Abstract
Rapid changes in sea ice extent and changes in freshwater inputs from land are rapidly changing the nature of Arctic estuarine ecosystems. In the Beaufort Sea, these nearshore habitats are known for their high productivity and mix of marine resident and diadromous fishes that have great subsistence value for Indigenous communities. There is, however, a lack of information on the spatial variation among Arctic nearshore fish communities as related to environmental drivers. In summers of 2017–2019, we sampled fishes in four estuarine ecosystems to assess community composition and relate fish abundance to temperature, salinity, and wind conditions. We found fish communities were heterogeneous over larger spatial extents with rivers forming fresh estuarine plumes that supported diadromous species (e.g., broad whitefish Coregonus nasus), while lagoons with reduced freshwater input and higher salinities were associated with marine species (e.g., saffron cod Eleginus gracilis). West–East directional winds accounted for up to 66% of the community variation, indicating importance of the wind-driven balance between fresh and marine water masses. Salinity and temperature accounted for up to 54% and 37% of the variation among lagoon communities, respectively. Recent sea ice declines provide more opportunity for wind to influence oceanographic conditions and biological communities. Current subsistence practices, future commercial fishing opportunities, and on-going oil and gas activities benefit from a better understanding of current fish community distributions. This work provides important data on fish spatial distributions and community composition, providing a basis for fish community response to changing climatic conditions and anthropogenic use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid warming in the Arctic has resulted in noticeable declines in the extent, thickness, and volume of sea ice over the last 40 years (Meredith et al. 2019; Thoman et al. 2020). On average, monthly sea ice declined 12.6% per decade from 1979 to 2022 compared to the 1981–2010 baseline [National Snow and Ice Data Center (NSIDC) 2023]. Ice loss has created an ocean environment with considerably more open water, which reduces the albedo effect of the ice and further enhances warming of marine ecosystems (Timmermans and Labe 2020). Additionally, storm related wave action is projected to increase along Arctic coastlines (Casas-Prat and Wang 2020), which could favor along-shore currents, lead to higher coastal erosion rates, and change nearshore water turbidity and primary production (Bonsell and Dunton 2018; Gibbs et al. 2021). Climate change effects on terrestrial landscapes also have measured consequences for Arctic estuarine systems (Meredith et al. 2019), including changes in quantity and form (i.e., snow versus rain) of over-land precipitation, magnitude and duration of the spring freshet, and delivery of carbon and nutrients from permafrost thaw to brackish systems (Ahmed et al. 2020; Beel et al. 2021). Longer ice-free seasons will also enable access to the Arctic, allowing for increased opportunities of resource development or commercial fishing (NPFMC 2009; Meredith et al. 2019). Little information, however, on the biotic response to marine and freshwater inputs is available for Arctic estuarine systems, even before effects of climate change are considered. Yet, this information can inform the management and conservation of estuarine fish species that are important to Indigenous communities, marine mammals, and birds (Rizzolo et al. 2015; Choy et al. 2020; Bilous et al. 2022).
The factors that organize fish communities in estuarine environments of the Beaufort Sea are relatively unknown; in part due to numerous challenges in studying Arctic estuarine fish communities, including accessibility of remote coastal locations and sampling highly mobile species (i.e., diadromous migrants) in shallow water (Roux et al. 2016; Khalsa et al. 2021; Bilous et al. 2022). A few foundational papers linked the presence and distribution of diadromous and marine species to various environmental factors, including temperature, the presence of rivers, wind-driven current systems, and the arrangement and size of barrier islands (e.g., Craig 1984; Craig et al. 1985; Fechhelm and Fissel 1988; Fechhelm et al. 1989). With few exceptions, however, there is little information on how fish community composition varies longitudinally among spatially distinct Beaufort Sea lagoons [for individual lagoons, see Barton et al. (2020) for Elson Lagoon, Alaska, U.S.A.; Craig et al. (1985) for Simpson Lagoon, Alaska, U.S.A.; Priest et al. (2022) for Prudhoe Bay, Alaska, U.S.A.; and Roux et al. (2016) for Husky Lakes, Northwest Territories, Canada].
Beaufort Sea lagoons offer relatively warm habitats compared to the adjacent marine environment (Craig 1984; Harris et al. 2017). For example, in August 2019, offshore sea surface temperatures were < 5 °C (Timmermans and Ladd 2019), while adjacent Alaskan lagoons ranged from 5 to 17 °C (Khalsa et al. 2021; Stanek et al. 2024). With air and sea surface temperatures increasing over several decades, fish are expected to respond by changing their habitat use and distribution (Ballinger et al. 2020; Timmermans and Labe 2020; von Biela et al. 2023). Recent investigations suggest that some fish species with boreal zoogeography are becoming more abundant [e.g., saffron cod, uugaq, (common name in English and Iñupiaq, upon introduction) Eleginus gracilis] or changing their seasonal distribution patterns (e.g., Dolly Varden, iqalukpik, Salvelinus malma) in response to warmer temperatures and sea ice extent (Gallagher et al. 2021; Priest et al. 2022; von Biela et al. 2023).
The presence of several species, including diadromous ciscoes (Coregonus spp.) and estuarine fourhorn sculpin, kanayuq (Myoxocephalus quadricornis), have been found to be limited by salinity gradients and are rarely captured outside barrier islands (Barton et al. 2020). Other species, like Dolly Varden, are tolerant to salinity variation and can be found in marine surface waters (< 2 m) up to 152 km from shore (Craig 1984; Gallagher et al. 2021). Winds also influence fish communities by moving water masses and juvenile fishes. For example, young-of-the-year Arctic cisco, qaaktaq (Coregonus autumnalis) rely on wind facilitated movement for recruitment to Alaska’s nearshore and river habitats from the Mackenzie River plume in the Canadian Beaufort Sea (Zimmerman et al. 2013). To improve our understanding, we ask, how do lagoon-specific environmental characteristics (i.e., temperature and salinity) and meteorology (i.e., wind speed and direction) influence fish community composition over 300 km of Alaska’s coastline? This question is important because recent changes in fish abundance have a direct effect on the ability of subsistence fishers and marine predators to access desired fish species (Divoky et al. 2021; von Biela et al. 2023). Further, variation in the physical, chemical, and ecological system can influence the structure of fish communities, which can have profound feedback effects on lagoon food web dynamics and energy flow (Stanek et al. 2022a).
We examined spatial variation in nearshore fish community composition among four Beaufort Sea lagoons in relation to local oceanic and meteorological conditions. The lagoons vary in their proximity to large rivers and connectivity to the Beaufort Sea. Specifically, we looked for correlations between fish relative abundance and community composition with gradients in water temperature, salinity, and wind speed and direction. Based on the importance of these abiotic factors to fish populations, we expected to see correlations between (1) warmer water temperatures and species with a boreal zoogeography, (2) higher salinities and marine species, and (3) wind velocity and species that rely on winds and currents for recruitment or movement. We aim to demonstrate how communities reflect their contemporary local environment, as generalized in an early review documenting fish use of estuarine water along the Alaskan coast prior to the mid-1980s and the now rapid pace of climate change (Craig 1984; Rantanen et al. 2022).
Methods
Study area
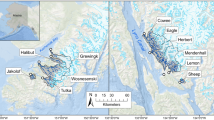
The lagoons sampled in this study are located along the Beaufort Sea coast of Alaska, U.S.A, and span ~300 km (Fig. 1). Stefansson Sound and Simpson Lagoon lie in the central region (Fig. 1b), near Prudhoe Bay, approximately halfway between Point Barrow and the border with Canada. Extensive oil and gas facilities have been developed around Simpson Lagoon. Drilling islands and causeways have also been constructed in Stefansson Sound. These structures and the fish communities around them have been the subject of study since the 1980s (Fechhelm et al. 1989; Priest et al. 2022). Jago and Kaktovik lagoons are to the east (Fig. 1c), within the bounds of the Arctic National Wildlife Refuge (ANWR) and near Kaktovik Village, Barter Island, Alaska. Lagoon regions will be classified as “central” and “eastern” hereafter. Within ANWR, the Alaska National Interest Land Conservation Act of 1980, 16 U.S.C. § 3101– 3233 aims to provide opportunities for subsistence and maintenance of healthy fish and wildlife populations. Currently, there is no offshore industrial development in marine waters adjacent to the 1002 area of ANWR.
