Abstract
Purpose
This study aimed to develop an unobtrusive method for home sleep apnea testing (HSAT) utilizing micromotion signals obtained by a piezoelectric rubber sheet sensor.
Methods
Algorithms were designated to extract respiratory and ballistocardiogram components from micromotion signals and to detect respiratory events as the characteristic separation of the fast envelope of the respiration component from the slow envelope. In 78 adults with diagnosed or suspected sleep apnea, micromotion signal was recorded with a piezoelectric rubber sheet sensor placed beneath the bedsheet during polysomnography. In a half of the subjects, the algorithms were optimized to calculate respiratory event index (REI), estimating apnea–hypopnea index (AHI). In the other half of subjects, the performance of REI in classifying sleep apnea severity was evaluated. Additionally, the predictive value of the frequency of cyclic variation in heart rate (Fcv) obtained from the ballistocardiogram was assessed.
Results
In the training group, the optimized REI showed a strong correlation with the AHI (r = 0.93). Using the optimal cutoff of REI ≥ 14/h, subjects with an AHI ≥ 15 were identified with 77.8% sensitivity and 90.5% specificity. When applying this REI to the test group, it correlated closely with the AHI (r = 0.92) and identified subjects with an AHI ≥ 15 with 87.5% sensitivity and 91.3% specificity. While Fcv showed a modest correlation with AHI (r = 0.46 and 0.66 in the training and test groups), it lacked independent predictive power for AHI.
Conclusion
The analysis of respiratory component of micromotion using piezoelectric rubber sheet sensors presents a promising approach for HSAT, providing a practical and effective means of estimating sleep apnea severity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Home sleep apnea testing (HSAT) [1], as a means of screening sleep apnea, plays a crucial role in efficiently utilizing the limited medical resources of polysomnographic examination. Various types of sensors are utilized in HSAT devices, including those for measuring nasal pressure/temperature [2], respiratory inductance plethysmography [3], peripheral arterial tonometry [4, 5], oximetry [6], electrocardiography [7, 8], pulse wave photo-plethysmography [9], radio-wave Doppler effect [10] and bed-embedded micromotion sensors [11,12,13,14]. Generally, it is believed that the accuracy of sleep apnea detection improves with an increased number of signals measured [15]. However, considering the convenience of HSAT, it is desirable to minimize the number of sensors used and the effort required to wear them [16]. Thus, the optimal signal and measurement method should be selected by considering the tradeoff between accuracy and convenience.
Among the sensors utilized in HSAT, micromotion sensing by sheet sensors positioned beneath the bedsheet presents the notable advantage of enabling users to sleep without the need for wearing sensors or electrodes while remaining unaware of their presence. Additionally, these sensors possess a unique feature of capturing respiration and ballisttocardioram as well as body movement using a single sensor device [13]. Thus, they could be a promising solution for HSAT, effectively meeting both accuracy and convenience requirements simultaneously. In this study, we developed an unobtrusive method for HSAT utilizing a piezoelectric rubber sheet sensor. We designated algorithms to extract respiration and ballistocardiogram signals, allowing for the scoring of the respiratory event index (REI) [16, 17], as well as measuring the frequency (Fcv) of cyclic variation of heart rate (CVHR) [7, 8, 18]. The performance of REI and Fcv in classifying sleep apnea severity was assessed using the apnea–hypopnea index (AHI) obtained from the simultaneous polysomnogram as a reference standard.
Methods
Ethics approval and consent to participate
All procedures were performed in accordance with the protocol that was approved by the Research Ethics Committee of the Center for Data-driven Science and Artificial Intelligence, Tohoku University, Japan (registration number 2022–7). All subjects participated in this study gave their written informed consent.
Subjects
The eligible subjects for this study were patients who underwent an overnight polysomnography due to suspected or diagnosed sleep disordered breathing at Gifu Mates Sleep Clinic in Gifu, Japan, between September 2022 and December 2022. The inclusion criterion was adulthood (age 20 years or older). Subjects were excluded if they had continuous atrial fibrillation, experienced acute illness, or had exacerbation of chronic diseases requiring hospitalization within the past three months. Additionally, individuals who were pregnant or breastfeeding were also excluded.
