Abstract
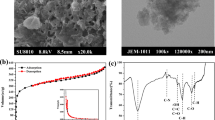
The electro-assisted adsorptive removal of Sr2+ and Cs+ ions from aqueous solution by capacitive deionization (CDI) was studied using activated carbon cloth (ACC) as electrode. Various influencing factors, including initial concentration and the applied voltage, on the removal efficiency of Sr2+ and Cs+ were examined. The results showed that ACC electrode had a large amount of oxygen- and nitrogen-containing functional groups. The removal efficiency of Sr2+ and Cs+ was 40.58% and 62.05%, respectively, which decreased when their initial concentration increased from 3 to 20 mg L−1. The removal efficiency of Sr2+ and Cs+ increased by 26.64% and 17.84% with increase of the applied voltage. CDI process is favorable to remove high valence ions due to the ion-exchange and charge interaction mechanisms. The mixed-order (MO) model could fit the adsorption kinetics of Sr2+ and Cs+ (R2 = 0.938). The Redlich-Peterson isotherm could be used for Sr2+ and Cs+ adsorption. After adsorption, Sr and Cs partly deposited on the surface of the ACC, which did not change the surface structure of the ACC electrode.












Similar content being viewed by others
Data availability
Not applicable.
References
Abdullah MA, Chiang L, Nadeem M (2009) Comparative evaluation of adsorption kinetics and isotherms of a natural product removal by amberlite polymeric adsorbents. Chem Eng J 146:370–376
Aguila B, Banerjee D, Nie Z, Shin Y, Ma S, Thallapally PK (2016) Selective removal of cesium and strontium using porous frameworks from high level nuclear waste. Chem Commun 52:5940–5942
Ahmed MA, Tewari S (2018) Capacitive deionization: processes, materials and state of the technology. J Electroanal Chem 813:178–192
Al Attar L, Safia B, Ghani BA (2018) Uptake of 137Cs and 85Sr onto thermally treated forms of bentonite. J Environ Radioact 193-194:36–43
Al-Jubouri SM, Curry NA, Holmes SM (2016) Hierarchical porous structured zeolite composite for removal of ionic contaminants from waste streams and effective encapsulation of hazardous waste. J Hazard Mater 320:241–251
Atoufi HD, Hasheminejad H, Lampert DJ (2020) Performance of activated carbon coated graphite bipolar electrodes on capacitive deionization method for salinity reduction. Front Environ Sci Eng 14:99
Chegrouche S, Mellah A, Barkat M (2009) Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination 235:306–318
Chen YW, Wang JL (2012) Removal of radionuclide Sr2+ ions from aqueous solution using synthesized magnetic chitosan beads. Nucl Eng Des 242:445–451
Chen, Y.W., Wang, J.L., 2016. Removal of cesium from radioactive wastewater using magnetic chitosan beads cross-linked with glutaraldehyde. Nucl. Sci. Tech. 2016. 27, 432.
Chen H, Shao D, Li J, Wang X (2014) The uptake of radionuclides from aqueous solution by poly(amidoxime) modified reduced graphene oxide. Chem Eng J 254:623–634
Choi J, Dorji P, Shon HK, Hong S (2019) Applications of capacitive deionization: desalination, softening, selective removal, and energy efficiency. Desalination 449:118–130
Dermentzis K (2010) Removal of nickel from electroplating rinse waters using electrostatic shielding electrodialysis/electrodeionization. J Hazard Mater 173:647–652
Dermentzis K, Davidis A, Papadopoulou D, Christoforidis A, Ouzounis K (2009) Copper removal from industrial wastewaters by means of electrostatic shielding driven electrodeionization. J Eng Sci Technol Rev 2:131–136
Divyapriya G, Vijayakumar KK, Nambi I (2019) Development of a novel graphene/Co3O4 composite for hybrid capacitive deionization system. Desalination 451:102–110
El-Kamash AM (2008) Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J Hazard Mater 151:432–445
Faiz Z, Fakhi S, Bouih A, Outayad R, Benkdad A, Hannache H (2017) Leaching study of cesium from spent ion-exchange resins and Portland cement package. Int J Environ Sci Technol 14:1019–1026
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Gerber SJ, Erasmus E (2018) Electronic effects of metal hexacyanoferrates: an XPS and FTIR study. Mater Chem Phys 203:73–81