Lagoons on a Alaska’s Beaufort Sea coast are found between Point Barrow and Canada. The b central region lagoons are adjacent to large rivers and are relatively exposed to the marine environment, while the c eastern region lagoons are adjacent to small rivers and are protected from the marine environment by barrier islands
The lagoons of the central region experience high influx of water from nearby rivers, with the largest of the North Slope rivers, the Colville River, discharging ~15 km to the west of Simpson Lagoon. The Kuparuk and Sagavanirktok rivers flow directly into Simpson Lagoon and Stefansson Sound, respectively. Simpson Lagoon and Stefansson Sound have a relatively open exchange with the Beaufort Sea due to wide spacing among barrier islands. The eastern region lagoons experience freshwater inflow from diffuse runoff and small rivers like the Jago River, which flows directly into Jago Lagoon. Kaktovik Lagoon is the least connected to freshwater and marine fish habitat as it is the only of the four lagoons without a major river and no direct connection to the open ocean, its waters pass through Jago Lagoon, which is connected to the Beaufort Sea through a 200 m wide channel. During the open water season, wind-driven currents bring water masses of varying salinity and temperature into the lagoon systems (Dunton et al. 2006). Moderate and strong easterly winds (> 5.4 m·s−1) favor coastal upwelling, pushing warmer fresh waters away and drawing cold, saline water from the deep into the nearshore (Okkonen et al. 2018). Fluctuating winds lead the lagoons to experience substantial seasonal variability, with temperatures ranging from – 2 to 14 °C and salinities from 0 to > 45 (Dunton et al. 2006, 2009; Harris et al. 2017). Substrate tends to be uniform in this region, mostly mud and sand (Craig 1984), interspersed with beaches of small gravel. There are no seagrass beds and macroalgae is limited to discrete boulder and cobble patches at depths > 5 m in Stefansson Sound where the protection of barrier islands limits ice scour of the seabed (Dunton 1982).
Data collection
Two stations, established in each of the four lagoons [see Craig and Haldorson (1981) and Underwood et al. (1995)] were sampled during this study. One station in Simpson Lagoon was exposed to extreme changes in wind-forced water depth that submerged the fyke net in 2018, preventing collection of comparable fish abundance data; it was relocated in 2019. In total, fish data were collected from fyke nets deployed in Simpson Lagoon during late July of 2019, in Stefansson Sound during late July 2018 and 2019, and in Jago and Kaktovik lagoons during early August 2017, 2018, and 2019. Nets consisted of a 2.5 cm stretch mesh for lead (61 m length) and wings (15 m) attached to a trap with 1.3 cm stretch mesh and an opening of 1.5 m by 1.2 m (Underwood et al. 1995). Traps had two collection chambers, one for each side of the lead net. Set within 60 m and perpendicular to shore in water depths of about 1 m, traps fished continuously for one to six days (weather depending) with checks made approximately every 24 h. Each 24 h set was considered a “net-day” of fishing. We counted and identified all captured fishes to species or lowest taxonomic level. Volumetric estimates of abundance were used as necessary for large numbers (1000s) of juvenile Arctic cisco (Underwood et al. 1995; Brown 2008). Fish catch data that support the findings of this publication are available in Stanek et al. (2022b).
Analytical methods
The catch per unit effort (CPUE) of each fish species was determined for each independent fyke net collection chamber (two per apparatus), which separated fish traveling from opposing directions along the shore, as the number of fish captured per net-day (CPUEnet). Any fish not identified to species were excluded from these analyses (0.14% of total catch). In addition to the CPUEnet, data were summarized to determine differences among lagoons and to examine the association between lagoon communities and the environment. First, species CPUEs were averaged across all nets and years within each lagoon (CPUElagoon), so that proportional differences in species composition among lagoons could be visualized over the time frame of the study. Second, to relate the communities to environmental variables, we examined the mean CPUE of each species at the station (two net-days at each station) and year when data were available (CPUEy,station). Using the average CPUEy,station allowed for spatial comparisons without confounding relationships of in-year temporal or spatial autocorrelation. All analyses were performed using R Statistical Software (v4.2.2; R Core Team 2021).
Community ordination
Using CPUEnet, we examined the community dissimilarity of all daily samples in ordination space. Community data were subject to a Wisconsin double standardization and square root transformation using the metaMDS function in R package vegan (R package version 2.5–7; Oksanen et al. 2020), and non-metric multidimensional scaling (NMDS) was used to examine communities in ordination space on three dimension (k = 3) using Bray–Curtis dissimilarities. Convex hulls were drawn around each of the lagoons to visualize separation of lagoon communities and show the spread of individual community samples. Species scores are shown (as the abbreviated species name) in the ordination at their optimum, with abundance decreasing in all directions from that point.
Species contributions
The contributions of individual species to community similarity (rather, the Bray–Curtis dissimilarity of the community matrix [Clarke 1993]) were determined using CPUEnet. Comparisons were made on a pairwise basis, for example, comparing Jago Lagoon to Kaktovik Lagoon, Jago Lagoon to Simpson Lagoon, and Jago Lagoon to Stefansson Sound. The function simper in R package vegan (R package version 2.5–7; Oksanen et al. 2020) provides a cumulative contribution based on species abundances. Permutations tests (n = 1000) were used to discover for which species the difference in abundance among lagoons were a consistently important component of their contribution to dissimilarities, rather than contributions due to variation in individual species abundance (e.g., 0–146 Arctic cisco per day in Jago Lagoon). Contributions were considered important for permutation p-values < 0.05.
Environmental associations
Measurements of water temperature and salinity associated with each station were collected using data loggers (HOBO Salt Water Conductivity/Salinity Data Logger, U24-002-C, Onset Computer Corporation, Bourne, Massachusetts, USA) attached to the fyke net. Data loggers recorded temperature (°C) and conductivity every 5 min during net deployment, the mean and maximum temperature and mean salinity were calculated for net-day. Salinity was determined from conductivity using the function gsw_SP_from_C in the gsw package (Kelley et al. 2022) with the corresponding temperature (°C) and given a pressure of 10.6351 dbar at 0.5 m depth. All temperature, conductivity, and salinity data that support the findings of this publication can be found in Stanek et al. (2024).
Wind speed and direction data, downloaded from MesoWest (University of Utah 2019), were gathered for Barter Island, Alaska (station PABA) and employed for fyke net stations in Kaktovik Lagoon and Jago Lagoon, and from Deadhorse, Alaska (station PASC) for Simpson Lagoon and Stefansson Sound. Data were downloaded beginning 72 h prior to the first net-day through to the end of sampling. Typically, measurements were available at 5-min intervals, although additional and missing times are present in the dataset. Data were converted to metric using standard formulas. Wind u (west–east) and wind v (south-north) vectors were calculated from the wind speed (m·s−1) and direction. Lagged wind u and v were determined as the average wind condition three days prior to the net-day of interest for all sampling events (Barton et al. 2020). Relationships between the variables were assessed using pairwise Spearman correlations between maximum temperature, mean temperature, mean salinity, mean wind speed, mean wind u, mean wind v, mean lag wind speed, mean lag wind u, and mean lag wind v.
A second NMDS ordination of the entire fish community (19 species) was evaluated using the same methods as above, but mean values (CPUEy,station) were used in order to meet the assumptions of independence for linear modeling. Here, a smoothed surface for each environmental variable was examined using the ordisurf function in R package vegan (R package version 2.5–7; Oksanen et al. 2020). For each station and year combination, we determined the average value of maximum temperature and mean temperature, salinity, wind speed, wind u, wind v, lagged wind speed, lagged wind u, and lagged wind v; individually, these variables were fit to the ordination surface using a generalized additive model (GAM). All GAMs used a thin plate regression spline with four knots. GAMs of environmental variables were examined on each pair of NMDS axes (i.e., 1 and 2, 2 and 3, 1 and 3) and diagnostic plots checked for goodness of fit. Select species scores, based on contributions, are shown in the ordinations at their optimum, with abundance decreasing in all directions from that point.
