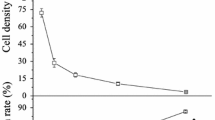
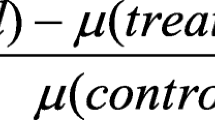Abstract
Background
Sensitivity differences to chemical pollutants in different phytoplankton species may potentially shape the community structure of phytoplankton. However, detailed information supporting the understanding of sensitivity variations between phytoplankton species is still limited.
Results
To investigate sensitivity differences between the cyanobacterium Microcystis aeruginosa, and the green alga Chlorella sp. to paraquat, multiple physiological parameters were measured and compared through acute and chronic toxicity assays. Early photosynthetic responses during acute toxicity assays showed that paraquat affects Photosynthesis System II energy fluxes in M. aeruginosa within 3 h of exposure, but not in Chlorella sp. After 5 h of cumulative exposure, an EC50 based on the maximum quantum yield for primary photochemistry of 0.54 mg L−1 was achieved and remained more or less constant, while the EC50 values for Chlorella fluctuated around 44.76 ± 3.13 mg L−1 after 24 h of exposure. During chronic 96 h exposure to paraquat, differences in antioxidant enzyme activities, reactive oxygen species (ROS) levels, and ultrastructure were observed in both M. aeruginosa and Chlorella sp. An increase in the intracellular levels of ROS and the number of plasma membrane damaged cells was observed in M. aeruginosa in the 0.2, 0.5, and 1.0 mg L−1 treatments (p < 0.01), but not for Chlorella. In addition, at an exposure level of 1.0 mg L−1, extensive disruption of cell structure was observed in M. aeruginosa. Conversely, little disarrangement of organelle structure was found in Chlorella sp.
Conclusion
These results confirm that paraquat is more toxic to M. aeruginosa than to Chlorella sp. The sensitivity differences between these two species (one a prokaryote and the other a eukaryote) to paraquat might be partially explained by the differences in cell structure (cell wall and photosynthetic structure), the enzymatic antioxidant system, and the physiological vulnerability. The multiple physiological endpoint analysis approach used in the current study provides more detailed information for understanding the mechanisms of sensitivity variation between these phytoplankton species.
Similar content being viewed by others
Background
Microalgae play a crucial role as primary producers in the aquatic food web and it is expected that any damage produced by environmental stressors (e.g., chemical pollutants) will likely affect higher trophic levels [1]. The phytoplankton community in a freshwater ecosystem is diverse and often includes cyanobacteria and green microalgae as major components [2]. The sensitivity of different phytoplankton species towards chemical pollutants is highly variable [3,4,5]. At a community level, chemical pollutants might exert selective pressure on phytoplankton community structure. Thus, understanding the physiological basis of sensitivity variation of different species provides the knowledge to explain the adverse outcome pathways of these chemical pressures at the ecological level.
Meanwhile, ecotoxicological assessment of chemical pollutants on phytoplankton has generally been focused on the growth inhibition as a common endpoint, because this is ecologically relevant [4, 6, 7]. However, several sub-cellular physiological and biochemical processes showed faster or stronger effects than apical growth inhibition in unicellular algae-based assays when exposed to toxicants [8]. For instance, Prado et al. reported that after only 24 h of exposure to 0.05 µM paraquat, significant DNA damage was observed in Chlamydomonas moewusii Gerloff, while the growth rate was not affected [9]. Parameters associated with oxidative stress, DNA damage, and cell apoptosis (the mitochondrial membrane potential parameter) have also been shown to be highly sensitive endpoints in unicellular algae exposed to different concentrations of prooxidant pollutants [10, 11]. Moreover, in comparison with single endpoint-based assays (i.e., growth inhibition of phytoplankton), multiple endpoint assays can offer a more comprehensive understanding of the risk presented by toxicants by providing important insights into their modes of action [8]. Chakraborty et al. have evaluated the toxicity of malathion on Anabaena sphaerica Bornet and Flahault using the multiple endpoint approach and found that toxicity resulted in oxidative stress within the cell [12]. However, A. sphaerica could ameliorate the toxicity through the production of superoxide dismutase (SOD), catalase (CAT), ascorbic acid peroxidase, and antioxidants including phenolics and flavonoids. In addition, multiple physiological endpoint experiments can improve our understanding of the mode of action of herbicides on different phytoplankton species [13]. Thus, in the present study, multiple cellular level endpoints were used to study the relative sensitivity of two different phytoplankton species to the photosynthesis inhibitor—paraquat (1, 1′-dimethyl-4, 4′-bipyridilium dichloride).
Furthermore, xenobiotic chemicals affect algal cells as a result of toxicokinetic and toxicodynamic processes. Chemicals can enter algal cells, and reach equilibrium between internal and external concentrations within minutes, while damage cumulatively increases over a period of hours [14, 15]. In addition, the previous research showed that the toxicological effects of a chemical on phytoplankton differ in acute and chronic tests [16, 17]. Therefore, to gain a better and more comprehensive understanding of the mechanisms underlying the modes of action of toxicants, comparative acute and chronic response experiments should be carried out.
Paraquat—a nonselective herbicide widely used to prevent the growth of weeds and grasses—was selected as the photosynthesis inhibitor “model chemical” in this study. It has been known to exert its toxic effects by catalyzing the transfer of electrons from photosystem I (PS I) within the thylakoid membrane, to molecular oxygen by producing oxygen radicals that cause lipid peroxidation and membrane damage [18]. This results in the organelle’s death [19, 20]. A previous study has shown growth sensitivity differences to paraquat exposure for cyanobacteria and green algae [21]. Nestler et al. concluded that the mode of action of paraquat was reflected in the differing responses of multiple endpoints in green alga [8]. However, research relating the mechanisms of sensitivity differences between cyanobacteria and green algae is not well explored. Microcystis is a cyanobacterium species which related to harmful algal blooms in worldwide freshwater ecosystems, and Chlorella is a representative chlorophyte microalga in the aquatic system. In our pre-screening test, we found that the Microcystis and Chlorella are the most sensitive species and the most tolerance species for paraquat, respectively (data were not shown). Thus, in the present study, we elucidated the mechanisms of sensitivity differences of these two representative species, one of which, Microcystis aeruginosa (Kützing) Kützing, is a prokaryote, and the other, Chlorella sp., is a eukaryote. Multiple endpoints were measured at different time scales following acute and chronic exposure. Acute effects were defined as occurring within 24 h (shorter than one cell division under the growth conditions used) [17] and assessed by measuring the response of photosynthetic processes. Chronic toxicity was measured after 96 h (longer than one reproduction cycle under the growth conditions used) [17], using antioxidant enzyme biomarkers, indicators of oxidative stress, photosynthetic processes, and morphological characters as the endpoints.
