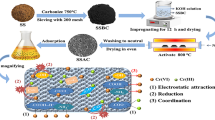
Abstract
Due to the environmental concerns and the potential hazards and risks posed by chromium (VI), its removal from aquatic habitats has become a serious demand. In this paper, novel modifications were employed to increase the adsorption performance of Cr (VI) using activated carbon (AC) derived from sugarcane bagasse activated with phosphoric acid. AC was consequently modified with triethoxysilane propylamine and diethylene triamine as silica (Si) and nitrogen (N) sources, respectively. SEM-EDX, N2 adsorption isotherms and FTIR analyses were performed to characterize the adsorbents. Equilibrium adsorption behavior of Cr(VI) ions with respect to varying in surface characteristics of resulting materials was investigated using Langmuir, Freundlich and Temkin isotherms. Kinetic and thermodynamic studies of Cr(VI) adsorption over AC, Si@AC and Si-N@AC were carried out. Freundlich model described the adsorption isotherm of AC and Si-N@AC severally, suggesting that the Cr(VI) removal was multi-layer adsorption. However, Langmuir model satisfied with adsorption of Cr (VI) over Si@AC. Pseudo-second order model fitted well the adsorption suggesting the chemical interaction. As a result of doping AC with Si and N, high adsorption capacities obtained at pH 2 and 30oC were to be 268 and 233 mg/g for Si@AC and Si-N@AC which are especially superior compared to others in literature.









Similar content being viewed by others
References
Gupta VK, Rastogi A, Nayak A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Coll Interf Sci 342:135–141
Uysal M, Irfan A (2007) Removal of cr(VI) from industrial wastewaters by adsorption part I: determination of optimum condition. J Hazard Mater 149:482–491
Fathy NA, El-Wakeel ST, Abd El-Latif RR (2015) Biosorption and desorption studies on chromium (VI) by novel biosorbents of raw rutin and rutin resin. J Environ Chem Eng 3:1137–1145
Shouman MA, Fathy NA, Khedr SA, Attia AA (2013) Comparative biosorption studies of hexavalent chromium ion onto raw and modified palm branches. Adv Phys Chem 2013:1–9
Wang H, Wang W, Zhou S, Gao X (2023) Adsorption mechanism of cr(VI) on woody-activated carbons. Heliyon 9:e13267
Kumar A, Jena HM (2017) Adsorption of cr(VI) from aqueous phase by high surface area activated carbon prepared by chemical activation with ZnCl2. Process Safe Environ Protect 109:63–71
Fang Y, Yang K, Zhang Y, Peng C, Robledo-Cabrera A, López-Valdivieso A (2021) Highly surface activated carbon to remove cr(VI) from aqueous solution with adsorbent recycling. Environ Res 197:111151
Kumar A, Jena HM (2017) Adsorption of cr(VI) from aqueous solution by prepared high surface area activated carbon from Fox nutshell by chemical activation with H3PO4. J Environ Chem Eng 5:2032–2041
Demiral H, Demiral I, Tümsek F, Karabacakoğlu B (2008) Adsorption of chromium (VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem Eng J 144:188–196
Karthikeyan T, Rajgopal S, Miranda LR (2005) Chromium (VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J Hazard Mater 124:192–199
Labied R, Benturki O, Hamitouche AE, Donnot A (2018) Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): kinetic, equilibrium, and thermodynamic study. Adsorp Sci Technol 36:1066–1099
Ammar NS, Fathy NA, Ibrahim HS, Mousa SM (2021) Micro-mesoporous modified activated carbon from corn husks for removal of hexavalent chromium ions. Appl Water Sci 11:1–12
Adebayo GB, Adegoke HI, Fauzeeyat S (2020) Adsorption of cr(VI) ions onto goethite, activated carbon and their composite: kinetic and thermodynamic studies. Appl Water Sci. https://doi.org/10.1007/s13201-020-01295-z.
