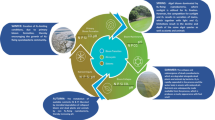
Abstract
Harmful algal blooms (HABs), which have expanded worldwide in their occurrence and frequency, are a serious menace to aquatic ecosystems and humans. The development of rapid, accurate and cost-effective detection systems for toxic algal monitoring in aquatic environments is urgently required. Although many efforts have been devoted to develop reliable tools to monitor the entire spectrum of existing toxic algae, a portable semi-automated system that enables HAB monitoring at a low cost is still not available for general purchase. This work reviews the challenges and opportunities in translating the remarkable progress of electrochemical genosensors-based methods towards practical in situ HAB monitoring applications. It is specifically focused on reviewing the optimised methods for a detection system based on a sandwich hybridisation assay (SHA) performed over transducer platforms of different materials, geometries and dimensions and presenting the diverse advantages and disadvantages among them. Probe design and specificity and optimisation of the genosensor in terms of hybridisation conditions and electrochemical signal are discussed as well as their long-term stability and storage and semi-automation attempts. With continuous innovation and attention to key challenges, we expect semi-automatic devices containing DNA-based electrochemical biosensors to have an important impact upon monitoring of serious HAB events.




Similar content being viewed by others
References
Anderson DM, Kulis D, Erdner D, Ahn S, Walt D (2006) Fibre optic microarrays for the detection and enumeration of harmful algal bloom species. Afr J Mar Sci 28:231–235
Anderson DM, Kulis DM, Keafer BA, Gribble KE, Marin R, Scholin CA (2005) Identification and enumeration of Alexandrium spp. from the Gulf of Maine using molecular probes. Deep-Sea Res II 52:2467–2490
Ayers K, Rhodes LL, Tyrrell J, Gladstone M, Scholin CA (2005) International accreditation of sandwich hybridization assay format DNA probes for micro-algae. New Zeal J Mar Fresh 39:1225–1231
Campuzano S, Kuralay F, Wang J (2012) Ternary monolayer interfaces for ultrasensitive and direct bioelectronic detection of nucleic acids in complex matrices. Electroanalysis 24:483–493
Castañeda MT, Merkoçi A, Pumera M, Alegret S (2007) Electrochemical genosensors for biomedical applications based on gold nanoparticles. Biosens Bioelectron 22:1961–1967
DeLong EF, Wickham GS, Pace NR (1989) Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science 243:1360–1363
Diercks S, Metfies K, Medlin, LK (2008a) Colorimetric detection of the toxic dinoflagellate Alexandrium minutum using sandwich hybridization in a microtiter plate assay. Harmful Algae 7:137–145
Diercks S, Metfies K, Medlin LK (2008b) Development and adaptation of a multiprobe biosensor for the use in a semi-automated device for the detection of toxic algae. Biosens Bioelectr 23:1527–1533
Diercks S, Metfies K, Medlin LK (2008c) Molecular probe sets for the detection of toxic algae for use in sandwich hybridisation formats. J Plankton Res 30(4):439–448
Diercks, S, Metfies, K, Jäckel, S, Medlin, LK (2008d) Development and adaptation of a multiprobe biosensor for the use in a semi-automated device for the detection of toxic algae. Biosens Bioelectron 23:1527–1533
Diercks S, Gescher C, Metfies K, Medlin LK (2009) Evaluation of locked nucleic acids for signal enhancement of oligonucleotide probes for microalgae miniaturised on solid surfaces. J Appl Phycol 21:657–668
Diercks-Horn S, Metfies K, Jäckel S, Medlin LK (2011) The ALGADEC device: a semi-automated rRNA biosensor for the detection of toxic algae. Harmful Algae 10:395–401
Díez B, Pedrós-Alió C, Massana R (2001) Study of genetic diversity of eukaryotic picoplankton in different oceanic regions by small-subunit rRNA gene cloning and sequencing. Appl Environ Microbiol 67:2942–2951
Gescher C, Metfies K, Medlin LK (2008) The ALEX CHIP—development of a DNA chip for identification and monitoring of Alexandrium. Harmful Algae 7:485–494
Giovannoni SJ, Britschgi TB, Moyer CL, Field KG (1990) Genetic diversity in Sargasso Sea bacterioplankton. Nature 345:60–63
