work and benefits), housing (density, quality, etc.), home appliances (air condition, personal computer, dishwasher, etc.), education, unemployment rate, amount and quality of motor vehicles, and additional socio-economic distress and demographic characteristics (ICBS 2021). A standardized test assessing general intelligence score obtained as a part of the military pre-enlistment evaluation was also considered. The test score ranges from 10 to 90 with intervals of 10 points, and highly correlates with the Wechsler Adult Intelligence Scale (WAIS) (Goldberg et al. 2011; Cukierman-Yaffe et al. 2015). For the subsequent analysis, the score was divided into three groups: low (10–30), medium (40–70), and high (80–90).
Lung function tests
Trained medical personnel at the INMI measured height and administered spirometry testing according to the ATS/ERS 2005 protocol (Miller 2005). LF tests were performed using a KoKo™ spirometer, which measured the forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), both derived from the best spirometry maneuver (the highest result of FVC + FEV1). We used percent predicted FEV1, percent predicted FVC, and the ratio of FEV1 to FVC (FEV1/FVC) in percentage as outcome variables. Predicted values were calculated according to the Global Lung Initiative (GLI) (Quanjer et al. 2012).
Ozone measurements
Ambient air quality in Israel is monitored through a network of over 150 air quality monitoring stations that continuously measure various air pollutants (Environment and Health Fund and Ministry of Health 2020). In particular, ozone concentrations are monitored by 63 monitoring stations using ultraviolet photometry according to the EN-14625 standard (EU 2012; MoEP 2022). As previously mentioned, we used ozone measurements from a local background monitoring station nearest the participant’s residence (≤ 2 km) to calculate ozone exposures by different metrics. The nearest monitoring station for each participant’s residence was determined using the ArcGIS software (Esri Inc. 2019).
Ozone exposure assessment metrics
To assess ozone exposure for each participant, we calculated several exposure metrics, including the two-year average, the peak-season average according to the recently published WHO guidelines (WHO 2021), the total number of peaks over the peak season, the peak intensity over the peak season, and the total excess of peak level. To calculate these exposure metrics, we used the maximum 8-hour daily running average ozone as per WHO Air Quality Guidelines (AQGs) level and US EPA National Ambient Air Quality Standards (NAAQS) (US EPA 2015; WHO 2021).
The two-year average was calculated by averaging the maximum daily 8-hour running average over the two years prior to the spirometry test. Similarly, we computed the peak-season average using daily 8-hour running average measurements from April to September in the year preceding the spirometry test.
Furthermore, we calculated the total number of peaks and the peak intensity during the peak-season for each participant in the study cohort. A peak was defined as an 8-hour maximum daily running average that exceeded the concertation of 100 µg/m3. The threshold of 100 µg/m3 was chosen based on its alignment with the WHO AQG level for short-term (8-hour) maximum (WHO 2021) and the target value in Israel’s clean air regulations (State of Israel 2008).
For participants with less than 100% daily ozone measurements (between 75 and 99% daily measurements), we estimated the missing peaks over the peak-season as follows:
The missing peaks, as calculated above, were added to the measured peaks to produce a comparable total number of peaks over peak-season value.
The peak intensity over the peak-season was calculated by averaging only the daily values in which the 8-hour daily maximum running average exceeded 100 µg/m3 concentrations, during the peak-season.
In addition, we calculated the total excess of peak level over the peak-season, reflecting the accumulated ozone concentration that exceeds the maximum 8-hour daily running average peak level of 100 µg/m³ during this period, following the SOMO (Sum Of Means Over) index developed by the WHO (WHO 2008). We adjusted the index for the 183-day peak-season duration and the 100 µg/m³ threshold. The calculation was performed as follows: \(\sum\nolimits_i {{\rm{max}}} \{ O,{C_i} - 100\mu {\rm{g}}/{m^3}\} \times \frac{{183}}{{{N_{valid}}}}\), where Ci represents the maximum daily 8-hour average concentration, and Nvalid is the number of valid daily values during peak-season. The summation is from day i = 1 to 183 over the peak-season period.