Materials and methods
Test organisms and culture condition
Eight species and strains of Microcystis spp. and seven species and strains of Chlorella spp. were tested in preliminary microplate assays. Detailed information is presented in Additional file. All the strains were provided by the Freshwater Algae Culture Collection at the Institute of Hydrobiology, Chinese Academy of Sciences (FACHB-Collection; Wuhan, China). The results of preliminary experiments showed that paraquat was more toxic to Microcystis than to Chlorella. Based on preliminary results, axenic strains of M. aeruginosa FACHB-469 and Chlorella sp. FACHB-1512 were selected. Cells were cultivated in BG11 medium at 25 ± 1 °C, and illuminated at white cool fluorescent light lamps (35 µmol photons m−2 s−1), with a light/dark cycle of 12 h: 12 h. The cultures were manually shaken 3 or 4 times each day during incubation.
Experimental design
Three hundred millilitres of BG11 medium spiked with 0 (control), 0.001, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, and 1 mg L−1 paraquat for M. aeruginosa and 0.1, 0.2, 0.5, 1, 5, 10, and 30 mg L−1 for Chlorella sp. was added to 500 mL Erlenmeyer flasks (n = 3). Paraquat (99.7%) was obtained from Macklin (Shanghai, China) and a stock solution made by dissolving 2 g in 1 L of sterile deionized water. The stock solution was stored at 4 °C. Treatment concentrations were established from the preliminary toxicity tests described above which are shown in Additional file 1: Fig. S2. Flasks were inoculated with log-phase cells to an initial cell density of 2.0 × 106 cells mL−1 and 1.2 × 106 cells mL−1 for M. aeruginosa and Chlorella sp., respectively. All the cultures were incubated for 96 h under the conditions as described above.
Measurement of the endpoints
Endpoints for growth performance
The growth performance was quantified via a commonly used method [17]. After each 24 h interval, optical density (OD680) was measured by in UV spectrophotometer (Shimadzu, Kyoto, Japan).
Endpoints for photosynthetic processes
To test the effect of acute exposure (5 min, 1 h, 3 h, 5 h, 9 h, and 24 h) on photosynthetic processes, polyphasic Chl a fluorescence transients were measured using a Handy-Plant Efficiency Analyzer (Handy-PEA, Hansatech, UK) with an actinic light of 3000 µmol photons m−2 s−1. All samples were dark-adapted for 10 min before measurement. The fluorescence signals were recorded within a time period from 10 µs to 2 s, and fluorescence kinetics showed a polyphasic rise over time known as the O–J–I–P transients [22]. The initial fluorescence level O, corresponds to the minimal Chl a fluorescence yield with all PSII reaction centers open, while the J–I transient is caused by the gradual reduction of primary electron acceptors, QA and QB. The P level is the maximal fluorescence yield (FM) when there is an accumulation of QA-Q 2−B [22, 23]. To better analyze the Chl a fluorescence differences between the treatments for different concentrations and exposure times, a total of 10 parameters were evaluated during the JIP test, as shown in Table 1, which shows the PSII energy fluxes [24, 25]. The data are presented as percentages of the corresponding control values, and their absolute value changes were plotted as a radar map. In addition, the maximum quantum yield for primary photochemistry (Fv/Fm) and the maximum relative electron transport rate (rETRmax) were analyzed daily by a Water–PAM fluorescence monitoring system (Walz, Effeltrich, Germany). All samples were dark-adapted for 10 min before measurement. The minimum fluorescence (F0) and the maximum fluorescence (Fm) were measured under a low light intensity and a saturating light pulse, respectively. Fv/Fm can be derived from the equations Fv/Fm = (Fm − F0)/Fm. The EC50 values based on Fv/Fm for different exposure time were calculated according to the method of Franz et al. [16]. The rapid light curve (RLC)—indicating the electron transport responses to increasing irradiance—were then plotted, and rETRmax was calculated according to the relative value of the plateau phase in RLC. During plotting RLC, 10 steps of actinic irradiance (0, 24, 124, 188, 276, 420, 625, 885, 1224, and 1427 µmol photons m−2 s−1) were included with a 20 s interval time between each adjacent step.
Endpoints for oxidative stress
For anti-oxidant relevant enzyme measurements, 40 mL cell suspensions of all treatments were centrifuged (4500×g, 4 °C) at the end of the bioassay (96 h) and washed twice with deionized water. The pellets were resuspended in 2 mL of 50 mM sodium phosphate buffer (PBS, pH 7.8). Cells were homogenized by the Continuous High-Pressure Cell Disrupter (with 0.2 mm zirconium beads) and then centrifuged (6000×g, 4 °C, for 10 min). The supernatant was used for biochemical analysis. Total soluble protein was measured using the BCA Protein Assay Kit (Beyotime, China). The concentration of malodialdehyde (MDA)—a lipid peroxidation index—was analyzed using the thiobarbituric acid-reacting substance method [26]. SOD activity was analyzed by monitoring the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT) according to Dhindsa and Matowe [27]. CAT activity was determined according to Aebi [28].
The ROS level in cells was measured after 96 h exposure using the cell permeable indicator 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (Sigma, St. Louis, Missouri, USA), according to the method described by Cheng et al. [29]. A final concentration of 10 µM DCFH-DA was added to the samples which were incubated at 25 °C in the dark for 1 h. Fluorescence was measured using a flow cytometer (FACS Verse BD Biosciences, Franklin Lakes, New Jersey, USA) with excitation and emission wavelengths of 488 nm and 525 nm, respectively. Data are expressed as a percentage of the respective control values and are presented as mean ± SE of the mean.