Greenfield DI, Marin R, Jensen S, Massion E, Roman B, Feldman J, Scholin CA (2006) Application of environmental sample processor (ESP) methodology for quantifying Pseudo-nitzschia australis using ribosomal RNA-targeted probes in sandwich and fluorescent in situ hybridisation formats. Limnol Oceanogr Meth 4:426–435
Groben R, Medlin LK (2005) In situ hybridisation of phytoplankton using fluorescently-labelled rRNA Probes. In: Zimmer EA, Roalson E (ed) Methods in Enzymology. Elsevier, San Diego, 395, 299–310
Groben R, John U, Eller G, Lange M, Medlin LK (2004) Using fluorescently labelled rRNA probes for hierarchical estimation of phytoplankton diversity. Nova Hedw 79:313–320
Guschin DY, Mobarry BK, Proudnikov D, Stahl DA, Rittmann BE, Mirzabekov AD (1997) Oligonucleotide microchips as genosensors for determinative and environmental studies in microbiology. Appl Environ Microbiol 63:2397–2402
Hallegraeff GM (1993) A review of harmful algal blooms and their apparent global increase. Phycologia 32:79–99
Haywood AJ, Scholin CA, Marin R, Steidinger KA, Heil C, Ray J (2007) Molecular detection of the brevetoxin-producing dinoflagellate Karenia brevis and closely related species using rRNA-targeted probes and a semiautomated sandwich hybridisation assay. J Phycol 43:1271–1286
John U, Cembella A, Hummert C, Ellbrächter M, Groben R, Medlin LK (2003) Discrimination of the toxigenic dinoflagellates Alexandrium tamarense and A. ostenfeldii in co-occurring natural populations from Scottish coastal waters. Eur J Phycol 38:25–40
John U, Medlin LK, Groben R (2005) Development of specific rRNA probes to distinguish between geographic clades of the Alexandrium tamarense species complex. J Plank Res 27:199–204
Keller MD, Selvin RC, Claus W, Guillard RRL (1987) Media for the culture of oceanic ultraphytoplankton. J Phycol 23:633–638
Ki J-S, Han M-S (2006) A low-density oligonucleotide array study for parallel detection of harmful algal species using hybridisation of consensus PCR products of LSU rDNA D2 domain. Biosens Bioelectron 21:1812–1821
Liao JC, Mastali M, Li Y, Gau V, Suchard MA, Babbitt J, Gornbein J, Landaw EM, McCabe ERB, Churchill BM, Haake DA (2007) Development of an advanced electrochemical DNA biosensor for bacterial pathogen detection. J Mol Diag 9:158–168
Lockhart DJ, Dong H, Byrne MC, Follettie MT, Gallo MV, Chee MS, Mittmann M, Wang C, Kobayashi M, Horton H, Brown EL (1996) Expression monitoring by hybridisation to high-density oligonucleotide arrays. Nat Biotechnol 14:1675–1680
Ludwig WSO, Westram R, Richter L, Meier H, Yadhukumar BA, Lai T, Steppi S, Jobb G, Förster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, König A, Liss T, Lüßmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer K-H (2004) ARB, a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Metfies K, Medlin LK (2004) DNA-microchips for phytoplankton. The fluorescent wave of the future. Nova Hedw 79:321–327
Metfies K, Huljic S, Lange M, Medlin LK (2005) Electrochemical detection of the toxic dinoflagellate Alexandrium ostenfeldii with a DNA-biosensor. Biosen Bioelec 20:1349–1357
Metfies K, Gescher C, Frickenhaus S, Niestroy R, Wichels A, Gerdts G, Knefelkamp B, Wiltshire K, Medlin LK (2010) Contribution of the Class Cryptophyceae to phytoplankton structure in the German Bight. J Phycol 46:1152–1160
Miller CA, Scholin PE (1998) Identification and enumeration of cultured and wild Pseudo-nitzschia (Bacillariophyceae) using species specific LSU rRNA-targeted fluorescent probes and filter-based whole cell hybridization. J Phycol 34:371–382
Moon-van der Staay SY, De Wachter R, Vaulot D (2001) Oceanic 18S rDNA sequences from picoplankton reveal unsuspected eukaryotic diversity. Nature 409:607–610
O’Halloran C, Silver MW, Holman TR, Scholin CA (2006) Heterosigma akashiwo in central California waters. Harmful Algae 5:124–132
Orozco J, Medlin L (2011) Electrochemical performance of a DNA-based sensor device for detecting toxic algae. Sensors and Actuators B: Chemical 153:71–77
Orozco J, Suárez G, Fernández-Sánchez C, McNeil C, Jiménez-Jorquera C (2007) Characterization of ultramicroelectrode arrays combining electrochemical techniques and optical microscopy imaging. Electrochim Acta 53:729–736
Orozco J, Jiménez-Jorquera C, Fernández-Sánchez C (2009) Gold nanoparticle ultramicroelectrode arrays for biosensing: a comparative assessment. Bioelectrochemistry 75:176–181