Statistical analysis
Descriptive statistics were applied to characterize the distribution of the study population and ozone exposure assessment metrics: averages, the total number of peaks, peak intensity, and the total excess of peak level. The variables of interest - ozone exposure assessment metrics, and the dependent variables - LF indices, were evaluated as continuous variables. We conduct multivariate linear regressions to evaluate the association between ozone exposure, calculated by different metrics, and lung function indices. To produce the estimates of differences of change in lung function per 10 units of ozone exposure (either 10 µg/m3 or 10 peaks), we multiplied the unstandardized regression coefficients by 10, for the metrics: two-year average, peak-season average, peak intensity over the peak-season, and the total number of peak over the peak-season. For the total excess of peak levels over the peak-season metric we multiplied the unstandardized regression coefficients by 100, to reflect an increase in 100 µg/m3•days units. Analyses were adjusted for BMI, childhood asthma, current or recent smoking, socio-economic level, and general intelligence score. The statistical analyses were performed using SPSS™ v.27 (IBM Corp. 2020).
Results
Study population
From an original pool of 6,013 recruits examined at the INMI, 536 were excluded due to incomplete medical questionnaires. Furthermore, we excluded 199 recruits lacking sufficient measurement data (the closest ozone monitoring station to their residence recorded less than 75% of the daily ozone measurements during the two-year period preceding the spirometry test or less than 75% of the daily ozone measurements during the peak-season). Recruits with a physician diagnosis of asthma (13 recruits) were excluded as well, resulting in 5,265 potential participants living at variable distances from ozone monitoring stations. Of these, 665 participants met the inclusion criteria of residing within 2 km of an ozone monitoring station and were eligible for participation in the study. Notably, 568 of 665 participants (85% of the study cohort) had more than 95% of the daily measurements available for the two exposure periods analyzed. Study population characteristics are presented in Table 1. On average, the percent predicted FEV1 was 98.13%, the percent predicted FVC was 96.44%, and the FEV1/FVC ratio in percentage was 87.67%.
Ozone exposure assessment metrics
Table 2 displays the distribution of ozone by different exposure assessment metrics. As shown in the table, the two-year average concentration of ambient ozone was 89.43 µg/m3 (± 8.08 SD), while the average concentration during the peak-season was 98.89 µg/m3 (± 10.99 SD). On average, each participant experienced 83 ozone peaks during the peak-season (± 48 SD), with an average peak intensity of 111.82 µg/m3 (± 4.80 SD). The average value of the total excess of peak level over peak-season was 1158.96 µg/m3•days (± 1039.75 SD).
Exposure to ozone and LF
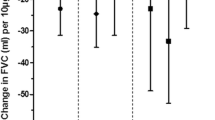
The results of the multivariate linear regression analyses are presented in Fig. 2. As shown in Fig. 2, among the LF indices examined, only the FEV1/FVC ratio exhibited a significant decrease in response to increases in ozone calculated by different metrics. Neither percent predicted FEV1 nor percent predicted FVC showed a significant effect for any of the exposure metrics evaluated. analysis of unadjusted values of FEV1 and FVC, which was also insignificant, presented in Table S1 in the supplementary material. The greatest reduction in the FEV1/FVC ratio was observed when the metric of average peak intensity over the peak-season was applied, with a decrease of 1.52% per 10 µg/m3 ozone (95%CI: -2.55, -0.49). For the remaining exposure assessment metrics, the decline in FEV1/FVC was smaller. A 10 µg/m3 increment in average ozone concentration during the two-year period prior to spirometry and across the peak-season was found to be significantly correlated with a reduction of 0.81% (95%CI: -1.43, -0.19) and 0.65% (95%CI: -1.10, -0.20), respectively. Furthermore, an increase of 10 peaks during the peak-season was associated with a decline of 0.17% (95%CI: -0.27, -0.07) in the FEV1/FVC ratio. Additionally, an increase in 100 µg/m3•days in the total excess of peak levels over the peak-season was significantly associated with a decrease of 0.08% (95%CI: -0.13, -0.03) in the FEV1/FVC ratio.