Results
In Jago and Kaktovik lagoons, Arctic cisco was the most abundant fish species, comprising 75% and 54% of the average catch, respectively (Fig. 2a, b). Other captured fishes with marine affinity included Arctic flounder, nataaġnaq (Liopsetta glacialis); Arctic smelt, iłhuaġniq (Osmerus dentex); fourhorn sculpin, and saffron cod. These fishes were prevalent in the eastern Beaufort Sea region, collectively making up 23% of the average catch in Jago Lagoon and 45% of the average catch in Kaktovik Lagoon; < 0.5% of the average catch in these lagoons included fish with a strong freshwater affinity [i.e., broad whitefish, aanaakłiq (Coregonus nasus); humpback whitefish, pikuktuuq (Coregonus pidschian); and least cisco, iqalusaaq (Coregonus sardinella)]. In the central Beaufort Sea region, fishes with a freshwater affinity had higher average CPUEs (Table 1) and comprised greater proportions of the catch (Fig. 2c, d) compared to eastern Beaufort Sea lagoons. Thirteen percent of the average catch in Simpson Lagoon and 60% of the average catch in Stefansson Sound included broad whitefish, humpback whitefish, least cisco, and threespine stickleback, kakiḷasak (Gasterosteus aculeatus). Simpson Lagoon had higher proportions of fourhorn sculpin (20%) and Arctic flounder (55%) than other lagoons. Polar cod, iqaluġaq (Boreogadus saida, sometimes called Arctic cod) comprised small proportions of the catch in the central region only (Table 1). Dolly Varden were captured in both regions, but made up less than 2% of the total catch. Other species, which cumulatively made up less than 1% of the catch in any lagoon, included Arctic grayling, sulukpaugaq (Thymallus arcticus); Arctic sand lance, paŋmaksraq (Ammodytes hexapterus); Arctic staghorn sculpin, kanayuq, (Gymnocanthus tricuspis); chum salmon, qalugruaq (Oncorhynchus keta); ninespine stickleback, kakalisauraq (Pungitius pungitius); Pacific capelin, paŋmaksraq (Mallotus catervarius); Pacific herring, uqsruqtuuk (Clupea pallasii); or pink salmon, amaqtuuq (Oncorhynchus gorbuscha) (see Table 1 for mean CPUEs across lagoons).
Fish species proportions in nearshore communities—a Jago Lagoon, b Kaktovik Lagoon, c Simpson Lagoon, and d Stefansson Sound—based on the mean catch per unit effort (CPUElagoon) of all sampled dates in 2017, 2018, and 2019. Species shaded in reds [broad whitefish (Coregonus nasus), humpback whitefish (Coregonus pidschian), least cisco (Coregonus sardinella), Dolly Varden (Salvelinus malma), threespine stickleback (Gasterosteus aculeatus), and Arctic cisco (Coregonus autumnalis)] are diadromous, likely using river habitats and lagoons as adults; species shaded in blues are marine [Arctic smelt (Osmerus dentex), saffron cod (Eleginus gracilis), Arctic flounder (Liopsetta glacialis), fourhorn sculpin (Myoxocephalus quadricornis), and polar cod (Boreogadus saida)]. Species in the “other” category may include < 1% combined of Arctic grayling (Thymallus arcticus), Arctic staghorn sculpin (Gymnocanthus tricuspis), Arctic sand lance (Ammodytes hexapterus), chum salmon (Oncorhynchus keta), pink salmon (Oncorhynchus gorbuscha), ninespine stickleback (Pungitius pungitius), Pacific capelin (Mallotus catervarius), or Pacific herring (Clupea pallasii). Fish CPUE data from Stanek et al. (2022b)
Community ordination & species contributions
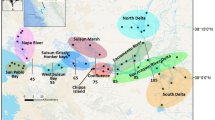
NMDS ordination (stress = 0.14) indicated clear separation of central (Simpson Lagoon and Stefansson Sound) and eastern (Jago and Kaktovik lagoons) lagoon communities along the first NMDS axis (Fig. 3a). Broad whitefish and humpback whitefish presence was associated with the central lagoons, while saffron cod and Arctic flounder presence was associated with the eastern lagoons. Among the freshwater associated fishes, least cisco contributed 18–24% and broad whitefish contributed 11–14% of the variation between the central Beaufort Sea and eastern Beaufort lagoon communities (Table 2; “Between regions”). Among the marine fishes, Arctic flounder contributed 29–33% and fourhorn sculpin contributed 12–15% of the variation between central Beaufort Sea lagoon communities and eastern Beaufort Sea lagoon communities (Table 2; “Between regions”). Polar cod, which was associated with Stefansson Sound, contributed little (1%) to the variation between regions and was captured only in the central Beaufort Sea lagoons.
Ordination of all axis pairs (a—NMDS 1 and 2, b—NMDS 1 and 3, and c—NMDS 2 and 3) for fish communities represented by individual sampling events during 2017, 2018, and 2019; each point represents the community based on the catch per unit effort (CPUEnet) of 19 species. Species include ARCI Arctic cisco (Coregonus autumnalis), ARFL Arctic flounder (Liopsetta glacialis), ARGR Arctic grayling (Thymallus arcticus), ARSA Arctic sand lance (Ammodytes hexapterus), ARSM Arctic smelt (Osmerus dentex), ARST Arctic staghorn sculpin (Gymnocanthus tricuspis), BRWH broad whitefish (Coregonus nasus), CHSA chum salmon (Oncorhynchus keta), DOVA Dolly Varden (Salvelinus malma), FOSC fourhorn sculpin (Myoxocephalus quadricornis), HUWH humpback whitefish (Coregonus pidschian), LECI least cisco (Coregonus sardinella), NIST ninespine stickleback (Pungitius pungitius), PACA Pacific capelin (Mallotus catervarius), PAHE Pacific herring (Clupea pallasii), PISA pink salmon (Oncorhynchus gorbuscha), POCO polar cod (Boreogadus saida), SACO saffron cod (Eleginus gracilis), and THST threespine stickleback (Gasterosteus aculeatus). Convex hulls encompass all points within a location: Jago Lagoon, Kaktovik Lagoon, Simpson Lagoon, and Stefansson Sound. POCO and BRWH (overlapped LECI), and HUWH and FOSC (overlapped ARFL) were removed from panel c for legibility
The convex hulls for the central Beaufort Sea lagoons were distinct along the third NMDS axis (Fig. 3b, c). Arctic flounder, fourhorn sculpin, threespine stickleback, and pink salmon contributed to differences between the Simpson Lagoon and Stefansson Sound communities (Table 2; “Within regions”). Arctic flounder accounted for the greatest percent contribution at 41%, followed by fourhorn sculpin (17%), threespine stickleback (2%) and pink salmon (< 1%). Species associations can be seen for pink salmon and fourhorn sculpin in Stefansson Sound and threespine stickleback in Simpson Lagoon (Fig. 3b, c). Arctic flounder, which was relatively abundant in Simpson Lagoon (Table 1), was an important contributor to the variation between Simpson Lagoon and all other lagoons (see also Table 2; “Between regions”).
The convex hulls for Jago and Kaktovik lagoons, where three years of data were collected, were larger than those for Simpson Lagoon (one year of data) and Stefansson Sound (two years of data); additionally, the eastern Beaufort Sea lagoons did not show clear separation on any axis (Fig. 3). Seven fish species contributed to minor differences between the communities in Kaktovik and Jago lagoons (Table 2; “Within regions”). The top three contributors from the similarity percentages were Arctic cisco (51%), saffron cod (22%) and Dolly Varden (3%). Several species, like Arctic sand lance and Pacific capelin, accounted for small proportions of the catch and small percent contributions to the variability (Table 2), but were captured only in Jago Lagoon. The association of Dolly Varden with Jago Lagoon also appeared to contribute to slight separation from the Kaktovik Lagoon community on the first and second axes (Fig. 3b, c), and the association of saffron cod in Kaktovik Lagoon contributed to separation on the second axis (Fig. 3c).