Ghaly M, El-Dars FMSE, Hegazy MM, Abdel Rahman RO (2016) Evaluation of synthetic birnessite utilization as a sorbent for cobalt and strontium removal from aqueous solution. Chem Eng J 284:1373–1385
Gimbert F, Morin-Crini N, Renault F, Badot P, Crini G (2008) Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: Error analysis. J Hazard Mater 157:34–46
Guo X, Wang JL (2019a) A general kinetic model for adsorption: theoretical analysis and modeling. J Mol Liq 288:111100
Guo X, Wang JL (2019b) Comparison of linearization methods for modeling the Langmuir adsorption isotherm. J Mol Liq 296:111850
Guo X, Wang JL (2019c) Sorption of antibiotics onto aged microplastics in freshwater and seawater. Mar Pollut Bull 14:110511
Han B, Cheng G, Wang Y, Wang X (2019) Structure and functionality design of novel carbon and faradaic electrode materials for high-performance capacitive deionization. Chem Eng J 360:364–384
Hsieh C, Teng H (2002) Influence of oxygen treatment on electric double-layer capacitance of activated carbon fabrics. Carbon 40:667–674
Hu C, Wang T, Dong J, Liu R, Liu H, Qu J (2018) Capacitive deionization from reconstruction of NiCoAl-mixed metal oxide film electrode based on the “memory effect”. Appl Surf Sci 459:767–773
Hu YM, Guo X, Chen C, Wang JL (2019) Algal sorbent derived from Sargassum horneri for adsorption of cesium and strontium ions: equilibrium, kinetics, and mass transfer. Appl Microbiol Biotechnol 103:2833–2843
Huang C, Su Y (2010) Removal of copper ions from wastewater by adsorption/electrosorption on modified activated carbon cloths. J Hazard Mater 175:477–483
Huang S, Fan C, Hou C (2014) Electro-enhanced removal of copper ions from aqueous solutions by capacitive deionization. J Hazard Mater 278:8–15
Huang Z, Lu L, Cai Z, Ren ZJ (2016) Individual and competitive removal of heavy metals using capacitive deionization. J Hazard Mater 302:323–331
Ibrahim HA, Hassan HS, Mekhamer HS, Kenawy SH (2019) Diffusion and sorption of Cs+ and Sr2+ ions onto synthetic mullite powder. J Radioanal Nucl Chem 319:1–12
Ito T, Xu Y, Kim S, Nagaishi R, Kimura T (2016) Adsorption behavior and radiation effects of a silica-based (Calix(4)+Dodecanol)/SiO2-P adsorbent for selective separation of Cs(I) from high level liquid waste. Sep Sci Technol 51:22–31
Jin QH, Cui CY, Chen HY, Wu J, Hu J, Xing X, Geng JF, Wu YH (2019) Effective removal of Cd2+ and Pb2+ pollutants from wastewater by dielectrophoresis-assisted adsorption. Front Environ Sci Eng 13:16
Kocaoba S (2009) Adsorption of Cd(II), Cr(III) and Mn(II) on natural sepiolite. Desalination 244:24–30
Leong ZY, Lu G, Yang HY (2019) Three-dimensional graphene oxide and polyvinyl alcohol composites as structured activated carbons for capacitive desalination. Desalination 451:172–181
Li H, GaoY PL, Zhang Y, Chen Y, Sun Z (2008) Electrosorptive desalination by carbon nanotubes and nanofibres electrodes and ion-exchange membranes. Water Res 42:4923–4928
Li H, Zou L, Pan L, Sun Z (2010) Using graphene nano-flakes as electrodes to remove ferric ions by capacitive deionization. Sep Purif Technol 75:8–14
Li T, Xiao K, Yang B, Peng GL, Liu FL, Tao LY, Chen SY, Wei HR, Yu G, Deng SB (2019) Recovery of Ni(II) from real electroplating wastewater using fixed-bed resin adsorption and subsequent electrodeposition. Front Environ Sci Eng 13:91
Li M, Liang S, Wu Y, Yang M, Huang X (2020) Cross-stacked super-aligned carbon nanotube/activated carbon composite electrodes for efficient water purification via capacitive deionization enhanced ultrafiltration. Front Environ Sci Eng 14:107
Ling J, Zou H, Yang W, Chen W, Lei K, Chen S (2018) Facile fabrication of polyaniline/molybdenum trioxide/activated carbon cloth composite for supercapacitors. J Energy Storage 20:92–100
Liu XJ, Wang JL (2020) Electro-assisted adsorption of Cs(I) and Co(II) from aqueous solution by capacitive deionization with activated carbon cloth/graphene oxide composite electrode. Sci Total Environ 749:141524