Estimates of differences in percent predicted FEV1 (A), percent predicted FVC (B), and FEV1/FVC ratio (C) associated with an increase in ozone assessed by different exposure metrics Notes: Results are presented as percent changes in lung function indices per increase in 10 μg/m3 ozone for the metrics: two-year average, peak-season averages, and peak intensity over the peak-season, for an increase of 10 peaks for the total number of peak over the peak-season metric, and for 100 μg/m3•days increase in the total excess of peak level over peak-season. Controlled for BMI, childhood asthma, smoking, socio-economic level, and general intelligence score. CI= confidence interval; *p<0.05, **p<0.01
Discussion
The results of this study demonstrate that an increase in peak intensity during the peak-season is associated with the highest decrease in FEV1/FVC, almost two-times more than the two-year average exposure assessment metric. To the best of our knowledge, this is the first study to assess ambient ozone exposures using both peak intensity and the total number of peaks during the peak-season as exposure parameters.
The US EPA (US EPA 1992) emphasized the importance of using the exposure profile, the pattern of concentration over time (including peak exposure, the periodicity of exposure, intensity, frequency, and duration) in conducting exposure assessments. According to the US EPA, “such profiles are very important for use in risk assessment where the severity of the effect is dependent on the pattern by which the exposure occurs rather than the total (integrated) exposure” (US EPA 1992). In addition, the importance of the seasonality effect regarding ozone exposure is described in the recently published WHO air quality guidelines, which established a long-term standard for ambient ozone for the first time (WHO 2021). As outlined in the guidelines’ development protocol, the recommendations for choosing the peak-season as the long-term standard period was based on a higher relative risk (RR) for respiratory mortality during the peak-season, compared with the RR for all non-accidental mortality (Huangfu and Atkinson 2020; WHO 2021). As described above, accounting for the exposure profile and seasonality as part of the exposure assessment has been consistently proposed to reveal greater adverse health effects, which coincides with our study findings.
While the potential mechanism of long-term health effects of chronic or repeated acute ozone exposures remains a matter of ongoing research (WHO 2021), and a causal relationship can not be established in our study cohort, the following explanation is suggested to elucidate our findings. Previous studies (Jang et al. 2002; Michaudel et al. 2018; Lee et al. 2021) have reported that exposure to high levels of ozone which exceed a certain threshold can trigger adverse reactions in the respiratory system through a dose-response relationship. Additionally, as previously described (Virji and Kurth 2021; Albano et al. 2022), repeated peak exposures might overcome the respiratory system’s defense mechanisms, leading to potential damage. Therefore, the combination of high ozone levels that frequently occurred during the peak-season, represented by the peak intensity over the peak season metric, could play a significant role in predicting potential ozone-related health effects, as supported by the present study findings.
The current study found a decrease in the obstructive markers, including a significant decline in the FEV1/FVC ratio and a non-significant reduction in percent predicted FEV1, both associated with increased ozone exposure assessed by different metrics. As previously described in the introduction, ozone is known to cause airways inflammation, structural and functional changes in the airways, potentially leading to airway obstruction (Watson et al. 1988; Mudway and Kelly 2000; WHO 2006; Mumby et al. 2019; US EPA 2020). The decreased ratio resulted from a decrease in FEV1 and an increase in FVC, an increase which was also observed in previous studies (Lee et al. 2011; Barone-Adesi et al. 2015; Gauderman et al. 2015; Ierodiakonou et al. 2016).
In the current study, we incorporated the general intelligence score variable as part of our analysis, given that several previous studies have demonstrated an association between intelligence and lung function (Taylor et al. 2005; Suglia et al. 2008; Vasilopoulos et al. 2015; Grenville et al. 2023). However, we did not find significant evidence regarding this association.