Protocol
The polysomnographic examination was performed with an Alice diagnostic sleep system (Philips-Respironics, Murrysville, PA, USA Philips Respironics, The Netherlands). The examination was initiated at the subject's customary bedtime and continued until the subject awoke the next morning, during which micromotions were continuously measured by a commercially available piezoelectric rubber sheet sensor device (Moni Life wellness®, Sumitomo Riko Company Limited, Komaki, Aichi, Japan).
Subjects were randomly allocated into a training group and a test group. Using the data from the training group, we developed and optimized the algorithms for sleep apnea detection, constructed regression models to estimate sleep apnea severity, and identified the optimal cutoff values for classifying the severity. Using the data from the test group, we evaluated the classification performance of the algorithms.
Measurements
The polysomnograms were recorded with the standard montages consisting of F4-M1, F4-M2, C4-M1, C3-M2, O2-M1, and O1-M2 electroencephalograms, left and right electrooculograms, a submental electromyogram, a nasal pressure cannula, oronasal airflows, left and right tibial electromyograms, thoracoabdominal inductance plethysmograms, pulse oximetric arterial blood oxygen saturation, a neck microphone, body position sensors, and a modified lead II ECG.
Sleep stages, apneic and hypopneic indices (AI and HI, respectively), and AHI were scored according to the American Association of Sleep Medicine (AASM) Manual for the Scoring of Sleep and Associated Events, Version 2.5 [19] by registered polysomnogram technicians. AHI was calculated both with the total recording time (TRT) as the denominator (AHITRT) and total sleep time (TST) as the denominator (AHITST). The AHITRT was used as the reference standard for developing algorithms for sleep apnea detection from the micromotion signal. The AHITST was used to classify sleep apnea severity, with < 5 defined as normal, 5–15 as mild, 15–30 as moderate, and ≥ 30 as severe sleep apnea. The ECG signal of the polygraph was sampled at a frequency of 100 Hz. All QRS complexes were identified and annotated as normal (sinus rhythm), ventricular ectopic beat, supraventricular ectopic beat, and artifact.
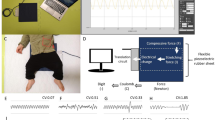
The piezoelectric rubber sheet sensor, depicted in Fig. 1, had dimensions of 811 mm in length, 60 mm in width, and 0.9 mm in thickness and a weight of 220 g. It was positioned beneath the bedsheet at a level from the subject's axilla to the lower end of the sternum body. The signal was digitized at 100 Hz with a 24-bit dynamic range (± 223, from -8,388,608 to + 8,388,608) and the device outputted the data to a CSV file.
Development of REI algorithms
The details of the algorithms are reported in Appendix. Briefly, first, the algorithm extracted respiratory, body movement, and ballistocardiogram components from the micromotion signal of the piezoelectric rubber sheet sensor using band-pass filters set to their respective frequency ranges (0.08–0.5, 2–3, and 4–11 Hz) (Fig. 2). Second, the respiratory component signals were rectified to reflect the magnitude of respiratory motion and natural logarithm transformation was performed to minimize the effects of large changes in respiratory amplitude and large noise. Third, two types of upper (95th percentile point) envelopes (fast and slow) were generated; the fast envelope depicted breath-by-breath amplitude changes, while the slow envelope presented the local trend of submaximum amplitude. The use of the 95th percentile points for the envelopes excluded outliers with an incidence < 5%. Fourth, periods in which the fast envelope separated downward from the slow envelope to an extent greater than a threshold (depth threshold) were detected. Fifth, when the length of a period was within a range (duration criteria), the period was considered to be a respiratory event. Finally, REI was calculated as the frequency of respiratory events per hour of TRT.