Orozco J, Jiménez-Jorquera C, Fernández-Sánchez C (2010) Ultramicroelectrode arrays: a promising analytical tool for environmental monitoring. Sensors 10:475–490
Orozco J, Baudart J, Medlin L (2011) Evaluation of probe orientation and effect of the digoxigenin-enzymatic label in a sandwich hybridisation format to develop toxic algal biosensors. Harmful Algae 10:489–494
Orozco J, Jiménez-Jorquera C, Fernández-Sánchez C (2012) Electrochemical performance of self-assembled monolayers at gold nanoparticle-modified ultramicroelectrode arrays architectures. Electroanalysis 24:635–642
Penna A, Magnani M (1999) Identification of Alexandrium (Dinophyceae) species using PCR and rDNA-targeted probes. J Phycol 35:615–621
Rautio FBKB, Lahdenpera J, Breinstein A, Molin S, Neubaure P (2003) Sandwich hybridisation assay for quantitative detection of yeast RNAs in crude cell lysates. Microbial Cell Factories 2:4
Scholin CA, Buck KR, Britschig T, Cangelosi G, Chavez FP (1996) Identification of Pseudo-nitzschia australis (Bacillariophyceae) using rRNA-targeted probes in whole cell and sandwich hybridisation formats. Phycologia 35:190–197
Scholin C, Miller P, Buck K, Chavez F, Harris P, Haydock P, Howard J, Cangelosi G (1997) Detection and quantification of Pseudo-nitzschia australis in cultured and natural populations using LSU rRNA-targeted probes. Limnol Oceanogr 42:1265–1272
Scholin C, Doucette G, Jensen S, Roman B, Pargett D, Marin R II, Preston C, Jones W, Feldman J, Everlove C, Harris A, Alvarado N, Massion E, Birch J, Greenfield D, Vrijenhoek R, Mikulski C, Jones K (2009) Remote detection of marine microbes, small invertebrates, harmful algae, and biotoxins using the environmental sample processor (ESP). Oceanography 22:158–167
Töbe K, Eller G, Medlin LK (2006) Automated detection and enumeration for toxic algae by solid-phase cytometry and the introduction of a new probe for Prymnesium parvum (Haptophyta: Prymnesiophyceae). J Plank Res 7:643–657
Touzet N, Keady E, Raine R, Maher M (2009) Evaluation of taxa-specific real time PCR, whole-cell FISH and morphotaxonomy analyses for the detection and quantification of the toxic microalgae Alexandrium minutums (Dinophyceae), Global Clade ribotype. FEMS Microbiol Ecol 67:329–341
Touzet N, Davidson K, Pete R, Flanagan K, McCoy GR, Amzil Z, Maher M, Chapelle A, Raine R (2010a) Co-occurrence of the West European (Gr.II) and North American (Gr.I) ribotypes of Alexandrium tamarense (Dinophyceae) in Shetland, Scotland. Protist 161:370–384
Touzet N, Farell H, Ní Rathaille A, Rodriguez P, Alfonso A, Botana L, Raine R (2010b) Dynamics of co-occurring Alexandrium minutum (Global Clade) and A. tamarense (West European) (Dinophyceae) during a summer bloom in Cork Harbour, Ireland. Deep-Sea Res II 57:268–278
Tyrrell JV, Connell LB, Scholin CA (2002) Monitoring for Heterosigma akashiwo using a sandwich hybridisation assay. Harmful Algae 1:205–214
van der Bos ES, Doelen AA, van Rooy N, Schuurs AHWM (1981) 3,3′, 5,5′-Tetramethylbenzidine as an Ames test negative chromogen for horse-radish peroxidase in enzyme-immunoassay. J Immunoass 2:187–204
Voogd CE, Vanderstel JJ, Jacobs J (1980) On the mutagenic action of some enzymeimmunoassay substrates. J Immunol Methods 36:55–61
Wang Q, Li NQ, Wu YH (2001) Direct electrochemistry of cytochrome c at a metallothioneins self-assembled gold electrode. Electroanalysis 13:149–154
Zammatteo N, Moris P, Alexandre I, Vaira D, Piette J, Remacle J (1995) DNAprobe hybridisation in microwells using a new bioluminiscent system for the detection of PCR-amplified HIV-1 proviral DNA. J Virological Meth 55:185–197
Acknowledgements
J.O. is supported by a Beatriu de Pinós Postdoctoral Fellowship from The Government of Catalonia. This work was partially supported by EU FP7 MIDTAL and BMBF TEPS. Martin Lange performed the RNA biosensor hybridisation within the context of the latter project. Figure 3 was taken from the final report of BMBF TEPS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Orozco, J., Medlin, L.K. Review: advances in electrochemical genosensors-based methods for monitoring blooms of toxic algae. Environ Sci Pollut Res 20, 6838–6850 (2013). https://doi.org/10.1007/s11356-012-1258-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1258-5