Abstract
As climate warming progresses, it becomes necessary to study the effects of water temperature on the basin-scale dynamic distribution of riverine fish. This study examined the spatiotemporal distribution of Plecoglossus altivelis using the environmental DNA approach and its relationship with water temperature from summer growth to autumn spawning periods in the Nagara River basin, central Japan. The overall results of the spatiotemporal distribution of P. altivelis were consistent with the known life history: a wide-range distribution in the basin during summer and aggregation in the middle mainstem during autumn. Additionally, this study found three intriguing distribution patterns depending on water temperature. During summer (August), the warmest period, P. altivelis was distributed in the upper mainstem, one tributary, and the mainstem downstream of the tributary confluences in relation to the relatively colder water (< 25 °C). During early autumn (September–early October), it spread widely in the middle and upper mainstem without the constraint of the upper limit of water temperature. During late autumn (late October–November), it steadily aggregated to the middle mainstem because of downstream migration for spawning at water temperatures below 20 °C. This study suggests the importance of river connectivity for P. altivelis migration to suitable habitats during its freshwater life stages, upper mainstem and tributaries as summer growth habitats, and cooling effects of tributaries on the mainstem during mid-summer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water temperature is critical for determining the behavior and distribution of riverine fish. Aquatic ectothermic animals such as riverine fish have species-specific water temperature sensitivity and tolerance ranges, which vary according to their life stages (Sunday et al. 2012; Dahlke et al. 2020). Therefore, riverine fish migrate between habitats and change their behavior in response to water temperature. Thus, the spatiotemporal distribution of riverine fish varies broadly and locally.
The ayu Plecoglossus altivelis is distributed in the Japanese Archipelago and along the Far East continental coast (Nakabo 2018). P. altivelis is an amphidromous annual fish that spawns in the middle to lower reaches of rivers in autumn, and the hatched larvae immediately drift to the sea and inhabit coastal marine waters in winter. The juveniles migrate to rivers in spring, grow and mature in the middle to upper reaches of rivers during summer to autumn, and mature fish migrate to spawning grounds in autumn. P. altivelis is one of the most valuable species in commercial and recreational fisheries in Japan. It is distributed both in the mainstem and tributaries during the growth period and mainly feeds on benthic alga. Dominant individuals defend their feeding territory and feed exclusively (Mizuno and Kawanabe 1957).
The relationship between water temperature and the migration and behavior of P. altivelis has been reported. Upstream migration of juveniles in spring begins when the river water temperatures reach 8–10 °C (Koizumi 1957; Tago 2004). In an experimental aquarium, the activity of territorial behavior was high between 20 and 24 °C, but extremely low below 14 °C (Shibuya et al. 1995). Aquafarming studies suggest that the upper water temperature limit suitable for fish growth is 25 °C (Suehiro 1951; Matsubara and Ochiai 1965). Downstream migration for spawning occurs at 14–20 °C (Koizumi 1957; Iguchi et al. 1998; Aizawa 2012).
Despite these findings, the effects of water temperature on the spatiotemporal distribution of P. altivelis in rivers are unknown. Recently, with the widespread use of environmental DNA (eDNA) analysis, the spatiotemporal distribution of P. altivelis has been studied in a broad range of river systems (Yamanaka and Minamoto 2016; Inui et al. 2021; Tenma et al. 2021). However, these studies did not focus on the relationship between P. altivelis distribution and the environment, including water temperature. There is growing concern regarding the impact of global warming on freshwater fish (Jarić et al. 2018; Dahlke et al. 2020). In the study river, some fishermen and ecologists believe that global warming may be responsible for the delay in the spawning season of P. altivelis and the poor catch at the lower sites of the river during summer. In fact, in Gujo City, located in the upper areas of the study basin, P. altivelis remains in late October; however, in the 1950s, it migrated downstream for spawning by the end of September (Koizumi 1957). In addition to the future conservation efforts of P. altivelis, the effects of water temperature on the basin-scale P. altivelis distribution should be examined along with its life history, to enhance the sustainability of fisheries.