Environmental associations
During sampling events, differences in temperature, salinity, and wind speed and direction were evident among years and sites (Fig. 4). Several variables were strongly correlated (|r|≥ 0.600) including daily maximum and mean water temperature (rs = 0.971, n = 16, p < 0.0001), lag wind u and lag wind speed (rs = 0.841, n = 16, p < 0.0001), wind u and salinity (rs = – 0.785, n = 16, p = 0.0005), wind v and maximum temperature (rs = 0.768, n = 16, p = 0.0008), lag wind v and maximum temperature (rs = 0.729, n = 16, p = 0.0019), lag wind v and mean temperature (rs = 0.726, n = 16, p = 0.0020), wind v and mean temperature (rs = 0.697, n = 16, p = 0.0036), lag wind u and wind speed (rs = 0.688, n = 16, p = 0.0042), wind speed and mean temperature (rs = − 0.612, n = 16, p = 0.0136), and wind speed and maximum temperature (rs = − 0.600, n = 16, p = 0.0160).
Environmental conditions of a maximum temperature, b salinity, and sign and magnitude of wind vectors (c—west–east, d—south-north) in nearshore lagoons of the Beaufort Sea during sampling in 2017, 2018, and 2019. Vectors of wind speed indicate the direction and magnitude (speed) of the wind; positive values indicate a west (u) or south (v) wind while negative values indicate an east (u) or north (v) wind. Box-and-whisker plots show the median (line in the box), interquartile range (IQR, box), and whiskers extending above and below to 1.5(IQR). Outliers included as points. Temperature and salinity data can be found in Stanek et al. (2024), wind data are from MesoWest (University of Utah 2019)
In Jago and Kaktovik lagoons, conditions in August 2017 were relatively warm [mean ± standard error (se) maximum temperatures: Jago Lagoon 12.9 ± 0.2 °C, n = 12; Kaktovik Lagoon 13.2 ± 0.2 °C, n = 12; Fig. 4a] and saline (mean ± se salinity: Jago Lagoon 19.6 ± 0.5, n = 12; Kaktovik Lagoon 23.6 ± 0.5, n = 12; Fig. 4b), when winds were relatively calm and vector components did not differ from zero (Fig. 4c, d). During sampling in August 2018, Jago and Kaktovik lagoons were cooler (mean ± se maximum temperatures: Jago Lagoon 5.6 ± 0.4 °C, n = 16; Kaktovik Lagoon 6.4 ± 0.2 °C, n = 16) and brackish (mean ± se salinity: Jago Lagoon 7.5 ± 1.1, n = 16; Kaktovik Lagoon 10.7 ± 0.5, n = 16; Fig. 4b), with stronger winds from the west and north (Fig. 4d). In August 2019, Jago and Kaktovik lagoons were relatively cold (mean ± se maximum temperatures: Jago Lagoon 7.7 ± 0.4 °C, n = 4; Kaktovik Lagoon 8.6 ± 0.3 °C, n = 4) and saline (mean ± se salinity: Jago Lagoon 24.7 ± 1.0, n = 4; Kaktovik Lagoon 22.7 ± 0.3, n = 4; Fig. 4b), with stronger winds from the east and north (Fig. 4c, d). In July 2018 and 2019, Stefansson Sound was warm (mean ± se maximum temperatures: 12.9 ± 0.5 °C, n = 12, in 2018; 12.3 ± 0.4 °C, n = 8, in 2019; Fig. 4a) and relatively fresh to brackish (mean ± se salinity: 2.8 ± 0.1, n = 12, in 2018; 10.2 ± 2.1, n = 8, in 2019; Fig. 4b). Simpson Lagoon was cooler (mean ± se maximum temperatures 9.9 ± 0.4 °C, n = 12) and saline (mean ± se salinity 19.3 ± 0.05, n = 12) compared to Stefansson Sound, but with variable northerly winds compared to other locations in 2019 (Fig. 4c, d).
The NMDS ordination (stress = 0.08) of the average fish community at all stations for each location and year, showed similar separation patterns as the ordination of all samples. Across all NMDS axes, GAMs were compared for each individually assessed variable (Table 3). Significant (p < 0.05) and marginally significant (p = 0.05) variables were visualized in plots (Figs. 5, 6, 7, 8, 9), with species that contributed to notable differences among locations and years indicated in multivariate space (not all species are shown). For six of nine variables – salinity, wind speed, wind u, lag wind u, wind v, and maximum water temperature – we found agreement between the community patterns (represented by species CPUEs) and the environment. On two NMDS axes pairs (1 and 2, 2 and 3), the correlated variables salinity and wind u both related to the community ordination, but their effects cannot be fully parsed. Surface fits of mean temperature, lagged wind speed and lagged wind v did not project meaningfully onto the community ordination, explaining little of the community patterns seen among lagoons (Table 3).
Contour lines represent the smoothed generalized additive model fit of salinity to the fish communities in ordination space for the a first and second non-metric multidimensional scaling (NMDS) axes and the b second and third NMDS axes. Each point represents the average fish community (includes 19 species) for a station within a location and year (CPUEy,station) when collection occurred. Select species associations are shown for visual clarity, including ARCI Arctic cisco (Coregonus autumnalis), ARFL Arctic flounder (Liopsetta glacialis), BRWH broad whitefish (Coregonus nasus), FOSC fourhorn sculpin (Myoxocephalus quadricornis), LECI least cisco (Coregonus sardinella), and SACO saffron cod (Eleginus gracilis)
Contour lines represent the smoothed generalized additive model fit of the wind u vector to the communities in ordination space for the a first and second non-metric multidimensional scaling (NMDS) axes and the b second and third NMDS axes, and c fit of the three-day lagged wind u vector to the communities for the first and third NMDS axes. Wind u describes west (+) and east (−) vectors of wind speed and direction; larger values are associate with stronger wind speeds. Each point represents the average fish community (includes 19 species) for a station within a location and year (CPUEy,station) when collection occurred. Select species associations are shown for visual clarity, including ARCI Arctic cisco (Coregonus autumnalis), DOVA Dolly Varden (Salvelinus malma), LECI least cisco (Coregonus sardinella), PISA pink salmon (Oncorhynchus gorbuscha), POCO polar cod (Boreogadus saida), SACO saffron cod (Eleginus gracilis), and THST threespine stickleback (Gasterosteus aculeatus)
Contour lines represent the smoothed generalized additive model fit of the wind v vector to the communities in ordination space on first and second non-metric multidimensional scaling axes. Wind v describes south (+) and north (–) vectors of wind speed and direction; larger values are associate with stronger wind speeds. Each point represents the average fish community (includes 19 species) for a station within a location and year (CPUEy,station) when collection occurred. Select species associations are shown for visual clarity, including ARFL Arctic flounder (Liopsetta glacialis), BRWH broad whitefish (Coregonus nasus), PISA pink salmon (Oncorhynchus gorbuscha), and SACO saffron cod (Eleginus gracilis)
Contour lines represent the smoothed generalized additive model fit of maximum temperature (°C) to the communities in ordination space on first and second non-metric multidimensional scaling axes. Each point represents the average fish community (includes 19 species) for a station within a location and year (CPUEy,station) when collection occurred. Select species associations are shown for visual clarity, including ARCI Arctic cisco (Coregonus autumnalis), ARFL Arctic flounder (Liopsetta glacialis), LECI least cisco (Coregonus sardinella), and SACO saffron cod (Eleginus gracilis)
Contour lines represent the smoothed generalized additive model fit of wind speed (m/s) to the communities in ordination space for the a first and second non-metric multidimensional scaling (NMDS) axes, the b second and third NMDS axes, and the c second and third NMDS axes. Each point represents the average fish community (includes 19 species) for a station within a location and year (CPUEy,station) when collection occurred. Select species associations are shown for visual clarity, including ARCI Arctic cisco (Coregonus autumnalis), ARSA Arctic sand lance (Ammodytes hexapterus), BRWH broad whitefish (Coregonus nasus), HUWH humpback whitefish (Coregonus pidschian), PACA Pacific capelin (Mallotus catervarius), and SACO saffron cod (Eleginus gracilis)
The surface fit of salinity was associated with the community ordination on two of three axes pairs and described about 53% of the community variation in ordination space (Table 3, Fig. 5). Communities containing higher abundances of least cisco and broad whitefish clustered together on the first and second axes, near points representing Stefansson Sound, Simpson Lagoon, and Kaktovik and Jago lagoons during 2018 at locations and time periods of lower salinity (Fig. 5a). Arctic cisco and saffron cod, which were separated from one another on the second and third ordination axes, were associated with higher salinities found in Kaktovik and Jago lagoons in 2017 and 2019 (Fig. 5b). Along the third axes, separation of Simpson Lagoon and Stefansson Sound communities were associated with high abundances of Arctic flounder and fourhorn sculpin in the relatively salty Simpson Lagoon, compared to fresher Stefansson Sound (Fig. 5b).