Aggarwal D, Goyal M, Bansal RC (1999) Adsorption of chromium by activated carbon from aqueous solution. Carbon 37:1989–1997
Chen F, Guo S, Wang Y, Ma L, Li B, Song Z, Huang L, Zhang W (2022) Concurrent adsorption and reduction of chromium (VI) to chromium(III) using nitrogen-doped porous carbon adsorbent derived from loofah sponge. Front Environ Sci Eng 16(5):57
Chu B, Amano Y, Machida M (2020) Preparation of bean dreg derived N-doped activated carbon with high adsorption for cr(VI). Coll Surf A: Physicochem Eng Aspects 586:124262
Fathy NA, El-Khouly SM, El-Shafey OI (2021) Modified carbon nanostructures obtained from sugarcane bagasse hydrochar for treating chromium-polluted water. Curr Anal Chem 17:975–988
Samuel MS, Bhattacharya J, Raj S, Santhanam N, Singh H, Singh ND (2019) Efficient removal of chromium(VI) from aqueous solution using chitosan grafted graphene oxide (CS-GO) nanocomposite. Int J Biol Macromol 121:285–292
Baig N, Chauhan DS, Saleh TA, Quraishi MA (2019) Diethylenetriamine functionalized graphene oxide as a novel corrosion inhibitor for mild steel in hydrochloric acid solutions. New J Chem 43:2328–2337
Karthik R, Meenakshi S (2014) Removal of hexavalent chromium ions using polyaniline/silica gel composite. J Water Proc Eng 1:37–45
Bilgiç A, Çimen A (2019) Removal of chromium(VI) from polluted wastewater by chemical modification of silica gel with 4-acetyl-3-hydroxyaniline. RSC Adv 9:37403–37414
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Temkin MI, Pyzhev V (1940) Kinetic of ammonia synthesis on promoted iron catalyst. Acta Phys Chem URSS 12:327–356
Lagergren S (1898) Zurtheorie der sogenannten adsorption gelosterstoffe. 591. Kungliga Svenska Vetenska psakademiens. Handlingar 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Fu L, Zhu I, Huang W, Fang J, Sun X, Wang X, Liao K (2020) Preparation of nano-porous carbon-silica composites and its adsorption capacity to volatile organic compounds. Processes 8:372
Chatterjee R, Sajjadi B, Chane W, Mattern DL, Hamner N, Raman V, Dorris A (2020) Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front Energy Res 8:85
Mohamed GM, Rashwan WE, Fathy NA, Ahmed SA (2019) Effect of nitrogen functionalization on the adsorption performance of commercial charcoal activated with phosphoric acid. Desal Water Treat 148:178–187
Nasseh N, Khosravi R, Abu Rumman G, Ghadirian M, Eslami H, Khoshnamv M, Al-Musawi TG, Khosravi A (2021) Adsorption of cr(VI) ions onto powdered activated carbon synthesized from Peganum harmala seeds by ultrasonic waves activation. Environ Technol Innov 21:101277
Hussain I, Qi J, Sun X, Wang L, Li J (2020) Melamine derived nitrogen doped carbon sheet for the efficient removal of chromium(VI). J Molec Liquids 318:114052
Guo C, Ding L, Jin X, Zhang H, Zhang D (2021) Application of response surface methodology to optimize chromium(VI) removal from aqueous solution by cassava sludge-based activated carbon. J Environ Chem Eng 9(1):104785
El-Sherif IY, Fathy NA (2013) Modification of the adsorptive characteristics of bagasse fly ash for uptaking cadmium from aqueous solution. Environ Res Eng Manag 2:19–28
Singh S, Anil AG, Khasnabis S, Kumar V, Nath B, Adiga V, Naik TS, Subramanian S, Kumar V, Singh J, Ramamurthy PC (2022) Sustainable removal of cr(VI) using graphene oxide-zinc oxide nanohybrid: adsorption kinetics, isotherms and thermodynamics. Environ Res 203:111891
Khan MA, Kim S-W, Khan Rao RA, Abou-Shanab RAI, Bhatnagar A, Song H, Jeon B-H (2010) Adsorption studies of dichloromethane on some commercially available GACs: effect of kinetics, thermodynamics and competitive ions. J Hazard Mater 178:963–972
Acknowledgements
Authors are thankful to the National Research Center, Egypt for supporting this work with technical facilities including chemicals and equipments.
Funding
The author(s) received no specific financial funding for this work.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally in this work.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohamed, G.M., Sayed Ahmed, S.A. & Fathy, N.A. Activated Carbon Doped with Silica and Nitrogen as Novel Adsorbent for Enhancing Adsorption Capacity of Cr(VI). Chemistry Africa 6, 3097–3107 (2023). https://doi.org/10.1007/s42250-023-00709-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00709-0