This study examined the spatiotemporal distribution of P. altivelis and its relationship with water temperature at the basin scale from summer growth to autumn spawning periods. eDNA analysis, which is a powerful tool for detecting aquatic organisms (Rees et al. 2014), was performed at broad and fine temporal scales to determine the distribution of P. altivelis. Recent studies have shown the applicability of eDNA for tracking the temporal distribution of P. altivelis within a river system (Yamanaka and Minamoto 2016; Inui et al. 2021; Tenma et al. 2021). In addition, the eDNA approach is useful for distribution surveys with repeated sampling throughout the river system while avoiding conflicts with commercial and recreational fishermen.
Materials and methods
Study river
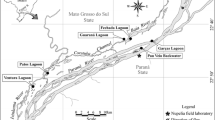
The study was conducted in a middle-upper segment (> 42.0 km from the mouth) of the Nagara River (mainstem) and its four tributaries (Yoshida, Itadori, Mugi, and Tsubo rivers) in central Japan in 2020 (Fig. 1). The region and P. altivelis were designated as “globally important agricultural heritage systems” by the UN Food and Agricultural Organization in 2015 for their value in combining livelihoods, nature, and culture (https://www.fao.org/giahs/giahsaroundtheworld/en/). The entire channel length of the Nagara River is 166 km, and its basin area is 1985 km2. The 42.0–53.0 km, 53.0–75.0 km, and > 75.0 km sections of the Nagara River, flow through the alluvial fan, valley bottom plain, and mountain area, respectively.
Usually, relatively high flows are observed from June to July (East Asian rainy season) and September to October (typhoon season). The flow is relatively low and stable from November to May. In 2020, the minimum and maximum water discharges observed at the Chusetsu water gauge station were 21 m3 s−1 on December 12 and 4 403 m3 s−1 on July 8, respectively. A total precipitation of 1923 mm was recorded at the Hachiman rain gauge station from June 10 to July 30, 2020, more than three times that from June to July in a normal year, and caused three large floods on June 15, July 8, and July 26 (Fig. 2). In September, the rainfall was intermittent, and the water level remained slightly high (Fig. 2). Small flooding events occurred on October 10 and 23 (Fig. 2).
In the Nagara River, the upstream migration of the native population of P. altivelis generally occurs from February to early June (mainly from April to May) according to the annual survey conducted by the Nagaragawa Estuary Barrage Operating & Maintenance Office, Japan Water Agency (https://www.water.go.jp/chubu/nagara/15_sojou/kakosojou.html). There are no dams in the study section of the mainstem of the Nagara River. Several check dams with fish passages are present in the Yoshida and Mugi rivers. Therefore, native P. altivelis spread throughout the river system, including its tributaries. In addition, plenty of hatchery-reared, Lake Biwa-originated, and amphidromous seed-originated stock (fry) are released in the Nagara River basin from April to July (mainly from April to May) by six fisheries cooperative associations (FCAs) (Aino et al. 2015). In 2020, approximately 40,550 kg (~ 4,055,000 individuals) of P. altivelis fry were released in the mainstem and tributaries by the FCAs. Therefore, both native and released fish are widely distributed throughout the study river system by the early summer. Incidentally, in 2010, a total of 41,450 kg of P. altivelis fry were released, and the native population from summer to autumn comprised over 70% of the total in the middle mainstem of the river (Aino et al. 2015).
eDNA sampling
eDNA analysis is a useful tool to estimate P. altivelis abundance (Doi et al. 2017). In this study, eDNA concentration was used as a relative indicator of P. altivelis population density among the sampling sites on each sampling date to evaluate the spatiotemporal changes in P. altivelis distribution.
Water samples were collected for the eDNA assay from 42 sites (mainstem, 20; Yoshida River, 6; Itadori River, 3; Tsubo River, 5; and Mugi River, 8) on nine sampling dates (August 11 and 25, September 14 and 23, October 6 and 20, November 4 and 17, and December 1) (Fig. 1). It took 2 days (the sampling date and following day) to sample water from the 42 sites. The sampling periods corresponded to the summer growth and autumn spawning seasons. The water level at the Chusetsu water gauge station on the eDNA sampling dates ranged from 8.91 to 10.12 m, with slightly high water level on September 14 and 23 (Fig. 2).