Similar surface fits were found for wind u (west–east) on the same axes (Fig. 6a, b), although wind u appeared to explain about 10% more variation than salinity alone—61% for the first and second axes and 66% on the second and third axes (Table 3). Positive values of wind u indicate a west wind, which was more often associated with Stefansson Sound and Kaktovik and Jago lagoons in 2018 and the presence of Dolly Varden (Fig. 6a, b). Strong easterly winds in 2017 and 2019 (negative wind u values) were associated with high abundances of Arctic cisco, saffron cod, and pink salmon in Jago and Kaktovik lagoons (Fig. 6a, b). Similarly, strong winds in Stefansson Sound during 2019 correlated with polar cod (Fig. 6a). Lagged wind u explained about 45% of the variation on the first and third axes. Stronger lagged west winds associated with least cisco in Stefansson Sound and saffron cod in Kaktovik Lagoon (Fig. 6c). Lagged easterly winds differentiated the Simpson Lagoon communities, which were associated with higher threespine stickleback abundance (Fig. 6c).
The wind v (south-north) component centered around the communities in Jago Lagoon in 2018, which were associated with Arctic flounder, and experienced the strongest average northerly winds. Communities in 2019 at all locations correlated with higher, but still negative, wind v values, but species associations varied across ordination space, with wind v explaining up to 38% of the variation on only the first and second axes (Table 3; Fig. 7). Lastly, average maximum water temperature, which was positively correlated with wind v, explained 37% of the community variation on the first and third axes (Table 3). Warmer water temperatures were associated with communities in Stefansson Sound, while cooler temperatures were associated with communities in Kaktovik Lagoon during 2019 (Fig. 8). This separation aligned with the arrangement of freshwater-leaning species (e.g., least cisco) and marine species (e.g., saffron cod). Average wind speed was the only variable correlated with the ordinations of fish communities on all three axes pairs, explaining between 47 and 55% of the variation in communities (Table 3; Fig. 9). The ordination of the fish community, particularly the first axes, showed clear separation between the central and eastern Beaufort Sea lagoons (Fig. 9a, c), and average wind speed correlated well with the distribution of marine and freshwater favoring species (Fig. 9b). Greater average wind speed was associated with marine species like Pacific capelin and Arctic sand lance that were encountered infrequently during sampling and only at Jago Lagoon. Higher wind speed was also associated to the presence of abundant Arctic cisco, a species that relies on winds for recruitment to the region.
Discussion
Composition of fishes in Beaufort Sea lagoons
The spatial distribution of fish communities highlighted the effects of wind, salinity, and temperature on the coexistence of diadromous and marine fishes among Beaufort Sea lagoons and demonstrated the importance of the local environment in supporting distinct lagoon communities. In agreement with earlier studies, diadromous species with strong freshwater affinity were common to the central region, where large coastal plain rivers are used for spawning, rearing, and overwintering (Craig 1984; Craig et al. 1985). Thus, landscape features like large Arctic rivers and their nearshore freshwater plumes may represent a dominant structuring force among Arctic estuarine communities that has been maintained during a period of rapid climate change (Rantanen et al. 2022). The recently documented community reorganizations, which are primarily driven by sea ice loss and warmer water temperatures (Priest et al. 2022; von Biela et al. 2023), appear to be nested within the spatial framework that exists along the Beaufort Sea coast.
The seasonal accessibility of freshwater habitats in the central region is critical to diadromous fishes. In winter, when lagoon water temperatures are subzero, large river deltas provide overwintering areas or overwintering access for ciscoes, whitefishes, and Dolly Varden (Craig et al. 1985; Carey et al. 2021; Gallagher et al. 2021). While the seasonal migration movements of some diadromous species or life stages (e.g., Dolly Varden and Arctic cisco) are documented, information on the amount of time spent in varying estuarine habitats remains limited for most Arctic fishes including marine species (Craig 1984; Zimmerman et al. 2013; Gallagher et al. 2021). The presence or abundance of marine fishes increased in lagoons with greater connection to marine waters, that is when lagoons were more saline–typically later in the summer after the spring freshet or during periods of easterly winds (Craig 1984; Khalsa et al. 2021; Priest et al. 2022). Marine species can make up large proportions of the fish community’s abundance in lagoons, though some species, like polar cod, are episodic in occurrence with their presence linked to strong winds, cold temperatures, and high salinity (Craig et al. 1982; Barton et al. 2020; von Biela et al. 2023). These findings support information generalized from early studies (Craig 1984) and expand on our knowledge of spatial distributions and environmental correlates in Arctic estuarine communities that are experiencing rapid climate change (Meredith et al. 2019).
Distribution of Arctic estuarine fishes in relation to abiotic variables
The results of the generalized additive models on each pair of the three NMDS axes revealed that estuarine communities are most responsive to the west–east (u) wind component, salinity, and wind speed. To a lesser extent, south-north (v) winds and maximum temperature were correlated to the spatial distribution of the fish communities. Multicollinearity among the environmental variables, however, makes it difficult to parse specific effects, yet these analyses indicated that local fish communities respond to wind influenced marine and freshwater masses that determine salinity and temperature in the lagoons (Craig 1984; Valesini et al. 2014). As expected, higher salinities correlated with marine species like saffron cod, while low salinity conditions in the central region near the Sagavanirktok (Stefansson Sound) and Kuparuk (Simpson Lagoon) rivers were associated with freshwater-leaning diadromous species like least cisco. Strong easterly winds were associated with Arctic cisco abundance, which we anticipated given the known recruitment patterns of young-of-the-year, which made up a large proportion of the catch in 2017 (Zimmerman et al. 2013; Stanek et al. 2022b). The response of fishes to the predominant regional water mass (i.e., river discharge versus marine water) and the accessibility of freshwater habitats for diadromous species may be the ultimate driver of coastal distribution patterns across Beaufort Sea lagoons (Craig 1984; Feyrer et al. 2015; Roux et al. 2016; Lundstrom et al. 2022). Changes to the timing or magnitude of river discharge events, or increased wave action from storms occurring over ice-free areas of the Arctic Ocean could have profound effects on the balance of water masses in lagoons (Casas-Prat and Wang 2020; Feng et al. 2021), potentially changing the relative abundance or presence of fish community members.
The west–east (u) wind component explained more community variation than temperature or salinity independently; temperature and salinity are important to the structure of fish communities in other Beaufort Sea estuarine studies (Roux et al. 2016; Barton et al. 2020; Priest et al. 2022). Strong easterly winds draw cold, saline water from depth onto the shelf (Schulze and Pickart 2012), drawing water away from the shoreline, resulting in decreased depth in lagoons and creating habitat for marine species (Okkonen et al. 2018). At Elson Lagoon, which lies directly east of Point Barrow, Alaska, easterly winds move water from the Beaufort Sea through barrier islands, advecting Arctic marine species (e.g., Pacific capelin) into the shallow water (Barton et al. 2020). Additionally, strong easterly winds in mid- to late-August promote transport of juvenile Arctic cisco into Alaskan waters (Fechhelm and Fissel 1988; Zimmerman et al. 2013; Priest et al. 2022), as witnessed through high abundance in Jago and Kaktovik lagoons in August of 2017 and 2019. Arctic cisco arrive in eastern lagoons earlier in August than to the central lagoons, which are farther from the Mackenzie River (Moulton 1989). July sampling in the central region likely resulted in missed detection of abundant recruits in Simpson Lagoon and Stefansson Sound. Still, our results are similar to those from season-long data sets (July–September), where marine species abundance is higher relative to freshwater/diadromous species in the eastern Beaufort Sea lagoons (Brown 2008) and marine species abundance is roughly equivalent to freshwater/diadromous species abundance in Prudhoe Bay (Priest et al. 2022).