Endpoints for cell-membrane integrity
Cell-membrane integrity was assessed by SYTOX™ Green Ready Flow™ Reagent (Life Technologies, Carlsbad, California, USA) after 96 h of exposure to paraquat. As a cell-impermeant dye, SYTROX Green enters the cell after loss of membrane integrity and binds with DNA to produce a bright green fluorescent complex which can be used to distinguishing plasma membrane damaged cells from integrated membrane cells in flow cytometry assays [30]. Two drops of reagent were added to 1 mL of the sample containing 106 cells mL−1 and the sample was incubated for 15 min in the dark at room temperature. The fluorescence was analyzed by flow cytometry with excitation and emission wavelengths of 488 nm and 523 nm, respectively, and data were presented as the percentage of cells that were stained with SYTOX™ green.
Endpoints for submicroscopic structure
At the end of the assays, the morphological effects of paraquat were determined in cells exposed to 0.1 mg L−1 and 1 mg L−1 and compared to the controls. Cells were collected by centrifugation (2000×g for 5 min) and fixed overnight at 4 °C with 2.5% glutaraldehyde. The pellets were washed three times for 15 min with phosphate buffer solution (0.1 M, pH 7.0) and then post-fixed with 1% osmium tetroxide for 2.5 h. Samples were dehydrated through a series of ethanol washes (30, 50, 70, 80, 90, and 100%, consecutively) for 10 min at each step and then transferred to absolute acetone for 20 min. Samples were then placed in a 2:1 mixture of absolute acetone: final resin for 1 h at room temperature then transferred to a 1:2 mixture of absolute acetone: final resin overnight, and finally into 100% final resin mixture for 1 h. Samples were then embedded in 100% EPON 812 resin (37 °C for 12 h) and heated at 60 °C for 48 h. Ultrathin sections (70 nm) were obtained with a Leica EM UC7 ultramicrotome contrasted with uranyl acetate and lead citrate and observed in a transmission electron microscope (Hitachi-7700, Hitachi, Tokyo, Japan) using an acceleration voltage of 80 kV.
Data analysis and statistics
All data are presented as mean ± standard error (SE). Flow cytometric analysis data were analyzed using FlowJo software (Tree Star Software, San Carlos, California, USA). One-way analysis of variance (ANOVA), followed by least-significant difference (LSD) (for homogeneity of variance), or Dunnett’s two-sided comparison test (for heterogeneity of variance) was applied to determine statistical differences between the different endpoints for different paraquat treatments and the control for each species. Independent sample t tests were performed to compare the M. aeruginosa and Chlorella sp. responses following exposure at the same paraquat concentration. Differences were considered to be significant at p < 0.05 unless otherwise stated.
Results
Distinct growth patterns of M. aeruginosa and Chlorella sp. following exposure to paraquat
Figure 1 shows dose- and time-dependent patterns of microalgal growth under paraquat exposure for both M. aeruginosa and Chlorella sp. Compared to the control, the growth of M. aeruginosa was significantly inhibited at concentrations of 0.2, 0.5, and 1 mg L−1 (p < 0.01, Fig. 1a). For Chlorella sp., growth inhibition was observed at concentrations of 5, 10, and 30 mg L−1 (p < 0.01, Fig. 1b), with inhibition rates of 39.85, 60.19, and 79.58%, respectively. Besides, the growth of M. aeruginosa was lower at 0.5 and 1 mg L−1 during the acute exposure stage (24 h, Fig. 1a), while for Chlorella sp., the declining growth curve was observed at 30 mg L−1 until 72 h (Fig. 1b).
Acute responses after exposed to paraquat
The fluorescence kinetics of M. aeruginosa and Chlorella sp. exposed to paraquat for 24 h are shown in Fig. 2. The fluorescence yield is presented as the concentration-dependent response of each species over 24 h. A concentration-dependent increase in the fluorescence yield was found in M. aeruginosa exposed to paraquat at concentrations higher than 0.1 mg L−1 (Fig. 2a). Conversely, a concentration-dependent decrease in the fluorescence yield was observed in Chlorella sp. at concentrations higher than 0.5 mg −1 with the lowest yield found in cultures exposed to 30 mg L−1 (Fig. 2b). When the fluorescence kinetics were plotted as the relative variable fluorescence to better reveal the changes in the transients (Fig. 2a, b, inserts), an increase in the J transient was found for M. aeruginosa at concentrations greater than 0.2 mg L−1 (Fig. 2a, inserts).
Chla fluorescence for each species changed in a concentration and time-dependent manner within 24 h (Fig. 3). Increases in Vj, ABS/RC, DIo/RC, and TR0/RC and decreases in ET0/RC, RC/CSo, ψ0, φEo, and φP0 were observed for both strains. Figure 3c shows significant changes in chlorophyll fluorescence after 3 h for M. aeruginosa at 1 mg L−1 (p < 0.05) and for Chlorella sp. after 9 h exposure at 30 mg L−1 (Fig. 3k). In addition, the PIabs index (Performance index based on absorption of light energy) decreased in a concentration-dependent manner after only 5 min for both M. aeruginosa (> 0.001 mg L−1) and Chlorella sp. (> 5 mg L−1). Similarly, a significant decrease in rETRmax was observed at concentrations of 0.2 mg L−1 and higher for M. aeruginosa, and for Chlorella sp. at concentrations of 10 mg L−1 (p < 0.05) and higher (Fig. 4).
Radar plots of the main OJIP fluorescence parameters following exposure at 5 min, 1 h, 3 h, 5 h, 9 h, and 24 h for Microcystis aeruginosa (a–f) and Chlorella sp. (g–l). The value in the plots is the ratio of the treatment to the control. The descriptions of fluorescence parameters are presented in Table 1
The EC50 based on Fv/Fm values decreased significantly with exposure time (Fig. 5). After 5 h cumulative exposure, the EC50 for M. aeruginosa was 0.54 mg L−1, while for Chlorella sp., it was higher at 44.76 ± 3.13 mg L−1 after 24 h.
Chronic responses after exposed to paraquat
To better explain the paraquat sensitivity variation between these two species, biochemical parameters were measured after 96 h of chronic exposure. Figure 6a shows that Fv/Fm and rETRmax were lower for M. aeruginosa when the exposure concentration was 0.2 mg L−1 or higher, but for Chlorella sp., Figure 6b shows that rETRmax was only lower when the exposure concentration exceeded 5 mg L−1.