We collected 1-L water samples from the water surface using a polypropylene bottle at locations with a current velocity of approximately 30–50 cm/s usually within 5 m from the shore. Benzalkonium chloride solution (0.1% w/v) was added to the bottles to preserve the eDNA (Yamanaka et al. 2017). The samples were stored in a cooler box along with a 1-L distilled water (DW) blank (cooler blank), taken to a laboratory, and refrigerated. The cooler blank was opened to add benzalkonium chloride solution in the same way as the sample in the field.
DNA filtration, extraction, and quantitative real-time polymerase chain reaction (qPCR)
The water samples were vacuum-filtered through a 0.7-µm membrane filter (47-mm GF/F glass fiber; GE Healthcare, Little Chalfont, UK) within 2 days of sampling. The cooler blank was filtered simultaneously. A filtered “equipment blank” (1 L of DNA-free water) was incorporated before and after each sample filtration day. The “cooler blank” and “equipment blank” were negative controls, and their DNA levels were measured to identify any DNA contamination from field preparation and transportation, filter equipment, or other sources. Bleaching of the filter funnel for more than 5 min was performed each time a new sample was handled. The filters were placed in a light-shielding bag and stored at − 20 °C until eDNA extraction.
eDNA extraction was performed using the QIAGEN DNeasy Blood and Tissue Kit (QIAGEN) as described in the “Environmental DNA Sampling and Experiment Manual” (http://ednasociety.org/en/manual) published by the eDNA Society (Minamoto et al. 2021). The final volume of each extracted DNA sample was 100 μL and all samples were stored at − 20 °C until the PCR assay.
The eDNA of P. altivelis was quantified by real-time TaqMan PCR (qPCR) using a PikoReal Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). Mitochondrial cytochrome b gene fragments (131 bp) were amplified and quantified with a TaqMan probe using the following primers: Paa-CytB-Forward: 5′-CCTAGTCTCCCTGGCTTTATTCTCT-3′, Paa-CytB-Reverse: 5′-GTAGAATGGCGTAGGCGAAAA-3′, and Paa-CytB-Probe: 5′-FAM-ACTTCACGGCAGCCAACCCCC-TAMRA-3′. Yamanaka and Minamoto (2016) confirmed the species specificity of the primer/probe set.
Each TaqMan reaction mixture contained 900 nM of each primer (Paa-CytB-Forward and Reverse), 125 nM Paa-CytB-Probe, 10 µL qPCR Master Mix (TaqMan Environmental Master Mix 2.0; Life Technologies, Carlsbad, CA, USA), 0.4 µL uracil N-glycosylase (Thermo Fisher Scientific, Waltham, MA, USA), and 4 µL DNA solution. The total volume of the reaction mixture was 20 µL, including DNA-free water. PCR conditions were as follows: 50 °C for 2 min, 95 °C for 10 min, 55 cycles of 95 °C for 15 s, and 60 °C for 1 min. Three replicates were performed per water sample and the average value of the replicates was used. Four no-template controls were included for each PCR run. The qPCR detection limit was one copy per reaction with three replicates.
A standard curve was constructed using dilution series of 10,000 (n = 2), 1,000 (n = 2), 100 (n = 2), and 10 copies (n = 2) per PCR. The R2 values of the standard curves ranged from 0.956 to 0.995. The standard samples contained the target DNA cloned into the plasmids.