Detection of sleep apnea by micromotion signal from piezoelectric rubber sheet sensor. A series of sleep apnea attacks began to appear at 04:28, with the fast upper envelope of respiration (red line in panel b) declining from the slow upper envelope (blue line in panel b) and showing periodic dips. Panel d is the enlarged image of frame b’ in panel b. Periods in which the dip of the fast upper envelope (red line) from the slow upper envelope (blue line) exceeds a depth threshold (ln (1.43), which corresponds to a 30% drop) are indicated by vertical lines (cyan). When the length (L) of a period meets duration criteria (10 to 70 s), the period is considered to be a respiratory event
The optimal values of depth threshold and duration criteria were determined in the training group using a grid-search method. This involved repeated analyses with small parameter adjustments until the closest correlation between REI and AHI was achieved. Because the amplitude of respiratory movement detected by the sheet sensor varied substantially with body position, a percentage reduction was employed as the depth threshold to adapt to the variations. The search for the optimal depth threshold was conducted in 1% increments, while the optimal lower and upper duration criteria were determined at 10-s intervals.
The source code for these algorithms was written in FORTRAN 95 and compiled on Microsoft Windows 10 by the Silverfrost Fortran (FTN95) compiler (Elgin, IL, USA).
Analysis of Fcv
Fcv was measured not only from the heartbeat interval of the ballistocardiogram but also from ECG R-R interval of the polysomnography (ECG-Fcv). The method for measuring the heartbeat interval from ballistocardiogram is reported in Appendix. CVHR was detected by previously published algorithm [7, 8, 18] and Fcv and ECG-Fcv was computed as the frequency of CVHR per hour of TRT. The ECG-Fcv was used to determine whether the association between ballistocardiogram Fcv and AHITRT is affected by the accuracy of ballistocardiogram-based heartbeat interval measurement.
Statistical analysis
The statistical analyses were performed using the program package of Statistical Analysis System (SAS Institute, Cary, NC, USA). Between-group differences in quantitative and categorical variables were assessed using the Wilcoxon rank sum test and χ2 test, respectively. The relationships between AHITRT and REI, Fcv, and ECG-Fcv were evaluated with Pearson's correlation coefficient. The multivariate regressions were performed using the SAS REG procedure. The classification performance of REI, Fcv, and ECG-Fcv for binary sleep apnea severity was evaluated by the area under the curve (AUC) of the receiver-operating characteristic (ROC) curve. The classification performance for four severity levels (normal, mild, moderate, and severe) was examined by the percentages of subjects correctly classified and misclassified off by one, two, and three classes. The optimal REI cutoff values for these classifications were determined by balancing sensitivity and specificity by ROC curve analysis in the training group and then evaluated in the test group. Statistical significance was defined as P < 0.05.
Results
Subjects’ characteristics
We enrolled a total of 78 consecutive subjects (21 females) with a median (interquartile range, IQR) age of 49 (38–62) years who underwent polysomnography for diagnostic purposes (n = 54, 69%) or evaluation of therapeutic effects (n = 24, 31%). The polysomnography revealed that the median (IQR) AHITST was 13.4 (3.6 to 23.8), with 34 (44%) subjects classified as having moderate-to-severe sleep apnea (AHITST ≥ 15) and 18 (23%) subjects classified as having severe sleep apnea (AHITST ≥ 30). Among apnea episodes, obstructive, central, and mixed types accounted for 72%, 4%, and 24%, respectively. Half (n = 39) of the subjects were assigned to the training group and the other half (n = 39) to the test group. There were no significant differences in the characteristics of the subjects between the two groups (Table 1).
Grid search for optimal parameters
In the training group, the grid search was conducted to determine the optimal parameter values. The results revealed that the closest correlation between REI and AHITRT was achieved when the depth threshold was set at 30% (equivalent to a natural-logarithmic transformed amplitude difference of ln (1.43)) and the duration criteria was configured to select dips with a length between 10 and 70 s as respiratory events. These parameters were used for analyzing the data in the test group.
Estimation of AHITRT by REI and Fcv
In the training group, the REI calculated with the optimized parameters closely correlated with AHITRT (r = 0.93), while the Fcv and ECG-Fcv correlated with correlation coefficients of 0.46 and 0.76, respectively (Fig. 3). When applying the same algorithms and parameters to the data in the test group, the correlation coefficients became 0.92, 0.66, and 0.77 for REI, Fcv, and ECG-Fcv, respectively. Notably, in both the training group and test group, the correlations of Fcv were lower compared to those of REI, as well as the correlations of ECG-Fcv. These differences were partly attributed to the impact of PLM on Fcv (Figs. 3 and 4). When excluding subjects with a PLM index of ≥ 15, the correlation coefficients of REI, Fcv, and ECG-Fcv became 0.92, 0.68, and 0.76, respectively, in the training group and 0.91, 0.70, and 0.79, respectively, in the test group.