Tables 2 and 3 show that the enzymatic activities of CAT and SOD for each strain were significantly differently between treatments (p < 0.01). Significantly higher CAT and SOD activities were measured in M. aeruginosa when exposure concentrations exceeded 0.1 mg L−1 and for Chlorella sp. when they exceeded 1 mg L−1. Significantly elevated MDA content (p < 0.05) was only observed in Chlorella sp. at a paraquat concentration of 1 mg L−1 (Table 3).
The relative results (control = 100%) for ROS and SYTRO green are presented in Fig. 7. For M. aeruginosa, significantly enhanced production of ROS occurred in cells, where exposure concentration was 0.2 mg L−1 or higher and the percentage of membrane damaged cells was around 20 times higher than the control at exposure concentrations greater than 0.2 mg L−1 after 96 h (p < 0.05). Conversely, for Chlorella sp., elevated ROS and membrane-damaged cells only occurred at exposure concentrations of 5 mg L−1 or higher and only around double that of the control.
The ultrastructure of both species exposed to two concentrations of paraquat for 96 h was compared using transmission electron microscopy (TEM) (Figs. 8, 9). For M. aeruginosa, the thylakoids and cytoplasm were shown to be distorted, and the cell walls damaged and detached from the cytoplasmic membrane in the 0.1 mg L−1 treatment (Fig. 8d–f), while in the 1.0 mg L−1 treatment, thylakoid membrane stacks, cytoplasmic vacuolation, and cytoclasis were evident (Fig. 8g–i). For Chlorella sp., the ultrastructures of cells were similar to the control treatment (Fig. 9a–c) for both 0.1 and 1.0 mg L−1 treatments, except that several outermost layers of the multi-layered cell walls appeared disrupted, and a disarrangement of organelles within cells was observed at in the 1.0 mg L−1 treatment (Fig. 9d–i).
Discussion
This study investigated the sensitivity differences to paraquat of two phytoplankton species M. aeruginosa and Chlorella sp. using time-dependent multi-endpoint assays. In general, M. aeruginosa was found to be tenfold more sensitive than Chlorella sp. to both chronic and acute exposures. These include photosynthetic processes, antioxidant response, oxidative stress, sub-microstructure changes, and growth inhibition.
Photosynthesis is a key physiological process for phototrophic organisms and is very sensitive to pollutants through the effects that some pollutants have on the electron transport chain [31, 32]. Chl a fluorescence is a rapid and non-invasive method for monitoring cellular stress and is commonly used to assess and quantify phytotoxicity in phytoplankton [32,33,34,35]. In the present study, the fluorescence yield is presented as the acute concentration-dependent response of M. aeruginosa and Chlorella sp. (Fig. 2) and shows that paraquat affected PSII photochemistry of both species [36]. Hess previously showed that the phytotoxic action of paraquat is based on interference with photosynthetic electron transport in PSI [18]. PSII is connected to PSI via the cytochrome b6/f complex [37], so if electron transfer from PSI is inhibited, PSII photochemistry would also be affected. In addition, the previous research has shown that paraquat caused up-regulation of the central component of PSII (D1 protein) in Chlamydomonas reinhardtii P.A.Dangeard [38].
The O–J–I–P fluorescence kinetics shows an increase at the J transient for both species after 24 h exposure to paraquat (Fig. 2, Insert). This indicates that paraquat appears to block electron transfer from QA to QB in both species. The similarity of the Chl a fluorescence response to paraquat in both species showed that the mode of action in PSII was similar. The absorption flux per reaction center (ABS/RC), which in a measure of PSII antennae size, was increased and may indicate an inactivation of PSII reaction centers by paraquat [25, 34, 39]. A concomitant decrease of ET0/RC indicated that the re-oxidation of QA through electron transport was reduced. Both species showed a decrease in ψ0 (the probability that an electron residing on QA enters the electron transport chain) and an increase in the J transient (Vj), thus confirming that electron transport was blocked between QA and QB (Fig. 3e, j). Similar results were found for other herbicides, including atrazine, diuron, and hexazinnone [33, 40, 41]. Furthermore, as shown in Fig. 3a, f, the PIabs index decreased in a concentration-dependent manner after only 5 min of exposure to paraquat for both species, indicating that PIabs is a sensitive indicator of early response to paraquat exposure.
Although the mode of action of paraquat towards each of the two species was similar, the toxicokinetic and toxicodynamic processes were different. In the present study, acute responses (i.e., within 24 h) such as the effect on chlorophyll fluorescence in cyanobacterial cells occurred within 3 h of exposure (p < 0.05; Fig. 3c). Higher acute susceptibility in PIabs, φEo, and ψ0 was found for M. aeruginosa with effects becoming evident within hours of exposure, but not for Chlorella sp. until after 24 h for the same concentrations (1.0 mg −1). In addition, the EC50 based on Fv/Fm for M. aeruginosa was lower than for Chlorella sp. and was obtained after only 5 h exposure (Fig. 5). Unlike Chlorella sp., a decrease of rETRmax was observed in M. aeruginosa after 24 h following exposure to paraquat at concentrations of 0.2 mg L−1 and higher (Fig. 4). This shows that the electron transport chain of M. aeruginosa was adversely affected by paraquat at relatively low concentrations, and photosynthetic responses to paraquat stress were significantly different between M. aeruginosa and Chlorella sp. As a result, growth inhibition of M. aeruginosa in this study occurred during the acute exposure stage (Fig. 1). Collectively, photosynthetic parameters can be used as sensitive cellular endpoints to study the early effects of paraquat on algae [8]. Our results provide supplementary evidence for differences in paraquat sensitivity between M. aeruginosa and Chlorella sp. in acute response.