Water temperature
The hourly water temperature was continuously recorded at 63 sites (Fig. 1) from July to November 2020 using an automated data logger (HOBO MX2201 and MX2203; Onset Computer Co., Massachusetts, USA). The measurement sites were set to encompass the sampling sites for eDNA, except for the uppermost sites of the Tsubo and Itadori Rivers. Most loggers were checked once every 1–2 month. If any logger drained or broke owing to flooding, a new logger was set. Nevertheless, the values prior to the installation of the new logger were lost. Daily water temperature was calculated as the mean of the 24-hourly data (0:00–23:00) and used to plot water temperature fluctuations. If even one value of any 24-hourly data was not available, the daily water temperature for that day was considered as a missing value. The missing values of daily water temperatures at a certain measurement site were interpolated using data from another site with the highest correlation (r > 0.99) of the available daily water temperatures. Water temperature for any eDNA sampling site was obtained from the nearest measurement site. However, if there was a tributary confluence between the nearest measurement and the eDNA sampling sites, the water temperature was obtained from another nearest measurement site in the opposite direction.
Statistical analyses
All statistical analyses were performed using R v. 3.6.3 (R Core Team, 2020). To evaluate the relationship between the temporal distribution of P. altivelis and water temperature, we constructed generalized linear models (GLMs) for each sampling date, with the response variable as the eDNA concentration (copies mL−1) and the explanatory variable as the mean daily water temperature, with a gamma distribution and log-link function. The mean daily water temperature was noted for 7 days prior to and including the sampling date. The quadratic term of the explanatory variable was also used in building the models. Model significance was evaluated using a likelihood ratio test against a null model at a significance level of 0.05. We excluded sites with eDNA concentrations of 0 from the GLMs because of the use of the gamma distribution. Samples from two of the sites on August 25 were lost during extraction. We did not build a GLM for the sampling date of December 1 because of the lack of water temperature data. Thus, the number of samples (n) used in building the models was 34 on August 11, 35 on August 25, 32 on September 14, 33 on September 23, 35 on October 6, 33 on October 20, 25 on November 4, and 27 on November 17.
Results
Water temperature of the mainstem and tributaries
The maximum water temperatures of the mainstem and tributaries among the sampling dates were recorded on August 25, following which the water temperatures steadily decreased over time, with a rapid drop between August 25 and September 14 (Fig. 3). Overall, the water temperature of the mainstem increased in a downward direction on all sampling dates, and the average difference between the uppermost and lowermost sites was 7.4 °C (range 6.0–8.3 °C) (Fig. 3a). However, particularly during summer (August) and early autumn (September), the water temperature of the mainstem dropped downstream of the confluences with the tributaries. For example, on August 25, the Yoshida, Itadori, and Mugi Rivers lowered the water temperature of the mainstem from 24.1 to 23.8 °C, from 25.4 to 25.0 °C, and from 26.5 to 25.9 °C, respectively. On August 11, the Itadori River cooled the mainstem from 24.5 to 24.1 °C. In contrast, the Tsubo River raised the water temperature of the mainstem on all sampling dates, with an average rise of 1.3 °C (Fig. 3a).
Daily water temperature derived from data loggers nearest to the eDNA sampling sites on each eDNA sampling date in the mainstem of the a Nagara River, b Yoshida River, c Itadori River, d Mugi River, and e Tsubo River. The number on the horizontal axis indicates the eDNA sampling site. The “L” on the horizontal axis indicates that the water temperature data was not used at any of the eDNA sampling sites. In the panel of the a Nagara River, the tributary confluences are also shown by gray belts
In tributaries other than the Tsubo River, the water temperature was less than 25 °C throughout all sites and sampling dates (Fig. 3b–d). During August, the water temperature at the lowermost sites of tributaries (Fig. 3b–d) other than the Tsubo River was lower than that of the mainstem immediately upstream of its confluence (Fig. 3a): 22.0, 24.5, and 24.4 °C for the Yoshida, Itadori, and Mugi Rivers, respectively, on August 25 (the warmest day). In contrast, the water temperature of the Tsubo River (Fig. 3e) was consistently higher than that of the mainstem (Fig. 3a) throughout the sampling period. On August 25, the water temperature was 27.8 °C at the lowermost site of the Tsubo River.
Spatiotemporal change in eDNA concentrations of P. altivelis
The number of positive detections of eDNA on each sampling date, except for December 1, was the same as the sample size used to build the GLM (see “Statistical Analyses”). That on December 1 was 14. All blank samples (cooler, equipment, and no-template control) were negative.