Lagoon salinity was correlated strongly with regional differences in fish community composition. Salinity gradients have been associated with species replacement in coastal lagoon systems, with stronger effects found at larger spatial scales (Feyrer et al. 2015; Menegotto et al. 2019). Investigations in other Beaufort Sea lagoon systems indicated the presence of or distance to the river mouth was pivotal to the distribution and abundance of species and that salinity played an important role in community composition (Roux et al. 2016; Barton et al. 2020; Priest et al. 2022). Freshwater species, like Arctic grayling, are found in oligohaline (salinity < 5) waters of lagoons (Roux et al. 2016) and were rarely captured during this study since salinities were often higher–oligohaline conditions occurred only in Jago Lagoon (Arctic grayling captured) and Stefansson Sound in 2018. Diadromous species with higher salinity tolerance (< 25) were common and widespread, but some species, like humpback whitefish and Arctic cisco, appeared to sort along a gradient from west to east, which may be strongly related to their life histories. Humpback whitefish occurred only in the central region, and their absence from Kaktovik and Jago lagoons likely reflects true differences in occurrence given their known distribution and reliance on large river systems to complete their life history (Alt 1979; Craig 1984). Arctic cisco abundance was ~11 times higher on average in the eastern region compared to the central region, which may be related to the congruence of August sampling and migration timing of young-of-the-year fish in Kaktovik and Jago lagoons (Moulton 1989).
Marine species rare to the lagoons during sampling (e.g., polar cod, Arctic sand lance) supported differences in fish community composition between individual lagoons within and across regions, and were likely associated with sporadic events like storms, when strong wind-driven currents facilitate the movement of small marine fishes into open lagoons (Valesini et al. 2014; Barton et al. 2020). We may have failed to detect species like Arctic sand lance that burrow in soft sediments and require active sampling for capture (e.g., beach seine). Among sites near Point Barrow, Alaska, Arctic sand lance CPUE was at least 40 times higher outside of the lagoon compared to capture rates in the lagoon (Barton et al. 2020), suggesting capture of this species in lagoons would be rare even using different sampling methods. The absence of polar cod in the lagoons is more likely due to current environmental conditions (i.e., warmer and less sea ice) than from sampling methods, which historically used the same gear and readily captured polar cod when present (Priest et al. 2022; von Biela et al. 2023). Reduction of polar cod in seabird diets also indicates their decline in nearshore areas as water temperatures increase and summer sea ice declines (Divoky et al. 2021).
In the Beaufort Sea’s coastal waters, wind speed is correlated with nearshore current speeds, which are coupled to the size of the brackish water band in the coastal zone and to the advection of water masses (Craig 1984; Hale 1990; Dunton et al. 2006). Stronger winds in 2017 were associated with high Arctic cisco abundance in Jago and Kaktovik lagoons and with the presence of Arctic sand lance and Pacific capelin in Jago Lagoon. The average wind speeds included in this study were limited in magnitude (3.4–5.0 m·s−1) due to operational constraints of field sampling; storms with strong westerly winds (22 m·s−1) prevented multiple days of sampling in 2019. Although sustained (> 72 h) strong winds have been shown to affect nearshore conditions and communities days later (Hale 1990; Barton et al. 2020), we saw limited evidence of association between the three-day lagged west–east wind component and community composition. The effects of strong winds in the nearshore Beaufort Sea can be moderated by sea ice cover, but summer sea ice extent and concentration in the nearshore Arctic has declined over the past four decades (Perovich et al. 2020). Summer and fall winds are now poised to have much stronger effects on oceanography and biological communities than they have in past decades with higher sea ice cover (Casas-Prat and Wang 2020; Falardeau et al. 2023).
Maximum temperature was less important to the distribution of fish communities across Beaufort Sea lagoons than anticipated, even though it was found to be important in studies within lagoons (e.g., Roux et al. 2016; Priest et al. 2022), or between lagoons and colder shelf waters (e.g., Barton et al. 2020). We attribute this to the relatively narrow range of average maximum temperatures (5.6 − 13.3 °C) experienced by fish during our July and August sampling windows or to the environmental tolerance of species present in our data set. In the Husky Lakes, some species that are encountered occasionally or rarely [e.g., pike (Esox lucius)] are cool-water fishes that reside primarily in freshwater and only visit Arctic lagoons during warmer periods (Roux et al. 2016). In warmer temperatures, upwards of 17 °C, like those experienced in Prudhoe Bay, Alaska, cold-water marine species likely remain outside of the lagoons (Barton et al. 2020; Khalsa et al. 2021). Polar cod, an ice-associated species, favors temperatures < 6 °C and the sea ice edge (Laurel et al. 2016; Marsh et al. 2020). In recent sampling, this species has been found less often in lagoons during late summer, and access to suitable lagoon habitat may be restricted as waters continue to warm during summer months (Marsh et al. 2020; von Biela et al. 2023). Certainly, small climate change induced increases in lagoon summer water temperatures (to > 10 °C) may affect the presence or distribution of fishes—promoting higher species richness in Arctic estuaries by way of range expansions or migrations of boreal species (Roux et al. 2016; Barton et al. 2020; Dunmall et al. 2022).
Conclusion
Our results showed fish community composition in Beaufort Sea lagoons depended on local geography and the wind-driven balance between freshwater and marine water masses that dictate salinity. As expected, higher salinities and associated easterly winds facilitated the arrival and abundance of marine species in the lagoons, but the relationship between community composition and warmer temperatures was weaker, and often covaried with winds and salinity making it difficult to parse these effects. The importance of easterly winds and salinity was evident during the brief summer period and agrees with other investigations in northern estuaries, some of which were conducted over longer time periods (e.g., Roux et al. 2016; Priest et al. 2022). Where long-term data collection exists, there is evidence of shifting communities, away from Arctic species and towards boreal species (Priest et al. 2022; von Biela et al. 2023). Changes in sea ice conditions and access to the Arctic, concurrent with increases in saffron cod abundance have added focus to potential commercial cod fisheries in U.S. Arctic waters (NPFMC 2009). Not only will sea ice loss be dire for polar cod and the ice-associated food web (Huserbråten et al. 2019), but ice reductions will alter nearshore currents, increase wave action, and erode shorelines, all of which will increase variability in nearshore salinity (Falardeau et al. 2023). The intrusion of marine waters to lagoons during storms may decrease the habitat suitability for diadromous fishes. Conversely, increasing freshwater inputs, from thawing permafrost or increased precipitation, would supply fresh water and additional carbon and nutrients from terrestrial sources for use by estuarine species (Stanek et al. 2022a). Additional freshwater inputs may make more nearshore areas available to the freshwater-leaning diadromous species. Together, changes to Arctic estuarine environments could have profound effects on the make up of the community, biological function, and energy flow. Improved understanding of lagoon community structure and the drivers supporting species presence and abundance may inform the management and conservation of estuarine fish species in a rapidly changing environment.
References
Ahmed R, Prowse T, Dibike Y, Bonsal B, O’Neil H (2020) Recent trends in freshwater influx to the Arctic Ocean from four major Arctic-draining rivers. Water 12:1189. https://doi.org/10.3390/w12041189
Alt KT (1979) Contributions to the life history of the humpback whitefish in Alaska. Trans Am Fish Soc 108:156–160
Ballinger TJ, Overland JE, Wang M, Bhatt US, Hanna E, Hanssen-Bauer I, Kim SJ, Thoman RL, Walsh JE (2020) Arctic Report Card 2020: Surface air temperature. https://doi.org/10.25923/gcw8-2z06. Accessed 20 Mar 2023.