The results of chronic response also showed sensitivity difference between the two species. One of the reported toxicity mechanisms for paraquat is its effect on cyclic reduction–oxidation reactions, through which reactive oxygen species (ROS) are induced in unicellular alga [42, 43]. SOD, peroxidase (POD), and CAT activities increased in response to paraquat exposure to remove excessive ROS [44,45,46]. Similarly, in the present study, significant increases in CAT and SOD activities were observed in M. aeruginosa after 96 h exposure to paraquat concentrations greater than 0.1 mg L−1 compared to the control (Table 2), whereas for Chlorella sp., a similar sensitivity was only found at paraquat concentrations of 1 mg L−1 or higher (Table 3). Nevertheless, the results of intracellular ROS levels and MDA content showed that the cells were still oxidatively damaged. An increase of intracellular ROS levels (Fig. 7) and MDA content (Tables 2, 3) was observed in M. aeruginosa at the 0.2, 0.5, and 1.0 mg L−1 treatments and in Chlorella sp. at 5, 10, and 30 mg L−1 treatments, respectively. The number of membrane damaged cells also increased significantly in M. aeruginosa at concentrations greater than 0.2 mg L−1 and in Chlorella sp. at concentrations greater than 5 mg L−1 (Fig. 7). This indicates that the cellular antioxidant defense mechanisms might not be capable of adequately protecting the cells following exposure to higher paraquat concentrations [8, 10]. Overproduction of ROS could trigger oxidative damage to proteins, nucleic acids, and lipids, and lead to the disruption of cellular structures, resulting in morphological changes and cell death [47, 48]. It could also explain the significantly higher growth inhibition at these concentrations, as shown in Fig. 1.
Interestingly, there were also several differences in the anti-oxidative responses of these two species. First, as shown in Tables 2 and 3, the response of the antioxidant systems was significantly different (p < 0.01). At exposure levels which caused significant growth inhibition for M. aeruginosa (> 0.2 mg L−1) and Chlorella sp. (> 5 mg L−1), the mean increase in CAT and SOD activity for M. aeruginosa cells was 11.25 and 7.14 times that of the control, whereas there was only 2.22 and 2.01 fold change in Chlorella sp. It has been reported that CAT and SOD vary among algal species when exposed under the same conditions to the same toxicant. For example, Martinez-Ruiz and Martinez-Jeronimo showed that 2, 4-dichlorophenoxyacetic acid (2, 4-D) induced a decrease of SOD activity, but did not affect the CAT activity in M. aeruginosa, while it produced significant differences compared to the control for Ankistrodesmus falcatus Corda Ralfs [49]. Second, compared to Chlorella sp., much higher increases in intracellular ROS levels and the percentage of membrane damaged cell were found for M. aeruginosa under the same treatment concentrations (i.e., 0.2, 0.5, and 1 mg L−1, Fig. 7). Third, TEM images confirmed the results of oxidative damage from paraquat exposure at the ultra-structural level in both species. At the same exposure concentrations, the morphological changes between the two species were also different. Therefore, paraquat can induce oxidative damage to both species but at different concentrations which depends on their sensitivity.
The similarity of the Chl a fluorescence response to paraquat in M. aeruginosa and Chlorella sp. indicated that the mode of action for paraquat in PSII was similar, that is, paraquat appears to block electron transfer from QA to QB in both species. However, the acute and chronic responses at the sub-cellular level showed that paraquat was more toxic to M. aeruginosa than to Chlorella sp. [21, 50]. Martinez-Ruiz and Martinez-Jeronimo suggested that differences in sensitivity could be related to species-specific responses [49]. There are at least four explanations for the species-specific responses to paraquat exposure observed in this study. First, the cell wall and cell membrane provide a self-protection barrier against xenobiotic chemicals, and this is different between the two species of phytoplankton. The cell envelope of M. aeruginosa (a prokaryote) is comprised of a cytoplasmic membrane, and a peptidoglycan layer composed of two sugar derivatives, N-acetylglucosamine and N-acetylmuramic acid, and several different amino acids, as well as an outer membrane [51]. Conversely, the cell wall of Chlorella sp. (a eukaryote) is composed of microfibrillar layers and an outer component that was thicker (up to 166 µm) than M. aeruginosa (about 45 nm thick, Figs. 8, 9) [52]. A highly chemical resistant cell wall found in coccoid green algae including Chlorella sp. contains the biopolymer algaenan [53,54,55] and this may also contribute to a higher paraquat tolerance by Chlorella sp. compared to M. aeruginosa. Second, compared to cyanobacteria (where photosynthesis occurs in the cytoplasm), paraquat needs to pass through an additional double-membrane chloroplast envelope before it arrives at the thylakoid membranes in Chlorella cells [34]. Third, paraquat resistance of plant cells is determined by paraquat uptake and efflux, sequestration, and catabolism, and through detoxification of the reactive oxygen species generated by paraquat [56]. Paraquat enters plant cells via a transport system that inherently functions as a transporter of polyamines which are structurally similar to paraquat [57, 58]. Incharoensakdi et al. proposed that the polyamine transport system in Synechocystis sp. PCC 6803 (which is a very rapid and energy-dependent process) is driven by a proton gradient and a membrane potential [59]. However, it is not universal, because studies on the green algae C. reinhardtii show that it does not contain short-living, high-affinity polyamine transporters [60]. Fourth, in comparison with eukaryotes, prokaryotes may have less elaborate enzymatic antioxidant pathways [34]. This may explain some of the sensitivity differences between cyanobacteria and green algae [61]. Esteves et al. showed that for atrazine, the sensitive strain decreased oxidative stress by increasing the activity of antioxidant enzymes such as SOD, but the tolerant strain invested in conjugation pathways and carotenoid maintenance [62]. Our results also showed that differences in response of the antioxidant system in the two species may explain differences in the extent of membrane and other ultrastructure damage. Therefore, differences between prokaryote and eukaryote physiology at the cellular level could contribute to the difference in toxicity, while the differences in inhibition efficiency might be largely dependent on the test species and incubation period. Y.