The eDNA concentrations of P. altivelis changed spatially and temporally in the Nagara River basin (Fig. 4). On August 11, eDNA concentrations were relatively high in the upper segment of the mainstem and Itadori River. On August 25, relatively high eDNA concentrations extended to the mainstem immediately downstream of the confluences with the Yoshida and Itadori Rivers. In September, when the water temperature dropped (Fig. 3), eDNA was entirely distributed throughout the mainstem, although the eDNA concentrations were low. On October 6, eDNA concentrations were evenly high throughout the mainstem and the lowermost sites of the Yoshida and Itadori Rivers, and then distinctly increased at some downstream sites of the mainstem on October 20. During October, eDNA concentrations were also relatively high in the downstream sites of the Yoshida and Itadori Rivers. In November, high eDNA concentrations were detected at the lower sites of the mainstem, which rapidly decreased on December 1.
The spatiotemporal distribution of eDNA concentration (copies/mL) of P. altivelis through the sampling dates. The eDNA concentration was a relative indicator of P. altivelis population density among the sampling sites on each sampling date. Cross-marks on August 25 indicate the samples lost during extraction
Relationship between the eDNA concentrations and water temperature
Statistically significant hump-shaped relationships were found between the eDNA concentrations and mean daily water temperatures from August 11 to October 6, except for September 23 (Fig. 5). Based on the models, the peaks of the eDNA concentrations were predicted at 20.7 °C, 22.8 °C, 20.7 °C, and 18.7 °C on August 11, August 25, September 14, and October 6, respectively. Since October 20, when the water temperature dropped to < 20 °C at all sites, statistically significant models predicted exponentially positive relationships between the eDNA concentrations and water temperature.
Relations between eDNA concentration of P. altivelis and daily water temperature on the sampling dates. Plots denote the available sampling sites on each sampling date. Solid lines indicate the significant models, with P values based on likelihood ratio tests. No significant model was not obtained on September 23. Water temperatures (dashed line) at which eDNA concentrations predicted by the models peaked are also shown
Discussion
Spatiotemporal distribution of P. altivelis in the Nagara River basin
Spatiotemporal changes in the eDNA concentration revealed the basin-scale dynamic distribution of P. altivelis from the summer growth to autumn spawning periods. The results were consistent with the known life history of P. altivelis: a wide-range distribution including the upper mainstem and tributaries in the mountain area during the summer growth period, aggregation in the lower mainstem on the alluvial fan during the autumn spawning period, and a population decline following spawning.
Additionally, this study detected three intriguing distribution patterns of P. altivelis during summer (August), early autumn (September–early October), and late autumn (late October–November) and their relationships with water temperatures. These distribution patterns are discussed in the following sections, especially in relation to water temperature.
Summer distribution
In August, the eDNA concentration was high in the upper mainstem, a tributary (Itadori River), and the mainstem downstream from two tributary confluences (Yoshida and Itadori Rivers), with the predicted peaks of eDNA concentration at 20.7 °C and 22.8 °C. These results indicate that P. altivelis is disproportionately distributed in reaches with colder water during the mid-summer. A water temperature of < 25 °C is suitable for P. altivelis growth (Suehiro 1951; Matsubara and Ochiai 1965), and the territorial behavior of P. altivelis becomes more active at temperatures > 20 °C (Shibuya et al. 1995). In August, a daily water temperature of > 25 °C was observed at the lower study sites (downstream from Site 14 and 15) of the mainstem. In addition, the original, hourly data before averaging showed temperatures exceeding 25 °C at more upstream sites, for example, 6 h at Site 11 on August 11 and two hours at Site 5 on August 25. Thus, the habitat unsuitable for P. altivelis growth, was widely distributed in the middle and lower study sites of the mainstem during August. However, the water temperatures were generally < 25 °C in the upper mainstem and tributaries (except for the Tsubo River), even during August. In addition, two tributaries (Yoshida and Itadori Rivers) lowered the water temperature of the mainstem downstream of the confluences to or below 25 °C. The upper mainstem and tributaries supplied relatively colder water and could therefore serve as refuge habitats from the high water temperature for P. altivelis during mid-summer at a basin scale. However, at a local scale, P. altivelis may have also used deep pools with colder upwelling water, although this was not evident in this study. This is well known among fishermen as “doyo-gakure” resulting from the avoidance of the high water temperature in the shallow habitats such as riffles and rapids in mid-summer, which is needed to be studied in the future.