Correlation of alternative measures of AHI with the true measure in the training group (panels a-c) and test group (panels d-f).
 Subjects with a PLM index ≥ 15. AHI = apnea–hypopnea index, ECG = electrocardiogram, Fcv = frequency of cyclic variation of heart rate, PLM periodic leg movement, PSG = polysomnography, REI = respiratory event index, TRT = total recording time
Subjects with a PLM index ≥ 15. AHI = apnea–hypopnea index, ECG = electrocardiogram, Fcv = frequency of cyclic variation of heart rate, PLM periodic leg movement, PSG = polysomnography, REI = respiratory event index, TRT = total recording time
Multiple regression analyses were performed to assess the relationship between AHITRT and the combination of REI and Fcv in the training and test groups. In either group, the inclusion of Fcv lead to no significant improvement in the multiple correlation when REI was already included, even when the subjects with PLM index of ≥ 15 were excluded.
Sleep apnea severity classification by REI
Table 2 displays the results of the ROC curve analysis evaluating the classification performance of sleep apnea severity using the REI, Fcv, and ECG-Fcv. In both the training and test groups, the REI demonstrated favorable classification performance, as indicated by AUC values exceeding 0.9, for both moderate-to-severe sleep apnea and severe sleep apnea. The AUC values of REI were higher than those of Fcv for both levels of severity and those of ECG-Fcv for severe sleep apnea.
The ROC curve analysis in the training group revealed that REI ≥ 14 and REI ≥ 24 were optimal cutoff values for detecting moderate-to-severe sleep apnea and severe sleep apnea, respectively (Table 3). When applying these cutoff values to the data in the test group, 87.5% sensitivity and 91.3% specificity for moderate-to-severe sleep apnea and 87.5% sensitivity and 96.8% specificity for severe sleep apnea were obtained (Table 3).
Finally, in the training group, the most favorable outcome for the classification of severity into four levels was achieved by incorporating cutoff values of REI < 9 to define the normal category, and 9 to 14 to define mild sleep apnea (Table 4). Subsequently, When applying these identical cutoff values to the test group, the classification accuracy reached 56.4% and 82% of the misclassifications were off by one class and the rest (18%) were off by two classes (Table 4).
Discussion
To develop an unobtrusive method for HSAT utilizing micromotion signals detected by a piezoelectric rubber sheet sensor, we developed algorithms to extract respiratory and ballistocardiogram components from these signals and detect respiratory events. In a group of 78 adult subjects with diagnosed or suspected sleep apnea, data from half of the subjects were used to optimize algorithms to calculate REI estimating AHITRT, while the other half was used to evaluate REI's performance in classifying sleep apnea severity. Additionally, the predictive value of Fcv from the ballistocardiogram was assessed. The optimized REI closely correlated with AHI, effectively identifying subjects with AHI ≥ 15 with high sensitivity and specificity in both training and test groups. However, while Fcv showed a modest correlation with AHI, it lacked independent predictive power. These results suggest that analyzing the respiratory component of micromotion using piezoelectric rubber sheet sensors offers a promising and practical avenue for HSAT, providing an effective means of estimating sleep apnea severity.