Table 3 summarizes studies conducted between 2012 and 2022 that evaluated the effect of long-term exposure to ozone (two months or more) on lung function in children and adolescents. As shown in Table 3, the exposure assessment metric used to evaluate the effect of ozone on lung function has been solely based on ozone averages. Among the studies reviewed in Table 3, some reported a statistically significant association between exposure to ozone and FEV1, FVC, or FEV1/FVC (Urman et al. 2014; Hwang et al. 2015; Ierodiakonou et al. 2016; Tsui et al. 2018; Dimakopoulou et al. 2020; Xing et al. 2020), while others (Barone-Adesi et al. 2015; Gauderman et al. 2015; Fuertes et al. 2015; Usemann et al. 2019) have not reported any significant effect. These inconsistent findings across studies may be attributed to the use of average as the exclusive exposure parameter. Among the studies mentioned above, four (Fuertes et al. 2015; Ierodiakonou et al. 2016; Tsui et al. 2018; Usemann et al. 2019) evaluated both FEV1/FVC ratio as well as FEV1 and FVC indices, similar to the present study. Ierodiakonou et al. (2016) found a significant decrease in the FEV1/FVC ratio of 0.4% (95%CI: -0.8, -0.1) in asthmatic children after bronchodilation with an increase in IQR (Inter Quartile Range) ozone. However, they did not observe any significant association with FEV1 and FVC. These findings are consistent with the present study, despite differences in the study population (our analysis is limited to healthy adolescents). In contrast, Tsui et al. (2018) observed a significant decline in FEV1 and FVC but not in FEV1/FVC, and both Fuertes et al. (2015) and Usemann et al. (2019) did not find any correlation between exposure to ozone and FEV1, FVC, or FEV1/FVC ratio. These contradictory results may be attributed to variations in the methods used to evaluate ozone exposure, as described in Table 3.
The current study was conducted in Israel, a Mediterranean country with relatively high ozone concentrations. During the peak-season, the average ozone concentration was 98.89 µg/m3 (± 10.99 SD), which significantly exceeded the newly recommended WHO AQG level for peak-season of 60 µg/m3, and almost reached the first interim target for peak season, set at 100 µg/m3 by WHO in 2021 (WHO 2021).
According to a recent publication by the Health Effects Institute (HEI 2022), in 2019, 92% of the world’s population lived in areas in which ozone concentrations during the peak-season exceeded the WHO AQG level, and 41% of the world’s population lived in areas where ozone levels exceeded the first interim target for the peak-season. Ozone levels have consistently increased over the past decade and are expected to continue to rise due to climate change and emissions of ozone precursors (Bell et al. 2007; Zhang et al. 2019; HEI 2020, 2022). Given the growing significance of this environmental pollutant, the present study may provide a valuable methodological consideration for assessing ozone exposures.
Finally, we should note that our study has several strengths and limitations. Notable strengths of the current study included the completeness of the exposure data set, as most participants (568 out of 665) had more than 95% of daily measurements for the two exposure period analyzed. Furthermore, our study included well-documented baseline information regarding each participant’s medical background, including asthma diagnosis by a physician, allowing for exclusion of participants with asthma to avoid bias. Another strength of this study concerns spirometry, a pulmonary test which greatly dependent on participant cooperation. Our study participants were highly motivated to obtain the best possible results, as spirometry tests were performed as part of medical screening for an elite military unit. In addition, the spirometry tests were performed using the same spirometer in the same clinic, maintaining uniformity in test implementation.
This study also has some limitations that should be considered. The main limitation of our study is that the exposure assessment is based on the best available monitoring station measurements, while personal exposure to ozone cannot be determined. Likewise, our analysis did not account for other ambient pollutants, such as PM2.5, NO2, CO, and SO2. Future research should consider including additional pollutants as part of the analysis, especially PM2.5, which may have a significant correlation with ozone (Zhu et al. 2019; US EPA 2020), potentially provide a more comprehensive understanding of ozone’s effect on LF. Moreover, in the current study, we used a 2 km distance threshold from the nearest monitoring station, consistent with previous studies on the effect of ozone on LF (Chang et al. 2012; Dimakopoulou et al. 2020; Xing et al. 2020). However, given that the distribution of ozone can be influenced by multiple factors (Lu et al. 2019; Chen et al. 2022), adopting a single distance threshold can be considered a limitation of the study. Additionally, the study exclusively analyzed one cut-off point of 100 µg/m³, which corresponds to the WHO AQG’s level for a daily 8-hour maximum and aligns with Israel’s clean air regulations as the 8-hour target value. Nevertheless, to comprehensively explore the potential peak thresholds, further studies should consider various other cut-off points. Furthermore, our study cohort consists of adolescent males, 16 to 18 years old, who underwent medical screening during recruitment into an elite military unit. The homogeneity of this cohort helps minimize confounding by limiting the number of potentially intervening variables. However, it must be acknowledged that this also limits the generalizability of our findings. Future studies investigating effects in women as well as men, and in older adults, would be useful to understanding potential health effects in a broader population.