Barton MB, Vollenveider JJ, Heintz RA, Norcross BL, Boswell KM (2020) Spatiotemporal variation of environmental conditions and prey availability that drive Arctic nearshore fish community structure in the Point Barrow, Alaska, region. Can J Fish Aquat Sci 77:1612–1624. https://doi.org/10.1139/cjfas-2019-0068
Beel CR, Heslop JK, Orwin JF, Pope MA, Schevers AJ, Hung JKY, Lafrenière MJ, Lamoureux SF (2021) Emerging dominance of summer rainfall driving High Arctic terrestrial-aquatic connectivity. Nat Commun 14:1448. https://doi.org/10.1038/s41467-021-21759-3
Bilous M, McNicholl DG, Dunmall KM (2022) Coastal marine biodiversity in the western Canadian Arctic. Arct Sci 8:1127–1147. https://doi.org/10.1139/as-2021-0022
Bonsell C, Dunton KH (2018) Long-term patterns of benthic irradiance and kelp production in the central Beaufort Sea reveal implications of warming for Arctic inner shelves. Prog Oceanogr 162:160–170. https://doi.org/10.1016/j.pocean.2018.02.016
Brown RJ (2008) Life history and demographic characteristics of Arctic cisco, Dolly Varden, and other fish species in the Barter Island region of northern Alaska. Alaska Fisheries Technical Report No. 101. U.S. Fish and Wildlife Service, Fairbanks, Alaska.
Carey MP, von Biela VR, Brown RJ, Zimmerman CE (2021) Migration strategies supporting salmonids in Arctic rivers: a case study of Arctic cisco and Dolly Varden. Anim Migr 8:132–143. https://doi.org/10.1515/ami-2020-0115
Casas-Prat M, Wang XL (2020) Projections of extreme ocean waves in the Arctic and potential implications for coastal inundation and erosion. J Geophys Res Ocean 125:e2019JC015745. https://doi.org/10.1029/2019JC015745
Choy ES, Giraldo C, Rosenberg B et al (2020) Variation in the diet of beluga whales in response to changes in prey availability: insights on changes in the Beaufort Sea ecosystem. Mar Ecol Prog Ser 647:195–210. https://doi.org/10.3354/meps13413
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Craig PC (1984) Fish use of coastal waters of the Alaskan Beaufort Sea: a review. Trans Am Fish Soc 113:265–282
Craig PC, Griffiths WB, Haldorson L, McElderry H (1982) Ecological studies of Arctic cod (Boreogadus saida) in Beaufort Sea coastal waters, Alaska. Can J Fish Aquat Sci 39:395–406
Craig PC, Griffiths WB, Haldorson L, McElderry H (1985) Distributional patterns of fishes in an Alaskan Arctic Lagoon. Polar Biol 4:9–18
Craig PC, Haldorson L (1981) Fish. In: Environmental Assessment of the Alaskan Continental Shelf, Final Reports of Principal Investigators Vol. 7. Biological Studies. U.S. Department of Commerce and U.S. Department of Interior, pp 384−678.
Divoky GJ, Brown E, Elliott KH (2021) Reduced seasonal sea ice and increased sea surface temperature change prey and foraging behavior in an ice-obligate Arctic seabird, Mandt’s black guillemot (Cepphus grylle mandtii). Polar Biol 44:701–715. https://doi.org/10.1007/s00300-021-02826-3
Dunmall KM, McNicholl DG, Zimmerman CE, Gilk-Baumer SE, Burril S, von Biela VR (2022) First juvenile chum salmon confirms successful reproduction for Pacific salmon in the North American Arctic. Can J Fish Aquat Sci 79:703–707. https://doi.org/10.1139/cjfas-2022-0006
Dunton KH, Reimnitz E, Schonberg S (1982) An Arctic kelp community in the Alaskan Beaufort Sea. Arctic 35:465–484
Dunton KH, Weingartner T, Carmack EC (2006) The nearshore western Beaufort Sea ecosystem: circulation and importance of terrestrial carbon in arctic coastal food webs. Prog Oceanogr 71:362–378. https://doi.org/10.1016/j.pocean.2006.09.011
Dunton KH, Schonberg SV, Funk DW (2009) Interannual and spatial variability in light attenuation: Evidence from three decades of growth in the Arctic kelp, Laminaria solidungula. In: Krupnik I, Lang MA, Miller SE (eds) Smithsonian at the poles: contributions to international polar year science. Scholarly Press, Washington
Falardeau J, de Vernal A, Fréchette B, Hillaire-Marcel C, Archambault P, Fritz M, Gallagher CP, Tanski G (2023) Impacts of stronger winds and less sea ice on Canadian Beaufort Sea shelf ecosystems since the late 1990s. Estuar Coast Shelf Sci 294:108520. https://doi.org/10.1016/j.ecss.2023.108520
Fechhelm RG, Fissel DB (1988) Wind-aided recruitment of Canadian Arctic cisco (Coregonus autumnalis) into Alaskan waters. Can J Fish Aquat Sci 45:906–910
Fechhelm RG, Baker JS, Griffiths WB, Schmidt DR (1989) Localized movement patterns of least cisco (Coregonus sardinella) and Arctic cisco (C. autumnalis) in the vicinity of a solid-fill causeway. Biol Pap Univ Alaska 24:75–106
Feng D, Gleason CJ, Lin P, Yang X, Pan M, Ishitsuka Y (2021) Recent changes to Arctic river discharge. Nat Commu 12:6917. https://doi.org/10.1038/s41467-021-27228-1
Feyrer F, Cloern JE, Brown LR, Fish MA, Heib KA, Baxter RD (2015) Estuarine fish communities responds to climate variability over both river and ocean basins. Glob Change Biol 21:3608–3619. https://doi.org/10.1111/gcb.12969
Gallagher CP, Courtney MB, Seitz AC, Lea EV, Howland KL (2021) Ocean-entry timing and marine habitat-use of Canadian Dolly Varden: dispersal among conservation, hydrocarbon exploration, and shipping areas in the Beaufort Sea. Estuar Coast Shelf Sci 262:107609. https://doi.org/10.1016/j.ecss.2021.107609
Gibbs AE, Erikson LH, Jones BM, Richmond BM, Engelstad AC (2021) Seven decades of coastal change at Barter Island, Alaska: exploring the importance of waves and temperature on erosion of coastal permafrost bluffs. Remote Sens 13:4420. https://doi.org/10.3390/rs13214420
Hale DA (1990) A description of the physical characteristics of nearshore and lagoonal waters in the eastern Beaufort Sea. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Anchorage, Alaska.