Although the analysis of micromotion signals has garnered increasing attention as a noninvasive method for obtaining respiration and heartbeat signals during sleep [11,12,13,14, 20], the use of these signals requires addressing two crucial challenges: the frequent inclusion of substantial noise, even with minimal body movements, and the notable variability in the magnitude of the respiratory signal with changes in body posture and position. The REI assessment algorithms developed in this study addressed these issues by a method utilizing the fast and slow envelopes of the 95th percentile points of logarithmically transformed respiratory amplitude. By employing the 95th percentile points for the envelopes, the influences of outliers with an incidence < 5% were excluded. Moreover, the logarithmic transformation minimized extensive deviations of the envelopes caused by large noise. As sleep apnea–hypopnea events were identified based on the relative percent reduction in the fast envelope compared to the slow envelope, the detection threshold automatically adapted to variations in respiratory amplitude reflected in the slow envelope. With these features, the algorithms provided an optimized REI that identified subjects with an AHI ≥ 15 with a sensitivity of 87.5% and specificity of 91.3% in the test group. This classification performance compared favorably even with the performance of Type 3 HSAT devices studied in the American Academy of Sleep Medicine's clinical guidelines for sleep apnea diagnosis (six devices studied in 457 participants, with sensitivity ranging from 62 to 94% and specificity ranging from 25 to 97%) [21].
In previous studies investigating sleep apnea–hypopnea detection through micromotion, these challenges were addressed using diverse approaches. Agatsuma et al. [11] evaluated the performance of a sheet sensor, SD-101, for HSAT. The SD-101 consisted of 162 membrane-type pressure sensors arranged on a sheet 1235 mm long, 555 mm wide, and 5 to 7 mm thick. The device had the ability to automatically find and select one of the 162 sensors that most significantly sensed respiratory motion and had the least noise. In 201 patients with suspected sleep apnea, the REI measured by the SD-101 correlated with AHITRT (r = 0.88); REI ≥ 14 identified patients with AHITST ≥ 15 with 89.5% sensitivity and 85.8% specificity. Their findings were replicated by Kobayashi et al. [22], with a correlation coefficient of 0.87 and a sensitivity of 87.5% and specificity of 85.7% for identification of patients with AHITST ≥ 15. Sadek et al. [13] conducted a study using a microbend fiber optic sensor mat measuring 20 cm × 50 cm × 0.5 cm in ten patients with obstructive sleep apnea. They devised an adaptive histogram-based thresholding approach for detecting respiratory events. This method involved creating a histogram from the absolute deviations of respiratory signal in overlapping 60-s segments and determined the optimal cutoff to detect segments with a respiratory event. Using this approach, they achieved a sensitivity of 57.07% and a specificity of 45.26% in detecting true individual respiratory events. Coluzzi et al. [14] developed a multi-scale algorithm for detecting sleep fragmentation. The algorithm analyzed the cumulative histogram of quiet sleep segment lengths derived from micromotion signals obtained by a pressure bed sensor measuring 64 cm × 64 cm. They reported that the ratio between the total fragmented sleep time and the total moving time had a correlation coefficient of 0.85 with AHI in 18 subjects who underwent polysomnography. Finally, Weinreich et al. [20] conducted a study in 57 patients with obstructive sleep apnea or PLM using Sleep Minder, a radio wave Doppler sensor, that enabled non-contact micromotion sensing. The detection algorithm employed by the device was not disclosed. While the AHI estimated by this device had a correlation coefficient of 0.57 with the true AHI, it displayed a correlation coefficient of 0.79 with the sum of the true AHI and PLMI by the polysomnography. Moreover, it successfully identified patients with a sum of AHI + PLMI ≥ 15/h with a sensitivity of 92.2% and a specificity of 95.8%. Due to differences in device type, study subjects, and taction target, comparing the performance of algorithms is not feasible. Nonetheless, the algorithms proposed in this study might be simpler and more straightforward, thereby enabling wide utilization across various types of micromotion sensors.
We analyzed heartbeat signal as well as respiration and evaluated the association between Fcv and AHITRT, but the correlation was modest. The disparity between ECG R-R intervals and ballistocardiogram heartbeat intervals may contribute the lower correlation of Fcv. In fact, the ECG-Fcv demonstrated a closer correlation with AHITRT than Fcv. Another potential factor for this difference might be the greater influence of PLM on ballistocardiogram CVHR than on ECG CVHR. PLM is known to be associated with CVHR, and differentiating CVHR caused by PLM from CVHR due to sleep apnea/hypopnea is generally challenging [7]. After excluding subjects with a PLM index of 15 or higher, the correlation between Fcv and AHITRT improved, but was still lower compared to that between REI and AHITRT. REI was not affected by PLM, and when combined with REI, Fcv had no significant predictive value for AHITRT. In terms of sleep apnea detection using the micromotion signal, the analysis of the Fcv may not be critical. However, the analysis of heartbeat and body movement would be valuable in evaluating sleep quality, as supported by earlier studies [13, 23, 24].