Conclusion
Our study findings suggest that exposure assessments to ambient ozone should consider not only ozone averages but also the exposure profile, including peaks and seasonality, as they may predict greater adverse health effects than averages alone. It is also important to note that the effect of exposure to ozone on LF may be overlooked when analyzing only averages as an exposure parameter, especially when the effect is of a small magnitude. Moreover, as exposures to ambient ozone are anticipated to increase with climate change, assessing ozone exposures by the optimal exposure metric is of greater importance. Further research in larger and more diverse cohorts is needed to confirm our results and gain a better understanding of the potential long-term health effects of chronic or repeated acute ozone exposures.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Albano GD, Gagliardo RP, Montalbano AM, Profita M (2022) Overview of the mechanisms of oxidative stress: impact in inflammation of the Airway diseases. Antioxidants 11:2237. https://doi.org/10.3390/antiox11112237
Barone-Adesi F, Dent JE, Dajnak D et al (2015) Long-term exposure to primary traffic pollutants and lung function in children: cross-sectional study and meta-analysis. PLoS ONE 10:1–16. https://doi.org/10.1371/journal.pone.0142565
Bell ML, Goldberg R, Hogrefe C et al (2007) Climate change, ambient ozone, and health in 50 US cities. Clim Change 82:61–76. https://doi.org/10.1007/s10584-006-9166-7
Berhane K, Gauderman WJ, Stram DO, Thomas DC (2004) Statistical issues in studies of the long-term effects of air pollution: the southern California children’s health study. Stat Sci 19:414–449. https://doi.org/10.1214/088342304000000413
Chang YK, Wu CC, Lee LT et al (2012) The short-term effects of air pollution on adolescent lung function in Taiwan. Chemosphere 87:26–30. https://doi.org/10.1016/j.chemosphere.2011.11.048
Chen CH, Chan CC, Chen BY et al (2015) Effects of particulate air pollution and ozone on lung function in non-asthmatic children. Environ Res 137:40–48. https://doi.org/10.1016/j.envres.2014.11.021
Chen B, Yang X, Xu J (2022) Spatio-temporal variation and influencing factors of ozone Pollution in Beijing. Atmos (Basel) 13:359. https://doi.org/10.3390/atmos13020359
Cukierman-Yaffe T, Kasher-Meron M, Fruchter E et al (2015) Cognitive performance at late adolescence and the risk for impaired fasting glucose among young adults. J Clin Endocrinol Metab 100:4409–4416. https://doi.org/10.1210/jc.2015-2012
Dimakopoulou K, Douros J, Samoli E et al (2020) Long-term exposure to ozone and children’s respiratory health: results from the RESPOZE study. Environ Res 182. https://doi.org/10.1016/j.envres.2019.109002
Environment and Health Fund and Ministry of Health (2020) Environmental Health in Israel 2020
Esri Inc (2019) ArcMap 10.7 Desktop
EU (2012) BS EN 14625:2012 Ambient air. Standard method for the measurement of the concentration of ozone by ultraviolet photometry
Finlayson-Pitts BJ, Pitts JN (1993) Atmospheric Chemistry of Tropospheric ozone formation: Scientific and Regulatory implications. Air Waste 43:1091–1100. https://doi.org/10.1080/1073161X.1993.10467187
Fuertes E, Bracher J, Flexeder C et al (2015) Long-term air pollution exposure and lung function in 15 year-old adolescents living in an urban and rural area in Germany: the GINIplus and LISAplus cohorts. Int J Hyg Environ Health 218:656–665. https://doi.org/10.1016/j.ijheh.2015.07.003
Gauderman WJ, Urman R, Avol E et al (2015) Association of improved air quality with lung development in children. N Engl J Med 372:905–913. https://doi.org/10.1056/NEJMoa1414123
Goldberg S, Fruchter E, Davidson M et al (2011) The relationship between risk of hospitalization for Schizophrenia, SES, and cognitive functioning. Schizophr Bull 37:664–670. https://doi.org/10.1093/schbul/sbr047