The cooling effect of tributaries on the water temperature of the mainstem was also exerted from 26.5 to 25.9 °C by the Mugi River. However, P. altivelis was scarce in the cooled site, probably because the water temperature was still high for P. altivelis. P. altivelis was also scarce in three tributaries (Yoshida, Mugi, and Tsubo Rivers), regardless of the low water temperature (< 25 °C). This might be attributed to factors other than water temperature. The Yoshida River experienced strong disturbances due to heavy rainfall events for 2 months prior to the eDNA sampling (Harada and Nagayama 2022), which may have caused the scarcity of P. altivelis. The reason for the scarcity of P. altivelis in the Mugi and Tsubo Rivers is unclear. The quality of water and/or habitat may affect the P. altivelis distribution in these tributaries, which needs to be examined in future studies.
The Tsubo River increased the water temperature of the mainstem from 25.8 to 26.8 °C on August 25. A high water temperature of > 25 °C was also observed in the mainstem downstream the confluence with the Tsubo River on August 11. The warming effect of the Tsubo River could have further degraded the temperature conditions for P. altivelis growth in the mainstem during the summer.
Early autumn distribution
From September to early October, when the water temperature dropped below 20 °C at most sites of the mainstem, the distribution of eDNA concentration was similar throughout the mainstem and the lowermost sites of two tributaries (Yoshida and Itadori Rivers). An increase in eDNA concentration of P. altivelis in the mainstem during autumn has also been reported in previous studies (Tenma et al. 2021). Such higher order sites generally have more abundant habitats and open canopies, which induce the high primary production attracting P. altivelis (Abe et al. 2007). Therefore, our results may be related to the dispersal of P. altivelis toward the sites with a large carrying capacity when they were freed from the constraint of water temperature. In addition, in the mainstem and tributaries of the Nagara River system, there were no heavy rains from August to October that caused severe disturbances (Harada and Nagayama 2022). This is another possible reason for P. altivelis to be able to disperse freely. When there are no constraints of water temperature and discharge (disturbance), the distribution of P. altivelis could be regulated by the abundance of foraging habitats. Thus, P. altivelis may be widely distributed in the mainstem with wide reaches containing the abundant foraging habitats.
Based on our models for early autumn, the peaks of eDNA concentration were predicted at approximately 20 °C. This may not indicate an optimal water temperature in early autumn; however, it represents the result of distribution to better foraging habitats under conditions without water temperature constraints. In September, the eDNA concentrations were generally low, probably due to dilution by increased water discharge on the sampling dates.
The wide-ranging distribution of P. altivelis mainly in the mainstem on October 6, may indicate a standby mode of downstream migration for spawning. In the Nagara River, P. altivelis grow until September (Aino et al. 2015). The water temperature of the mainstem on October 6 was less than 20 °C, which is a reasonable temperature for P. altivelis downstream migration and spawning (Koizumi 1957; Iguchi et al. 1998; Aizawa 2012). However, it stayed upstream, where it could not spawn. Iguchi et al. (1998) found that when the water temperature was below 20 °C, the number of P. altivelis migrating downstream increased the day following every rainfall event. Based on the experiences of fishermen and ecologists, the downstream migration for spawning is believed to be triggered by increasing water discharge due to rain (e.g., Koizumi 1957). Therefore, P. altivelis might have been waiting for flooding for downstream migration.