Liu Y, Nie C, Liu X, Xu X, Sun Z, Pan L (2015) Review on carbon-based composite materials for capacitive deionization. RSC Adv 5:15205–15225
Liu XJ, Wu JL, Wang JL (2019) Electro-enhanced removal of cobalt ions from aqueous solution by capacitive deionization. Sci Total Environ 697:134144
Ma F, Li Z, Zhao H, Geng Y, Zhou W, Li Q, Zhang L (2017) Potential application of graphene oxide membranes for removal of Cs(I) and Sr(II) from high level-liquid waste. Sep Purif Technol 188:523–529
Mahmood Z, Azam M, Mushtaq A, Kausar R, Kausar S, Gilani SR (2006) Comparative vapour phase FTIR spectra and vibrational assignment of manganese pentacarbonyls derivatives of the type XMn(CO)5: (where X=Br, Cl, I, H, D, CH3, CD3, CF3). Spectrochim Acta A 65:445–452
Miao Z, Huang Y, Xin J, Su X, Sang Y, Liu H, Wang J (2019) High-performance symmetric supercapacitor constructed using carbon cloth boosted by engineering oxygen-containing functional groups. Appl Mater Interfaces 11:18044–18050
Mossad M, Zou L (2012) A study of the capacitive deionisation performance under various operational conditions. J Hazard Mater 213-214:491–497
Mu W, Yu Q, Li X, Wei H, Jian Y (2017) Efficient removal of Cs+ and Sr2+ from aqueous solution using hierarchically structured hexagonal tungsten trioxide coated Fe3O4. Chem Eng J 319:170–178
Mu W, Du S, Li X, Yu Q, Hu R, Wei H, Yang Y, Peng S (2019) Efficient and irreversible capture of strontium ions from aqueous solution using metal-organic frameworks with ion trapping groups. Dalton Trans 48:3284–3290
Ng JCY, Cheung WH, McKay G (2002) Equilibrium studies of the sorption of Cu(II) ions onto chitosan. J Colloid Interface Sci 255:64–74
Nie Z, Finck N, Heberling F, Pruessmann T, Liu C, Lützenkirchen J (2017) Adsorption of selenium and strontium on goethite: EXAFS study and surface somplexation modeling of the ternary systems. Environ Sci Technol 51:3751–3758
Oladunni J, Zain JH, Hai A, Banat F, Bharath G, Alhseinat E (2018) A comprehensive review on recently developed carbon based nanocomposites for capacitive deionization: from theory to practice. Sep Purif Technol 207:291–320
Ryu J, Kim S, Hong H, Hong J, Kim M, Ryu T, Park I, Chung K, Jang JS, Kim B (2016) Strontium ion (Sr2+) separation from seawater by hydrothermally structured titanate nanotubes: removal vs. recovery. Chem Eng J 304:503–510
Seneca SM, Rabideau AJ (2013) Natural zeolite permeable treatment wall for removing Sr-90 from groundwater. Environ Sci Technol 47:1550–1556
Seo S, Jeon H, Lee JK, Kim G, Park D, Nojima H, Lee J, Moon S (2010) Investigation on removal of hardness ions by capacitive deionization (CDI) for water softening applications. Water Res 44:2267–2275
Shen W, Fan W (2013) Nitrogen-containing porous carbons: synthesis and application. J Mater Chem A 1:999–1013
Sun Y, Shao D, Chen C, Yang S, Wang X (2013) Highly efficient enrichment of radionuclides on graphene oxide-supported polyaniline. Environ Sci Technol 47:9904–9910
Tamura K, Kogure T, Watanabe Y, Nagai C, Yamada H (2014) Uptake of cesium and strontium ions by artificially altered phlogopite. Environ Sci Technol 48:5808–5815
Tang W, Liang J, He D, Gong J, Tang L, Liu Z, Wang D, Zeng G (2019a) Various cell architectures of capacitive deionization: recent advances and future trends. Water Res 150:225–251
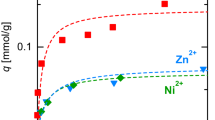
Tang W, Wang X, Zeng G, Liang J, Li X, Xing W, He D, Tang L, Liu Z (2019b) Electro-assisted adsorption of Zn(II) on activated carbon cloth in batch-flow mode: experimental and theoretical investigations. Environ Sci Technol 53:2670–2678
Tayyebi A, Outokesh M, Moradi S, Doram A (2015) Synthesis and characterization of ultrasound assisted “graphene oxide–magnetite” hybrid, and investigation of its adsorption properties for Sr(II) and Co(II) ions. Appl Surf Sci 353:350–362
Trypidis D, García-González DL, Lobo-Prieto A, Nenadis N, Tsimidou MZ, Tena N (2019) Real time monitoring of the combined effect of chlorophyll content and light filtering packaging on virgin olive oil photo-stability using mesh cell-FTIR spectroscopy. Food Chem 295:94–100