Conclusion
In this study, we found that paraquat affects population growth and photosynthetic processes, as well as inducing oxidative stress and anti-oxidative responses in two different types of phytoplankton, as shown in Fig. 10 (1 mg L−1 paraquat treatment as sample). For M. aeruginosa, Chl a fluorescence was affected after 3 h of exposure to paraquat. After a 96 h chronic exposure, significant increases in ROS levels, lipid peroxidation, growth inhibition, and the percentage of dead cells as well as comprehensive collapse of the cell structure occurred in M. aeruginosa. For Chlorella sp., only minimal effect on Chl a fluorescence was found following an acute exposure period (24 h), and after 96 h of chronic exposure to low concentrations of paraquat (< 1 mg L−1), relative low levels of ROS and growth inhibition were observed. These results show that multiple endpoints measured for both acute and chronic exposures provide more comprehensive information about the sensitivity differences of M. aeruginosa and Chlorella sp. The difference in cell structure of the two species plays a pivotal role in their response to paraquat, suggesting that physiology differences between prokaryotes and eukaryotes need to be considered when the toxic effect of chemical pollutants is studied. This study provides a “departure point” to investigate the composition of phytoplankton communities under a potential selective pressure from an herbicide such as paraquat in a natural ecosystem.
Proposed mechanistic scheme for paraquat toxicity (1 mg L−1) in Microcystis aeruginosa and Chlorella sp. The change of photosynthetic processes (Chl a fluorescence, rETRmax) and cell-membrane integrity (SYTRO green positive) in 24 h are defined as acute responses. The variation of oxidative stress (SOD, CAT, MDA, ROS), cell-membrane integrity (SYTRO green positive), and growth (OD680) at 96 h are defined as chronic responses. Significant increased endpoints, significantly decreased endpoints, and no significant changed endpoints are presented in red, green, and dark blue, respectively
Availability of data and materials
The data sets supporting the conclusions of this article are included within the article and its additional file.
Abbreviations
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- CAT:
-
catalase
- PS I:
-
photosystem I
- F M :
-
the maximal fluorescence yield
- Fv/F m :
-
the maximum quantum yield for primary photochemistry
- rETRmax :
-
the maximum relative electron transport rate
- F0 :
-
the minimum fluorescence
- F m :
-
the maximum fluorescence
- RLC:
-
the rapid light curve
- MDA:
-
malodialdehyde
- NBT:
-
nitroblue tetrazolium
- SE:
-
standard error
- ANOVA:
-
one-way analysis of variance
- LSD:
-
least-significant difference
- TEM:
-
transmission electron microscopy
- POD:
-
peroxidase
References
Pesce S, Bouchez A, Montuelle B (2011) Effects of organic herbicides on phototrophic microbial communities in freshwater ecosystems. Rev Environ Contam Toxicol 214:87–124. https://doi.org/10.1007/978-1-4614-0668-6_5
El-Dib MA, Abou-Waly HF, El-Naby AMH (2000) Impact of pentachlorophenol on growth and community structure of Nile River water microalgae. Int J Environ Health Res 10:239–250. https://doi.org/10.1080/09603120050127185
Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstein A, Cunningham V (2002) Aquatic toxicity of triclosan. Environ Toxicol Chem 21:1338–1349. https://doi.org/10.1002/etc.5620210703
Ma JY (2005) Differential sensitivity of three cyanobacterial and five green algal species to organotins and pyrethroids pesticides. Sci Total Environ 341:109–117. https://doi.org/10.1016/j.scitotenv.2004.09.028
Dupraz V, Coquille N, Menard D, Sussarellu R, Haugarreau L, Stachowski-Haberkorn S (2016) Microalgal sensitivity varies between a diuron-resistant strain and two wild strains when exposed to diuron and irgarol, alone and in mixtures. Chemosphere 151:241–252. https://doi.org/10.1016/j.chemosphere.2016.02.073
Relyea RA (2005) The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol Appl 15:618–627. https://doi.org/10.1890/03-5342
Fu L, Huang T, Wang S, Wang XH, Su LM, Li C, Zhao YH (2017) Toxicity of 13 different antibiotics towards freshwater green algae Pseudokirchneriella subcapitata and their modes of action. Chemosphere 168:217–222. https://doi.org/10.1016/j.chemosphere.2016.10.043
Nestler H, Groh KJ, Schönenberger R, Behra R, Schirmer K, Eggen RIL, Suter MJF (2012) Multiple-endpoint assay provides a detailed mechanistic view of responses to herbicide exposure in Chlamydomonas reinhardtii. Aquat Toxicol 110–111:214–224. https://doi.org/10.1016/j.aquatox.2012.01.014
Prado R, Rioboo C, Herrero C, Abalde J, Cid A (2009) Comparison of the sensitivity of different toxicity test endpoints in a microalga exposed to the herbicide paraquat. Environ Int 35:240–247. https://doi.org/10.1016/j.envint.2008.06.012
Esperanza M, Cid A, Herrero C, Rioboo C (2015) Acute effects of a prooxidant herbicide on the microalga Chlamydomonas reinhardtii: screening cytotoxicity and genotoxicity endpoints. Aquat Toxicol 165:210–221. https://doi.org/10.1016/j.aquatox.2015.06.004
Almeida AC, Gomes T, Langford K, Thomas KV, Tollefsen KE (2017) Oxidative stress in the algae Chlamydomonas reinhardtii exposed to biocides. Aquat Toxicol 189:50–59. https://doi.org/10.1016/j.aquatox.2017.05.014
Chakraborty S, Tiwari B, Singh SS, Srivastava AK, Mishra AK (2017) Differential physiological, oxidative and antioxidative responses of cyanobacterium Anabaena sphaerica to attenuate malathion pesticide toxicity. Biocatal Agric Biotechnol 11:56–63. https://doi.org/10.1016/j.bcab.2017.05.011
Coquillé N, Ménard D, Rouxel J, Dupraz V, Éon M, Pardon P, Budzinski H, Morin S, Parlanti É, Stachowski-Haberkorn S (2018) The influence of natural dissolved organic matter on herbicide toxicity to marine microalgae is species-dependent. Aquat Toxicol 198:103–117. https://doi.org/10.1016/j.aquatox.2018.02.019