Greenberg N, Carel RS, Derazne E et al (2017) Modeling long-term effects attributed to nitrogen dioxide (NO2) and sulfur dioxide (SO2) exposure on asthma morbidity in a nationwide cohort in Israel. J Toxicol Environ Heal - Part Curr Issues 80:326–337. https://doi.org/10.1080/15287394.2017.1313800
Grenville J, Granell R, Dodd J (2023) Lung function and cognitive ability in children: a UK birth cohort study. BMJ Open Respir Res 10:e001528. https://doi.org/10.1136/bmjresp-2022-001528
HEI (2020) State of Global Air 2020. Special Report. Boston, M.A
HEI (2022) How Does Your Air Measure Up Against the WHO Air Quality Guidelines? A State of Global Air Special Analysis. Boston, MA
Holm SM, Balmes JR (2022) Systematic review of ozone effects on human lung function, 2013 through 2020. Chest 161:190–201. https://doi.org/10.1016/j.chest.2021.07.2170
Huangfu P, Atkinson R (2020) Long-term exposure to NO2 and O3 and all-cause and respiratory mortality: a systematic review and meta-analysis. Environ Int 144:105998. https://doi.org/10.1016/j.envint.2020.105998
Hwang BF, Chen YH, Lin YT et al (2015) Relationship between exposure to fine particulates and ozone and reduced lung function in children. Environ Res 137:382–390. https://doi.org/10.1016/j.envres.2015.01.009
IBM Corp (2020) IBM SPSS Statistics for Windows
ICBS (2021) Characterization and classification of geographical units by the Socio-Economic Level of the Population 2017. Jerusalem, Israel
Ierodiakonou D, Zanobetti A, Coull BA et al (2016) Ambient air pollution, lung function, and airway responsiveness in asthmatic children. J Allergy Clin Immunol 137:390–399. https://doi.org/10.1016/j.jaci.2015.05.028
Jang A-S, Choi I-S, Koh Y-I et al (2002) The relationship between alveolar epithelial proliferation and airway obstruction after ozone exposure. Allergy 57:737–740. https://doi.org/10.1034/j.1398-9995.2002.23569.x
Lee YL, Wang WH, Lu CW et al (2011) Effects of ambient air pollution on pulmonary function among schoolchildren. Int J Hyg Environ Health 214:369–375. https://doi.org/10.1016/j.ijheh.2011.05.004
Lee YG, Lee PH, Choi SM et al (2021) Effects of air pollutants on airway diseases. Int J Environ Res Public Health 18. https://doi.org/10.3390/ijerph18189905
Lu X, Zhang L, Shen L (2019) Meteorology and Climate influences on Tropospheric ozone: a review of natural sources, Chemistry, and transport patterns. Curr Pollut Rep 5:238–260. https://doi.org/10.1007/s40726-019-00118-3
Michaudel C, Mackowiak C, Maillet I et al (2018) Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL-33. J Allergy Clin Immunol 142:942–958. https://doi.org/10.1016/j.jaci.2017.11.044
Miller MR (2005) Standardisation of spirometry. Eur Respir J 26:319–338. https://doi.org/10.1183/09031936.05.00034805
MoEP (2022) Air monitoring in Israel. https://www.svivaaqm.net/. Accessed 10 Dec 2022
Mudway IS, Kelly FJ (2000) Ozone and the lung: a sensitive issue. Mol Aspects Med 21:1–48. https://doi.org/10.1016/s0098-2997(00)00003-0
Mumby S, Chung KF, Adcock IM (2019) Transcriptional effects of ozone and impact on airway inflammation. Front Immunol 10:1–14. https://doi.org/10.3389/fimmu.2019.01610
Nuvolone D, Petri D, Voller F (2018) The effects of ozone on human health. Environ Sci Pollut Res 25:8074–8088. https://doi.org/10.1007/s11356-017-9239-3
Pusede SE, Steiner AL, Cohen RC (2015) Temperature and recent trends in the Chemistry of Continental Surface ozone. Chem Rev 115:3898–3918. https://doi.org/10.1021/cr5006815
Quanjer PH, Stanojevic S, Cole TJ et al (2012) Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 40:1324–1343. https://doi.org/10.1183/09031936.00080312
State of Israel (2008) Clean Air Law
Suglia SF, Wright RO, Schwartz J, Wright RJ (2008) Association between lung function and cognition among children in a prospective birth Cohort Study. Psychosom Med 70:356–362. https://doi.org/10.1097/PSY.0b013e3181656a5a