Villard A, Siboulet B, Toquer G, Merceille A, Grandjean A, Dufrêche J (2015) Strontium selectivity in sodium nonatitanate Na4Ti9O20·xH2O. J Hazard Mater 283:432–438
Wang JL, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wang JL, Chen C (2014) Chitosan-based biosorbents: modification and application for biosorption of heavy metals and radionuclides. Bioresour Technol 160:129–141
Wang JL, Guo X (2020a) Adsorption isotherm models: classification, physical meaning, application and solving method. Chemosphere 258:127279
Wang JL, Guo X (2020b) Adsorption kinetic models: physical meanings, applications, and solving methods. J Hazard Mater 390:122156
Wang JL, Zhuang ST (2019) Removal of cesium ions from aqueous solutions using various separation technologies. Rev Environ Sci Biotechnol 18:231–269
Wang JL, Zhuang ST (2020) Covalent organic frameworks (COFs) for environmental applications. Coord Chem Rev 400:213046
Wang JL, Zhan XM, Qian Y (2000) Removal of Cr(VI) from aqueous solution by macroporous resin adsorption. J Environ Sci Health Part A 35:1211–1230
Wang JL, Zhan XM, Ding DC, Zhou D (2001) Biosorption of lead(II) from aqueous solution by fungal biomass of Aspergillus niger. J Biotechnol 87:273–277
Wang JL, Zhuang ST, Liu Y (2018) Metal hexacyanoferrates-based adsorbents for cesium removal. Coord Chem Rev 374:430–438
Wen T, Zhao Z, Shen C, Li J, Tan X, Zeb A, Wang X, Xu A (2016) Multifunctional flexible free-standing titanate nanobelt membranes as efficient sorbents for the removal of radioactive 90Sr2+ and 137Cs+ ions and oils. Sci Rep:1–10
Xu LJ, Wang JL (2017) The application of graphene-based materials for the removal of heavy metals and radionuclides from water and wastewater. Crit Rev Environ Sci Technol 47:1042–1105
Yang Y, Chen T, Li P, Liu H, Xie J, Xie Q, Zhan XM (2014) Removal and recovery of Cu and Pb from single and Cu–Pb–Cd–Zn multi-metal solutions by modified pyrite: fixed bed columns. Ind Eng Chem Res 53:18810–18188
Yu F, Wang L, Wang Y, Shen X, Cheng Y, Ma J (2019) Faradaic reactions in capacitive deionization for desalination and ion separation. J Mater Chem A 7:15999–16027
Zhan XM, Zhao X (2003) Mechanism of lead adsorption from aqueous solutions using an adsorbent synthesized from natural condensed tannin. Water Res 37:3905–3912
Zhan XM, Zhao X, Miyazaki A, Nakano Y (2003) Removal of lead from aqueous solutions by condensed tannin gel adsorbent. J Environ Sci 15:102–106
Zhang H, Liang P, Bian Y, Jiang Y, Sun X, Zhang C, Huang X, Wei F (2016a) Moderately oxidized graphene–carbon nanotubes hybrid for high performance capacitive deionization. RSC Adv 6:58907–58915
Zhang A, Zhang W, Wang Y, Ding X (2016b) Effective separation of cesium with a new silica-calix [4] biscrown material by extraction chromatography. Sep Purif Technol 171:17–25
Zhang M, Gu P, Zhang Z, Liu J, Dong L, Zhang G (2018) Effective, rapid and selective adsorption of radioactive Sr2+ from aqueous solution by a novel metal sulfide adsorbent. Chem Eng J 351:668–677
Zhao Y, Hu X, Jiang B, Li L (2014) Optimization of the operational parameters for desalination with response surface methodology during a capacitive deionization process. Desalination 336:64–71
Funding
The research was supported by the National Key Research and Development Program (2016YFC1402507) and the Program for Changjiang Scholars and Innovative Research Team in University (IRT-13026).
Author information
Authors and Affiliations
Contributions
Li Xiaojing: investigation, formal analysis, writing and editing; Wang Jianlong: conceptualization, funding acquisition, methodology, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Consent to participate
Not applicable
Consent to publish
Not applicable
Competing interest
The authors declare that they have no competing interest.
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Wang, J. Adsorptive removal of Sr2+ and Cs+ from aqueous solution by capacitive deionization. Environ Sci Pollut Res 28, 3182–3195 (2021). https://doi.org/10.1007/s11356-020-10691-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10691-6