Abstract
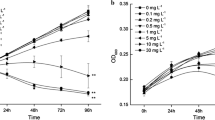
The interaction of natural organic matter with phytoplankton communities in freshwater ecosystems is an intensively studied subject matter. Previous studies showed that apparently plant-derived phenols were able to inhibit algal and cyanobacterial growth. Furthermore, it was also assumed that humic substances (HS), which comprise the major part of dissolved organic carbon in freshwater ecosystems, directly interact with freshwater phototrophs. For example, quinoid building blocks of HS were thought to be algicidal. To identify key environmental variable for the toxic action of potential quinone algicides, we tested the toxicity of hydroquinone (HQ) to different eukaryotic and prokaryotic freshwater phototrophs in terms of growth performance and investigated also the effect of HQ oxidation at different pH values on its algicidal potential. It was shown that cyanobacterial species were much more susceptible to hydroquinone than coccal green algal species were, with Microcystis aeruginosa being the most sensitive species by far. In addition, it was obvious that the aging of hydroquinone-stock solution at pH 11 led to polymerization and, by this process, to a total loss of toxicity; whereas the algicidal potential sustained if the polyphenol was kept at pH 7. Since most lakes with heavy blooms of phototrophs possess pH values clearly above 7.0, it is questionable, if polyphenols in general and quinones in particular are the effective chemicals and if litter and straw leachates are applied as means to combat algal and cyanobacterial blooms.





Similar content being viewed by others
References
Appel HM (1993) Phenolics in ecological interactions—the importance of oxidation. J Chem Ecol 19(7):1521–1552
Bährs H, Steinberg CEW (2012) Impact of two different humic substances on selected coccal green algae and cyanobacteria—changes in growth and photosynthetic performance. Environ Sci Poll Res 19(2):335–346
Bährs H, Menzel R, Kubsch G, Stößer R, Putschew A, Heinze T, Steinberg CEW (2012) Does quinone or phenol enrichment of humic substances alter the primary compound from a non-algicidal to an algicidal preparation? Chemosphere 87:1193–1200
Cason J (1976) Synthesis of benzoquinones by oxidation. In: Adams R et al (eds) Organic reactions, vol IV. John Wiley, New York, pp 305–361
Choe S, Jung IH (2002) Growth inhibition of freshwater algae by ester compounds released from rotted plants. J Ind Eng Chem 8(4):297–304
Dedonder A, van Sumer C (1971) Effect of phenolics and related compounds on growth and respiration of Chlorella vulgaris. Z Pflanzenphysiol 65(1):70–80
DEV (1982) Deutsches Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung: DIN 38412 - Testverfahren mit Wasserorganismen. Band III
Everall NC, Lees DR (1996) The use of barley-straw to control general and blue-green algal growth in a Derbyshire reservoir. Wat Res 30:269–276
Gross EM, Meyer H, Schilling G (1996) Release and ecological impact of algicidal hydrolysable polyphenols in Miriophyllum spicatum. Phytochemistry 41:133–138
Hong Y, Hu HY, Xie X, Sakoda A, Sagehashi M, Li FM (2009) Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat Toxicol 91(3):262–269. doi:10.1016/j.aquatox.2008.11.014
Islami HR, Filizadeh Y (2011) Use of barley straw to control nuisance freshwater algae. J Am Water Work Assoc 103 (5):111-+
Kim HK, Saifullah KS, Wilson EG, Kricun SDP, Meissner A, Goraler S, Deelder AM, Choi YH, Verpoorte R (2010) Metabolic classification of South American Ilex species by NMR-based metabolomics. Phytochemistry 71(7):773–784
Kostadinova EP, Alipieva KI, Kokubun T, Taskova RM, Handjieva NV (2007) Phenylethanoids, iridoids and a spirostanol saponin from Veronica turrilliana. Phytochemistry 68(9):1321–1326
Lyr H (1965) On the toxicity of oxidized polyphenols. J Phytopath 52:229–240
Martin D, Ridge I (1999) The relative sensitivity of algae to decomposing barley straw. J Appl Phycol 11(3):285–291
Meinelt T, Paul A, Phan TM, Zwirnmann E, Kruger A, Wienke A, Steinberg CE (2007) Reduction in vegetative growth of the water mold Saprolegnia parasitica (Coker) by humic substance of different qualities. Aquat Toxicol 83(2):93–103. doi:10.1016/j.aquatox.2007.03.013