Late autumn distribution
From October to November, the distribution of high eDNA concentrations shifted downstream, indicating downstream migration of P. altivelis for spawning, associated with water temperature and discharge in late autumn. From October 6 to November 4, the water temperature at the lower sites of the mainstem (downstream from Site 12) was below 20 °C and dropped steadily, which matched the range of water temperatures suitable for downstream migration and spawning (see previous section). Moreover, two small floods due to rain occurred on October 11 and 24. The combined factors of suitable water temperature and the two small floods probably induced the downstream migration of P. altivelis for spawning (see previous section) and steadily shifted its distribution to the middle reaches of the river.
Our models for late autumn showed positive relationships between eDNA concentration and water temperature when the water temperature at all sites dropped to < 20 °C. This can be attributed to the biased distribution of P. altivelis due to the downstream migration for spawning, and at the same time indicate that the downstream migration became active below 20 °C, which is consistent with previous reports.
On December 1, eDNA concentrations rapidly decreased at the lower sites of the mainstem. This result indicates that most P. altivelis individuals spawned and died in November.
Conclusion
This study suggests the necessity for the connectivity of river channel networks consisting of the mainstem and its tributaries for the dynamic distribution of P. altivelis throughout its freshwater life stages, the importance of the upper mainstem and tributaries as summer growth habitats with relatively colder water, and the cooling effect of tributaries on the mainstem during mid-summer. These suggestions will become increasingly important because climate warming is expected to promote an increase in the river water temperature (van Vliet et al. 2013). The water temperature of the middle segment of the Nagara River nearly reached and partly exceeded 25 °C during the summer of 2020, even though the tributaries cooled the mainstem. A water temperature of 25 °C is the upper limit of the summer growth habitat for P. altivelis (Suehiro 1951; Matsubara and Ochiai 1965). Therefore, as climate warming progresses, the P. altivelis population in the Nagara River may be threatened, or their summer habitat may narrow. In addition, the summer distribution and autumn downstream migration, which might be related to water discharge and water temperature, may also be impacted by climate warming, causing a change in the hydraulic regime with increased floods in rivers (Döll and Zhang 2010). These effects on P. altivelis could further affect the fishery and tourism industry related to P. altivelis in the Nagara River. It is important to further examine the complex effects of water temperature and hydraulic regime on the basin-scale spatiotemporal distribution of P. altivelis and its life history.
References
Abe S, Yodo T, Matsubara N, Iguchi K (2007) Distribution of two sympatric amphidromous grazing fish Plecoglossus altivelis Temminck & Schlegel and Sicyopterus japonicus (Tanaka) along the course of a temperate river. Hydrobiologia 575:415–422
Aino S, Yodo T, Yoshioka M (2015) Changes in the composition of stock origin and standard length of ayu Plecoglossus altivelis altivelis during the Tomozuri angling season in the Nagara River, central Japan. Fish Sci 81:37–42
Aizawa Y (2012) Distribution of the spawning ground and spawning season of ayu Plecoglossus altivelis in the Tamagawa River. Bull Kanagawa Pref Fish Technol Cent 5:15–20 (in Japanese)
Dahlke FT, Wohlrab S, Butzin M, Pörtner H-O (2020) Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science 369:65–70
Doi H, Inui R, Akamatsu Y, Kanno K, Yamanaka H, Takahara T, Minamoto T (2017) Environmental DNA analysis for estimating the abundance and biomass of stream fish. Freshw Biol 62:30–39
Döll P, Zhang J (2010) Impact of climate change on freshwater ecosystems: a global-scale analysis of ecologically relevant river flow alterations. Hydrol Earth Syst Sci 14:783–799