Taylor MD, Hart CL, Smith GD et al (2005) Childhood IQ and social factors on smoking behaviour, lung function and smoking-related outcomes in adulthood: linking the Scottish Mental Survey 1932 and the midspan studies. Br J Health Psychol 10:399–410. https://doi.org/10.1348/135910705X25075
Tsui HC, Chen CH, Wu YH et al (2018) Lifetime exposure to particulate air pollutants is negatively associated with lung function in non-asthmatic children. Environ Pollut 236:953–961. https://doi.org/10.1016/j.envpol.2017.10.092
Urman R, McConnell R, Islam T et al (2014) Associations of children’s lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax 69:540–547. https://doi.org/10.1136/thoraxjnl-2012-203159
US EPA (2020) Integrated Science Assessment (ISA) for ozone and related photochemical oxidants (Final Report). US Environ Prot Agency Washington, DC, EPA/600/R-20/012, 2020
US EPA (2021) Health Effects of Ozone in the General Population | US EPA. https://www.epa.gov/ozone-pollution-and-your-patients-health/health-effects-ozone-general-population. Accessed 5 Feb 2022
US EPA (1992) Guidelines for Exposure Assessment, EPA/600/Z-92/001. Risk Assess Forum, Washington, DC, 1992 57:22888–22938
US EPA (2015) 80 FR 65292 - National Ambient Air Quality Standards for Ozone - Content Details – 2015–26594
Usemann J, Decrue F, Korten I et al (2019) Exposure to moderate air pollution and associations with lung function at school-age: a birth cohort study. Environ Int 126:682–689. https://doi.org/10.1016/j.envint.2018.12.019
Vasilopoulos T, Kremen WS, Grant MD et al (2015) Individual differences in cognitive ability at age 20 predict pulmonary function 35 years later. J Epidemiol Community Health 69:261–265. https://doi.org/10.1136/jech-2014-204143
Virji MA, Kurth L (2021) Peak inhalation exposure Metrics used in occupational epidemiologic and exposure studies. Front Public Heal 8. https://doi.org/10.3389/FPUBH.2020.611693/FULL
Watson AY, Bates RR, Kennedy D (1988) Transport and Uptake of Inhaled Gases
WHO (2008) Health risks of ozone from long-range transboundary air pollution. Copenhagen, Denmark
WHO (2013) Health risks of air pollution in Europe – HRAPIE project. World Heal Organ Organ 60
WHO (2021) WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide
WHO (2006) Air Quality Guidelines Global Update 2005. Particulate matter, ozone, nitrogen dioxide and sulfur dioxide. 22:485. https://doi.org/10.1016/0004-6981(88)90109-6
Xing X, Hu L, Guo Y et al (2020) Interactions between ambient air pollution and obesity on lung function in children: the seven northeastern Chinese cities (SNEC) study. Sci Total Environ 699:134397. https://doi.org/10.1016/j.scitotenv.2019.134397
Zhang JJ, Wei Y, Fang Z (2019) Ozone pollution: a major health hazard worldwide. Front Immunol 10:1–10. https://doi.org/10.3389/fimmu.2019.02518
Zhu J, Chen L, Liao H, Dang R (2019) Correlations between PM2.5 and ozone over China and Associated underlying reasons. Atmos (Basel) 10:352. https://doi.org/10.3390/atmos10070352
Funding
The authors did not receive financial support from any organization for the submitted work.
Open access funding provided by University of Haifa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of the Israel Defense Forces Medical Corps (Protocol number: 2195 − 2021, June 2021). In accordance with the institutional review board’s approval, individual consent was not necessary as it was a retrospective study which utilized pre-existing data, and all data were anonymized such that there was no identifying information in the analyzed dataset.
The authors declare that they agree with the publication of this paper at Air Quality, Atmosphere & Health.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raz-Maman, C., Borochov-Greenberg, N., Lefkowitz, R.Y. et al. Evaluating the effect of long-term exposure to ozone on lung function by different metrics. Air Qual Atmos Health (2024). https://doi.org/10.1007/s11869-024-01546-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11869-024-01546-x