Murray D, Jefferson B, Jarvis P, Parsons SA (2010) Inhibition of three algae species using chemicals released from barley straw. Environ Technol 31(4):455–466
Nakai S, Inoue Y, Hosomi M, Murakami A (2000) Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res 34(11):3026–3032
Nakai S, Inoue Y, Hosomi M (2001) Algal growth inhibition effects and inducement modes by plant-producing phenols. Water Res 35(7):1855–1859
Newman JR, Barrett PRF (1993) Control of Microcystis aeruginosa by decomposing barley straw. J Aquat Plant Manag 31:203–206
OECD (1984) Guideline for testing chemicals: alga, growth inhibition test. Section 2. doi:10.1787/20745761
Park MH, Han MS, Ahn CY, Kim HS, Yoon BD, Oh HM (2006) Growth inhibition of bloom-forming cyanobacterium Microcystis aeruginosa by rice straw extract. Lett Appl Microbiol 43(3):307–312. doi:10.1111/j.1472-765X.2006.01951.x
Pillinger JM, Cooper JA, Ridge I (1994) Role of phenolic compounds in the antialgal activity of barley straw. Chem Ecol 20:1557–1569
Pörs Y, Wüstenberg A, Ehwald R (2010) A batch culture method for microalgae and cyanobacteria with CO2 supply through polyethylene membranes. J Phycol 46:825–830
Prokhotskaya VY, Steinberg CEW (2007) Differential sensitivity of a coccal green algal and a cyanobacterial species to dissolved natural organic matter (NOM). Environ Sci Poll Res 14(1):11–18
Sachse A, Henrion R, Gelbrecht J, Steinberg CEW (2005) Classification of dissolved organic carbon (DOC) in river systems: influence of catchment characteristics and autochthonous processes. Org Geochem 36(6):923–935
Saito K, Matsumoto M, Sekine T, Murakoshi I, Morisaki N, Iwasaki S (1989) Inhibitory substances from Myriophyllum brasiliense on growth of blue-green algae. J Nat Prod 52(6):1221–1226
Skulberg OM (1967) Algal cultures as a means to assess the fertilizing influence of pollution. Wat Poll Control Fed, Washington, DC:113-127
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (Order Chroococcales). Bacteriol Rev 35:171–205
Suau R, Cuevas A, Valpuesta V, Reid MS (1991) Arbutin and sucrose in the leaves of the resurrection plant Myrothamnus flabellifolia. Phytochemistry 30(8):2555–2556
Sun BK, Tanji Y, Unno H (2006) Extinction of cells of cyanobacterium Anabaena circinalis in the presence of humic acid under illumination. Appl Microbiol Biotechnol 72(4):823–828
Tang CS, Waiss AC (1978) Short-chain fatty-acids as growth inhibitors in decomposing wheat straw. J Chem Ecol 4(2):225–232
Waybright TJ, Terlizzi DE, Ferrier MD (2009) Chemical characterization of the aqueous algistatic fraction of barley straw (Hordeum vulgare) inhibiting Microcystis aeruginosa. J Appl Phycol 21(3):333–340
Weidenhamer J, Romeo J (2004) Allelochemicals of Polygonella myriophylla: chemistry and soil degradation. J Chem Ecol 30(5):1067–1082
Wetzel RG (2001) Limnology: Lake and river ecosystems, 3rd edn. Academic, San Diego, 708
Xiao X, Chen YX, Liang XQ, Lou LP, Tang XJ (2010) Effects of Tibetan hulless barley on bloom-forming cyanobacterium (Microcystis aeruginosa) measured by different physiological and morphologic parameters. Chemosphere 81(9):1118–1123. doi:10.1016/j.chemosphere.2010.09.001
Zhao P, Tanaka T, Hirabayashi K, Zhang Y-J, Yang C-R, Kouno I (2008) Caffeoyl arbutin and related compounds from the buds of Vaccinium dunalianum. Phytochemistry 69(18):3087–3094
Acknowledgments
We gratefully acknowledge help from the people of the stress ecology laboratory, particularly Shumon Chakrabarti for assisting with the laboratory work. We particularly thank the Deutsche Forschungsgemeinschaft for supporting this study (Grant STE 673/17-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Braunbeck
Rights and permissions
About this article
Cite this article
Bährs, H., Putschew, A. & Steinberg, C.E.W. Toxicity of hydroquinone to different freshwater phototrophs is influenced by time of exposure and pH. Environ Sci Pollut Res 20, 146–154 (2013). https://doi.org/10.1007/s11356-012-1132-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1132-5