Harada M, Nagayama S (2022) Impacts of flood disturbance on the dynamics of basin-scale swimming fish migration in mountainous streams. Water 14:538
Iguchi K, Ito F, Yamaguchi M, Matsubara N (1998) Spawning downstream migration of ayu in the Chikuma River. Bull Natl Res Inst Fish Sci 11:75–84 (in Japanese with English summary)
Inui R, Akamatsu Y, Kono T, Saito M, Miyazono S, Nakao R (2021) Spatiotemporal changes of the environmental DNA concentrations of amphidromous fish Plecoglossus altiveli altiveli in the spawning grounds in the Takatsu River, western Japan. Front Ecol Evol 9:622149
Jarić I, Lennox RJ, Kalinkat G, Cvijanović G, Radinger J (2018) Susceptibility of European freshwater fish to climate change: Species profiling based on life-history and environmental characteristics. Glob Change Biol 25:448–458
Koizumi K (1957) Organisms in the Nagara River (Nagaragawa no seibutsu). Government of Gifu Prefecture, Gifu (in Japanese)
Matsubara K, Ochiai A (1965) Ichtyology (Part 2). Kouseisha Kouseikaku, Tokyo (in Japanese)
Minamoto T, Miya M, Sado T, Seino S, Doi H, Kondoh M, Nakamura K, Takahara T, Yamamoto S, Yamanaka H, Araki H, Iwasaki W, Kasai A, Masuda R, Uchii K (2021) An illustrated manual for environmental DNA research: water sampling guidelines and experimental protocols. Environ DNA 3:8–13
Mizuno N, Kawanabe H (1957) Behaviour of salmon-like fish “ayu” in an area with closely established territories. Jpn J Ecol 7:26–30 (in Japanese with English summary)
Nakabo T (2018) The natural history of the fishes of Japan. Shogakukan, Tokyo (in Japanese)
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Rees HC, Maddison BC, Middleditch DJ, Patmore JRM, Gough KC (2014) The detection of aquatic animal species using environmental DNA—a review of eDNA as a survey tool in ecology. J Appl Ecol 51:1450–1459
Shibuya R, Seki S, Taniguchi N (1995) Comparison of the territorial behavior between amphidromous and landlocked forms by water temperature in ayu, Plecoglossus altivelis. Aquac Sci 43:415–421 (in Japanese with English summary)
Suehiro Y (1951) Ichthyology. Iwanami Shoten, Tokyo (in Japanese)
Sunday JM, Bates AE, Dulvy NK (2012) Thermal tolerance and the global redistribution of animals. Nat Clim Change 2:686–690
Tago Y (2004) Relationship between body size of ayu migrating up rivers flowing into Toyama Bay and water temperature. Aquac Sci 52:315–323 (in Japanese with English summary)
Tenma H, Tsunekawa K, Fujiyoshi R, Takai H, Hirose M, Masai N, Sumi K, Takihana Y, Yanagisawa S, Tsuchida K, Ohara K, Jo T, Takagi M, Ota A, Iwata H, Yaoi Y, Minamoto T (2021) Spatiotemporal distribution of Flavobacterium psychrophilum and ayu Plecoglossus altivelis in rivers revealed by environmental DNA analysis. Fish Sci 87:321–330
van Vliet MTH, Franssen WHP, Yearsley JR, Ludwig F, Haddeland I, Lettenmaier DP, Kabat P (2013) Global river discharge and water temperature under climate change. Global Environ Chang 23:450–464
Yamanaka H, Minamoto T (2016) The use of environmental DNA of fishes as an efficient method of determining habitat connectivity. Ecol Indic 62:147–153
Yamanaka H, Minamoto T, Matsuura J, Sakurai S, Tsuji S, Motozawa H, Hongo M, Sogo Y, Kakimi N, Teramura I, Sugita M, Baba M, Kondo A (2017) A simple method for preserving environmental DNA in water samples at ambient temperature by addition of cationic surfactant. Limnology 18:233–241
Acknowledgements
This study was supported by the Environment Research and Technology Development Fund (JPMEERF20202004) of the Environmental Restoration and Conservation Agency of Japan and JSPS KAKENHI Grant-in-Aid for Scientific Research (19H04314). The authors thank the fisheries cooperative associations for supporting the measurement of the water temperature.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagayama, S., Sueyoshi, M., Fujii, R. et al. Basin-scale spatiotemporal distribution of ayu Plecoglossus altivelis and its relationship with water temperature from summer growth to autumn spawning periods. Landscape Ecol Eng 19, 21–31 (2023). https://doi.org/10.1007/s11355-022-00509-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11355-022-00509-7