Finally, regarding HSAT devices, piezoelectric rubber sheet sensors have excellent characteristics: small size, light weight, and thin profile. These features would improve not only the ease of incorporating the sensor into bedding but also portability and practicality, reduce the burden of transfers between beds and patient rooms, and facilitate lending from the clinic to patients. Given the significant variability in sleep apnea severity from night to night [18], prolonged monitoring over multiple nights in a home setting is desirable to accurately assess the disease's characteristics in real-world conditions. In such scenarios, the small and lightweight design of the device becomes crucial for convenient handling and ensuring effective maintenance of cleanliness and hygiene.
This study has several limitations. First, the participants were patients who required polysomnography, and the pretest probability of moderate-to-severe sleep apnea was 41% in the test group. When applying to a population with a lower probability, it is expected that the positive predictive accuracy will decrease accordingly. Second, while the sheet sensor was uniformly positioned from the subject's axilla to the lower end of the sternum, there is a possibility for further optimization of its placement for more effective detection of sleep apnea–hypopnea. Third, due to the limited number of cases, the analysis of the effects of comorbidities and of the type of sleep apnea (obstructive, central, or mixed) on the classification performance were not conducted. Fourth, differences in sleep apnea–hypopnea detection effectiveness by sleep stage could not be evaluated because they are disturbed by large changes in respiratory amplitude due to body posture/position. Fifth, the combined predictive value of Fcv and REI may be further improved by machine learning approaches, but they also require a larger sample size. Lastly, the study was conducted in a sleep laboratory, but the product is intended for home use. Future researches are needed to address these limitations and investigate the applicability of the findings in a home setting.
Conclusions
This study demonstrates that the analysis of the respiratory component, detected using piezoelectric rubber sheet sensors to measure REI, provides a valuable method for HSAT. The optimized REI closely correlated with the AHITRT, displaying strong sensitivity and specificity in identifying subjects with varying severity of sleep apnea–hypopnea. Additionally, while the Fcv showed some correlation with AHITRT, its incorporation did not enhance the predictive capability of the regression model when combined with REI. These findings suggest that the analysis of the respiratory component using piezoelectric rubber sheet sensors presents a promising approach for HSAT, offering a practical and effective means of estimating sleep apnea severity.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request and with permission of Gifu Mates Sleep Clinic.
Abbreviations
- AASM :
-
American Association of Sleep Medicine
- AHI :
-
Apnea–hypopnea index
- AI :
-
Apnea index
- AUC :
-
Area under the curve
- BMI :
-
Body mass index
- CAI :
-
Central apnea index
- CI :
-
Confidence interval
- CPAP :
-
Continuous positive airway pressure
- ECG :
-
Electrocardiography
- Fcv :
-
Frequency of cyclic variation of heart rate
- HI :
-
Hypopnea index
- HSAT :
-
Home sleep apnea test
- IQR :
-
Interquartile range
- MAI :
-
Mixed apnea index
- NPA :
-
Negative predictive accuracy
- OHI :
-
Obstructive apnea index
- PC :
-
Personal computer
- PLM :
-
Periodic leg movement
- PPV :
-
Positive predictive accuracy
- REI :
-
Respiratory event index
- ROC :
-
Receiver operating characteristic
- TRT :
-
Total recording time
- TST :
-
Total sleep time
References
Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R (2007) Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 3(7):737–747