Harris CM, McClelland JW, Connelly TL, Crump BC, Dunton KH (2017) Salinity and temperature regimes in eastern Alaskan Beaufort Sea lagoons in relation to source water contributions. Estuar Coast 40:50–62. https://doi.org/10.1007/s12237-016-0123-z
Huserbråten MBO, Eriksen E, Gjøsæter H, Vikebø F (2019) Polar cod in jeopardy under the retreating Arctic sea ice. Commun Biol 2:407. https://doi.org/10.1038/s42003-019-0649-2
Kelley D, Richards C (2022) gsw: Gibbs Sea Water Functions. R package version 1.1–1, https://CRAN.R-project.org/package=gsw. Accessed 1 Apr 2023
Khalsa NS, Gatt KP, Sutton TM, Kelley AL (2021) Characterization of the abiotic drivers of abundance of nearshore Arctic fishes. Ecol Evol 11:11491–11506. https://doi.org/10.1002/ece3.7940
Laurel BJ, Spencer M, Iseri P, Copeman LA (2016) Temperature-dependent growth and behavior of juvenile Arctic cod (Boreogadus saida) and co-occurring North Pacific gadids. Polar Biol 39:1127–1135. https://doi.org/10.1007/s00300-015-1761-5
Lundstrom NC, Beaudreau AH, Mueter FJ, Konar B (2022) Environmental drivers of nearshore fish community composition and size structure in glacially influenced Gulf of Alaska Estuaries. Estuar Coast 45:2151–2665. https://doi.org/10.1007/s12237-022-01057-x
Marsh JM, Mueter FJ, Quinn TJ (2020) Environmental and biological influences on the distribution and population dynamics of polar cod (Boreogadus saida) in the US Chukchi Sea. Polar Biol 43:1055–1072
Menegotto A, Dambros CS, Netto SA (2019) The scale-dependent effect of environmental filters on species turnover and nestedness in an estuarine benthic community. Ecology 100(7):e02721. https://doi.org/10.1002/ecy.2721
Meredith M, Sommerkorn M, Cssotta S et al (2019) Polar Regions, IPCC special report on the ocean and cryosphere in a changing climate. Intergovernmental Panel Climate Change. https://doi.org/10.1017/9781009157964.005
Moulton L (1989) Recruitment of Arctic cisco (Coregonus autumnalis) into the Colville Delta, Alaska, in 1985. Biol Pap Univ Alaska 24:107–111
NPFMC (2009) Fishery Management Plan for fish Resources of the Arctic Management Area. North Pacific Fishery Management Council. https://www.npfmc.org/wp-content/PDFdocuments/fmp/Arctic/ArcticFMP.pdf. Accessed 27 Apr 2023
NSIDC (2023) Sea Ice Index, Version 3 [Data Set]. National Snow and Ice Data Center, Boulder, Colorado USA. https://doi.org/10.7265/N5K072F8. Accessed 20 Mar 2023
Okkonen SR, Clarke JT, Potter RA (2018) Relationships among high river discharges, upwelling events, and bowhead whale (Balaena mysticetus) occurrence in the central Alaskan Beaufort Sea. Deep Sea Res Part II Top Stud Oceanogr 152:195–202. https://doi.org/10.1016/j.dsr2.2016.11.015
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens HH, Szoecs E, Wagner H (2020) vegan: Community Ecology Package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan. Accessed 1 Apr 2023
Perovich D, Meier W, Tschudi M, Hendricks S, Petty AA, Divine D, Farrell S, Gerland S, Haas C, Kaleschke L, Pavlova O, Ricker R, Tian-Kunze X, Webster M, Wood K (2020) Arctic Report Card 2020: Sea Ice. https://doi.org/10.25923/n170-9h57. Accessed 20 Mar 2023
Priest JT, Mueter FJ, Raborn SW, Sutton TM (2022) Effects of environmental variables on a nearshore arctic fish community, 2001–2018. Polar Biol 45:585–599. https://doi.org/10.1007/s00300-022-03013-8
R Core Team (2021) R: A language and envrionment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org
Rantanen M, Karpechko AY, Lipponen A, Nordling K, Hyvärinen O, Ruosteenoja K, Vihma T, Laaksonen A (2022) The Arctic has warmed nearly four times faster than the globe since 1979. Commun Earth Environ 3:168. https://doi.org/10.1038/s3247-022-00498-3
Rizzolo DJ, Schmutz JA, Speakman JR (2015) Fast and efficient: postnatal growth and energy expenditure in an Arctic-breeding waterbird, the Red-throated Loon (Gavia stellata). Auk 132:657–670. https://doi.org/10.1642/AUK-14-261.1
Roux M-J, Harwood LA, Zhu X, Sparling P (2016) Early summer near-shore fish assemblage and environmenta correlates in an Arctic estuary. J Great Lakes Res 42:256–266. https://doi.org/10.1016/j.jglr.2015.04.005
Schulze LM, Pickart RS (2012) Seasonal variation of upwelling in the Alaskan Beaufort Sea: Impact of sea ice cover. J Geophys Res 117:C06022. https://doi.org/10.1029/2012JC007985
Stanek AE, von Biela VR, Laske SM, Taylor RL, Dunton KH (2022a) Barrier islands influence the assimilation of terrestrial energy in nearshore fishes. Estuar Coast Shelf Sci 278:108094. https://doi.org/10.1016/j.ecss.2022.108094
Stanek AE, von Biela VR, Brown RJ (2022b) Fish communities of the nearshore Beaufort Sea, Alaska, across three decades, 1988–2019. U.S. Geological Survey data release, https://doi.org/10.5066/P9120X5B
Stanek AE, Laske SM, von Biela VR (2024) Water temperature, electrical conductivity, and salinity of lagoons in the Beaufort Sea, Alaska, 2017–2019. U.S. Geological Survey data release, https://doi.org/10.5066/P9V8N8FE
Thoman RL, Richter-Menge J, Druckenmiller ML (2020) Arctic Report Card 2020. https://doi.org/10.25923/mn5p-t549. Accessed 20 Mar 2023
Thorsteinson LK, Love MS (2016). Alaska Arctic marine fish ecology catalog: U.S. Geological Survey Scientific Investigations Report 2016−5038 (OCS Study, BOEM 2016−048). https://doi.org/10.3133/sir20165038
Timmermans ML, Labe Z (2020) Arctic Report Card 2020: Sea surface temperature. https://doi.org/10.25923/v0fs-m920. Accessed 20 Mar 2023
Timmermans ML, Ladd C (2019) Arctic Report Card 2019: Sea surface temperature. https://arctic.noaa.gov/report-card/report-card-2019/sea-surface-temperature-4/. Accessed 14 Dec 2023
Underwood TJ, Gordon JA, Millard MJ, Thorpe LA, Osborne BM (1995) Characteristics of selected fish populations of Arctic National Wildlife Refuge coastal waters, Final Report, 1988–1991. U.S. Fish and Wildlife Service Report, Fairbanks Fishery Resource Office, Alaska Fisheries Technical Report Number 28, Fairbanks, Alaska.
University of Utah (2019) MesoWest Data. https://mesowest.utah.edu. Accessed 1 Sept 2019
Valesini FJ, Tweedley JR, Clarke KR, Potter IC (2014) The importance of regional, system-wide and local spatial scales in structuring temperate estuarine fish communities. Estuar Coast 37:525–547. https://doi.org/10.1007/s12237-013-9720-2
von Biela VR, Laske SM, Stanek AE, Brown RJ, Dunton KH (2023) Borealization of nearshore fishes on an interior Arctic shelf over multiple decades. Glob Change Biol 29:1822–1838. https://doi.org/10.1111/gcb.16576
Zimmerman CE, Ramey AM, Turner SA, Mueter FJ, Murphy SM, Nielsen JL (2013) Genetics, recruitment, and migration patterns of Arctic cisco (Coregonus autumnalis) in the Colville River, Alaska, and Mackenzie River, Canada. Polar Biol 36:1543–1555. https://doi.org/10.1007/s00300-013-1372-y
Acknowledgements
We thank the knowledgeable people of Kaktovik Village and the staff and students of Harold Kaveolook School for their hospitality during the Kaktovik Oceanography Program. We would like to thank R/V Proteus captains Ted Dunton and John Dunton, Catherine Coon, Sean Burril, Daniel Donnelly, Cody Dawson, Arctic National Wildlife Refuge staff, the Beaufort Lagoon Ecosystem Long Term Ecological Research program, and Hilcorp Alaska, LLC for access to boat launch facilities. Thank you to Sarah Traiger and two anonymous reviewers for their feedback on earlier drafts of this manuscript. This work was supported by funding from U.S. Geological Survey Outer Continental Shelf Program and the U.S. Geological Survey Changing Arctic Ecosystems Initiative. Work was conducted under Alaska Department of Fish and Game Aquatic Resource Use permits (CF-2017-100, CF-2018-074, CF-2019-096) and Fish and Wildlife Service Special Use Permit 2017-03 from the Arctic National Wildlife Refuge. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Contributions
SML, VRV, KHD conceived the research. SML, VRV, AES conducted field investigations and curated data. AES assisted with visualization and coding. SML analyzed the data and wrote the manuscript. All authors contributed to reviewing and editing. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Sample collection and animal handling were conducted in accordance with the Animal Care and Use Policy of the Institutional Animal Care and Use Committee (IACUC), U.S. Geological Survey Alaska Science Center (2017-07).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laske, S.M., von Biela, V.R., Stanek, A.E. et al. Local environmental conditions structured discrete fish assemblages in Arctic lagoons. Polar Biol 47, 551–568 (2024). https://doi.org/10.1007/s00300-024-03239-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-024-03239-8