Vogs C, Kühnert A, Hug C, Küster E, Altenburger RA (2015) Toxicokinetic study of specifically acting and reactive organic chemicals for the prediction of internal effect concentrations in Scenedesmus vacuolatus. Environ Toxicol Chem 34:100–111. https://doi.org/10.1002/etc.2764
Vogs C, Altenburger R (2016) Time-dependent effects in algae for chemicals with different adverse outcome pathways: a novel approach. Environ Sci Technol 50:7770–7780. https://doi.org/10.1021/acs.est.6b00529
Franz S, Altenburger R, Heilmeier H, Schmitt-Jansen M (2008) What contributes to the sensitivity of microalgae to triclosan? Aquat Toxicol 90:102–108. https://doi.org/10.1016/j.aquatox.2008.08.003
Zhang W, Xiong B, Sun WF, An S, Lin KF, Guo MJ, Cui XH (2014) Acute and chronic toxic effects of bisphenol a on Chlorella pyrenoidosa and Scenedesmus obliquus. Environ Toxicol 29:714–722. https://doi.org/10.1002/tox.21806
Hess FD (2000) Light-dependent herbicides: an overview. Weed Sci 48:160-170. https://www.jstor.org/stable/4046249
Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A (2004) Pesticides and oxidative stress: a review. Med Sci Monit 6:RA141
Aksakal O (2013) Assessment of paraquat genotoxicity on barley (Hordeum vulgare L.) seedlings using molecular and biochemical parameters. Acta Physiol Plant 35:2281–2287. https://doi.org/10.1007/s11738-013-1265-2
Zhang H, Li J, Ge FP, Zhou JY, Guo KN (2013) Effects of paraquat on the growth of cyanobacteria and green algae. Acta Sci Circum 33(5):1441–1445
Strasser RJ, Srivastava A, Govindjee G (1995) Polyphasic chlorophyll a fluorescence transients in plants and cyanobacteria. Photochem Photobiol 61:32–42. https://doi.org/10.1111/j.1751-1097.1995.tb09240.x
Samson G, Prášil O, Yaakoubd B (1999) Photochemical and thermal phases of chlorophyll a fluorescence. Photosynthetica 37:163–182. https://doi.org/10.1023/A:1007095619317
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP-test. In: Mathis P (ed) Photosynthesis: from light to biosphere. KAP Press, Dordrecht, pp 977–980. https://doi.org/10.1007/978-94-009-0173-5_1142
Force L, Critchley C, VanRensen JJS (2003) New fluorescence parameters for monitoring photosynthesis in plants. 1. The effect of illumination on the fluorescence parameters of the JIP-test. Photosynth Res 78:17–33. https://doi.org/10.1023/A:1026012116709
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Chem 86:271–278. https://doi.org/10.1016/0003-2697(78)90342-1
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: with enzymatic defense against lipid peroxidation. J Exp Bot 32:79–91. https://doi.org/10.1093/jxb/32.1.79
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Cheng L, He Y, Tian Y, Liu BY, Zhang YY, Zhou QH, Wu ZB (2017) Comparative biotoxicity of N-Phenyl-1-naphthylamine and N-Phenyl-2-naphthylamine on cyanobacteria Microcystis aeruginosa. Chemosphere 176:183–191. https://doi.org/10.1016/j.chemosphere.2017.02.110
Roth BL, Poot M, Yue ST, Millard PJ (1997) Bacterial viability and antibiotic susceptibility testing with SYTOX green nucleic acid stain. Appl Environ Microbiol 63:2421–2431
Juneau P, Qiu B, Deblois CP (2007) Use of chlorophyll fluorescence as a tool for determination of herbicide toxic effect: review. Toxicol Environ Chem 89:609–625. https://doi.org/10.1080/02772240701561569
Schreiber U, Quayle P, Schmidt S, Escher BI, Mueller JF (2007) Methodology and evaluation of a highly sensitive algae toxicity test based on multiwell chlorophyll fluorescence imaging. Biosens Bioelectron 22:2554–2563. https://doi.org/10.1016/j.bios.2006.10.018
Kumar KS, Dahms HU, Lee JS, Kim HC, Lee WC, Shin KH (2014) Algal photosynthetic responses to toxic metals and herbicides assessed by chlorophyll a fluorescence. Ecotoxicol Environ Saf 104:51–71. https://doi.org/10.1016/j.ecoenv.2014.01.042
Perron MC, Juneau P (2011) Effect of endocrine disrupters on photosystem II energy fluxes of green algae and cyanobacteria. Environ Res 111:520–529. https://doi.org/10.1016/j.envres.2011.02.013
Prado R, Garicia R, Rioboo C, Herrero C, Cid A (2015) Suitability of cytotoxicity endpoints and test microalgal species to disclose the toxic effect of common aquatic pollutants. Ecotox Environ Safe 114:117–125. https://doi.org/10.1016/j.ecoenv.2015.01.021
Küster A, Pohl K, Altenburger R (2007) A Fluorescence-Based bioassay for aquatic macrophytes and its suitability for effect analysis of non-photosystem II inhibitors. Env Sci Pollut Res 14:377–383. https://doi.org/10.1065/espr2007.04.410
Zhan J, Zhu X, Zhou W, Chen H, He C, Wang Q (2016) Thf1 interacts with PS I and stabilizes the PS I complex in Synechococcus sp. PCC7942. Mol Microbiol 102:738–751. https://doi.org/10.1111/mmi.13488
Nestler H, Groh KJ, Schönenberger R, Eggen RIL, Suter MJF (2012) Linking proteome responses with physiological and biochemical effects in herbicide-exposed Chlamydomonas reinhardtii. J Proteome 75:5370–5385. https://doi.org/10.1016/j.jprot.2012.06.017
Eullaffroy P, Frankart C, Aziz A, Couderchet M, Blaise C (2009) Energy fluxes and driving forces for photosynthesis in Lemna minor exposed to herbicides. Aquat Bot 90:172–178. https://doi.org/10.1016/j.aquabot.2008.09.002
Trebst A (2007) Inhibitors in the functional dissection of the photosynthetic electron transport system. Photosynth Res 92:217–224. https://doi.org/10.1007/s11120-007-9213-x
Kumar KS, Han T (2010) Physiological response of Lemna species to herbicides and its probable use in toxicity testing. Toxicol Environ Health Sci 2:39–49. https://doi.org/10.1007/BF03216512
Jamers A, Coen WD (2010) Effect assessment of the herbicide paraquat on a green alga using differential gene expression and biochemical biomarkers. Environ Toxicol Chem 29:893–901. https://doi.org/10.1002/etc.102
Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F (2008) Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 38:13–71. https://doi.org/10.1080/10408440701669959
Ananieva EA, Christov KN, Popova LP (2004) Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to paraquat. J Plant Physiol 161:319–328. https://doi.org/10.1078/0176-1617-01022
Qian HF, Chen W, Sun LW, Jin YX, Liu WP, Fu ZW (2009) Inhibitory effects of paraquat on photosynthesis and the response to oxidative stress in Chlorella vulgaris. Ecotoxicology 18:537–543. https://doi.org/10.1007/s10646-009-0311-8
Zhang WG, Liu M, Zhang PL, Yu FG, Lu S, Li PF, Zhou JY (2014) Effects of paraquat on photosynthetic pigments, antioxidant enzymes, and gene expression in Chlorella pyrenoidosa under mixotrophic compared with autotrophic conditions. Arch Environ Con Toxicol 67:593–600. https://doi.org/10.1007/s00244-014-0067-x
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071. https://doi.org/10.1111/j.1365-3040.2005.01327.x
Martinez-Ruiz EB, Martinez-Jeronimo F (2017) Exposure to the herbicide 2,4-D produces different toxic effects in two different phytoplankters: a green microalga (Ankistrodesmus falcatus) and a toxigenic cyanobacterium (Microcystis aeruginosa). Sci Total Environ 619:1566–1578. https://doi.org/10.1016/j.scitotenv.2017.10.145
Yu XB, Hao K, Ling F, Wang GX (2014) Aquatic environmental safety assessment and inhibition mechanism of chemicals for targeting Microcystis aeruginosa. Ecotoxicology 23:1638–1647. https://doi.org/10.1007/s10646-014-1303-x
Jürgens UJ, Weckesser J (1985) The fine structure and chemical composition of the cell wall and sheath layers of cyanobacteria. Annales de l’Institut Pasteur/Microbiologie. 136(1):41–44. https://doi.org/10.1016/S0769-2609(85)80019-3
Atkinson AW, Gunning BES, John PCL (1972) Sporopollenin in the cell wall of Chlorella and other algae: ultrastructure, chemistry, and incorporation of (14) C-acetate, studied in synchronous cultures. Planta 107:1–32. https://doi.org/10.1007/BF00398011
Allard B, Templier J (2000) Comparison of neutral lipid profile of various trilaminar outer cell wall (TLS)-containing microalgae with emphasis on algaenan occurrence. Phytochemistry 54:369–380. https://doi.org/10.1016/S0031-9422(00)00135-7
Scholz MJ, Weiss TL, Jinkerson RE, Jing J, Roth R, Goodenough U, Posewitz MC, Gerken HG (2014) Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot Cell 13:1450–1464. https://doi.org/10.1128/EC.00183-14
Gartner G, Uzunov B, Ingolic E, Kofler W, Gacheva G, Pilarski P, Zagorchev L, Odjakova M, Stoyneva M (2015) Microscopic investigations (LM, TEM and SEM) and identification of Chlorella isolate R-06/2 from extreme habitat in Bulgaria with a strong biological activity and resistance to environmental stress factors. Biotechnol Biote Eq 29:536–540. https://doi.org/10.1080/13102818.2015.1013283
Xi J, Xu P, Xiang CB (2012) Loss of AtPDR11, a plasma membrane-localized ABC transporter, confers paraquat tolerance in Arabidopsis thaliana. Plant J 69:782–791. https://doi.org/10.1111/j.1365-313X.2011.04830.x
Fujita M, Fujita Y, Iuchid S, Yamada K, Kobayashid Y, Uranoa K, Kobayashid M, Yamaguchi-Shinozaki K, Shinozaki K (2012) Natural variation in a polyamine transporter determines paraquat tolerance in Arabidopsis. Proc Natl Acad Sci 109:6343–6347. https://doi.org/10.1073/pnas.1121406109
Fujita M, Shinozaki K (2014) Identification of polyamine transporters in plants: paraquat transport provides crucial clues. Plant Cell Physiol 55:855–861. https://doi.org/10.1093/pcp/pcu032
Incharoensakdi A, Jantaro S, Raksajit W, Mäenpää P (2010) Polyamines in cyanobacteria: biosynthesis, transport and abiotic stress response. In: Méndez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex, Badajoz, pp 23–32
Theiss C, Bohley P, Bisswanger H, Voigt J (2004) Uptake of polyamines by the unicellular green alga Chlamydomonas reinhardtii and their effect on ornithine decarboxylase activity. J Plant Physiol 161:3–14. https://doi.org/10.1078/0176-1617-00987
Drábková M, Matthijs HCP, Admiraal W, Maršálek B (2007) Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 45:363–369. https://doi.org/10.1007/s11099-007-0062-9
Esteves SM, Almeida SFP, Gonçalves S, Rimet F, Bouchez A, Figueira E (2018) Sensitive vs. tolerant Nitzschia palea (Kützing) W. Smith strains to atrazine: a biochemical perspective. Ecotoxicology 27:860–870. https://doi.org/10.1007/s10646-018-1953-1
Acknowledgements
The authors thank Lingling Zheng (Freshwater Algae Culture Collection at the Institute of Hydrobiology, Chinese Academy of Sciences) for kind help in algal cultivation and Yan Wang (the Center for Instrumental Analysis and Metrology, Institute of Hydrobiology, Chinese Academy of Science) for technical assistance in flow cytometry The authors also thank Dr. Philip Orr and Dr. Sarah E. Crawford for their help with linguistic assistance of the manuscript and for suggesting useful changes.
Funding
This work was supported by the National Key Research and Development Project (Nos: 2017YFE0125700 and 2018YFD0900701).
Author information
Authors and Affiliations
Contributions
BF, JY, and YC performed the experiments. BF, JY, and SL wrote the manuscript. LJ participated in the preliminary test. WZ contributed to the manuscript correction. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
The preliminary Microplate assay and the preliminary toxicity test for concentrations setting.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bai, F., Jia, Y., Yang, C. et al. Multiple physiological response analyses aid the understanding of sensitivity variation between Microcystis aeruginosa and Chlorella sp. under paraquat exposures. Environ Sci Eur 31, 83 (2019). https://doi.org/10.1186/s12302-019-0255-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-019-0255-4