Campbell AJ, Neill AM (2011) Home set-up polysomnography in the assessment of suspected obstructive sleep apnea. J Sleep Res 20(1 Pt 2):207–213. https://doi.org/10.1111/j.1365-2869.2010.00854.x
Dingli K, Coleman EL, Vennelle M, Finch SP, Wraith PK, Mackay TW, Douglas NJ (2003) Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J 21(2):253–259. https://doi.org/10.1183/09031936.03.00298103
Massie F, Van Pee B, Bergmann J (2022) Correlations between home sleep apnea tests and polysomnography outcomes do not fully reflect the diagnostic accuracy of these tests. J Clin Sleep Med 18(3):871–876. https://doi.org/10.5664/jcsm.9744
Jagielski JT, Bibi N, Gay PC, Junna MR, Carvalho DZ, Williams JA, Morgenthaler TI (2022) Evaluating an under-mattress sleep monitor compared to a peripheral arterial tonometry home sleep apnea test device in the diagnosis of obstructive sleep apnea. Sleep Breath. https://doi.org/10.1007/s11325-022-02751-7
Heneghan C, Chua CP, Garvey JF, de Chazal P, Shouldice R, Boyle P, McNicholas WT (2008) A portable automated assessment tool for sleep apnea using a combined Holter-oximeter. Sleep 31(10):1432–1439
Hayano J, Watanabe E, Saito Y, Sasaki F, Fujimoto K, Nomiyama T, Kawai K, Kodama I, Sakakibara H (2011) Screening for obstructive sleep apnea by cyclic variation of heart rate. Circ Arrhythm Electrophysiol 4(1):64–72. https://doi.org/10.1161/CIRCEP.110.958009
Hayano J, Tsukahara T, Watanabe E, Sasaki F, Kawai K, Sakakibara H, Kodama I, Nomiyama T, Fujimoto K (2013) Accuracy of ECG-based screening for sleep-disordered breathing: a survey of all male workers in a transport company. Sleep Breath 17(1):243–251. https://doi.org/10.1007/s11325-012-0681-7
Hayano J, Yamamoto H, Nonaka I, Komazawa M, Itao K, Ueda N, Tanaka H, Yuda E (2020) Quantitative detection of sleep apnea with wearable watch device. PLoS One 15(11):e0237279. https://doi.org/10.1371/journal.pone.0237279
Westenberg JN, Petrof BJ, Noel F, Zielinski D, Constantin E, Oskoui M, Kaminska M (2021) Validation of home portable monitoring for the diagnosis of sleep-disordered breathing in adolescents and adults with neuromuscular disorders. J Clin Sleep Med 17(8):1579–1590. https://doi.org/10.5664/jcsm.9254
Agatsuma T, Fujimoto K, Komatsu Y, Urushihata K, Honda T, Tsukahara T, Nomiyama T (2009) A novel device (SD-101) with high accuracy for screening sleep apnoea-hypopnoea syndrome. Respirology 14(8):1143–1150. https://doi.org/10.1111/j.1440-1843.2009.01627.x
Meng L, Xu H, Guan J, Yi H, Wu H, Yin S (2016) Validation of a novel sleep-monitoring system for diagnosing obstructive sleep apnea: A comparison with polysomnography. Exp Ther Med 12(5):2937–2941. https://doi.org/10.3892/etm.2016.3721
Sadek I, Heng TTS, Seet E, Abdulrazak B (2020) A New Approach for Detecting Sleep Apnea Using a Contactless Bed Sensor: Comparison Study. J Med Internet Res 22(9):e18297. https://doi.org/10.2196/18297
Coluzzi D, Baselli G, Bianchi AM, Guerrero-Mora G, Kortelainen JM, Tenhunen ML, Mendez MO (2022) Multi-Scale Evaluation of Sleep Quality Based on Motion Signal from Unobtrusive Device. Sensors (Basel) 22(14). https://doi.org/10.3390/s22145295
Collop NA, Tracy SL, Kapur V, Mehra R, Kuhlmann D, Fleishman SA, Ojile JM (2011) Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. J Clin Sleep Med 7(5):531–548. https://doi.org/10.5664/jcsm.1328
Tran NT, Tran HN, Mai AT (2023) A wearable device for at-home obstructive sleep apnea assessment: State-of-the-art and research challenges. Front Neurol 14:1123227. https://doi.org/10.3389/fneur.2023.1123227
Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV, Medicine ftAAoS (2015) The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Version 2.2. American Academy of Sleep Medicine, Darien, Illinois
Hayano J, Yuda E (2021) Night-to-night variability of sleep apnea detected by cyclic variation of heart rate during long-term continuous ECG monitoring. Ann Noninvasive Electrocardiol e12901. https://doi.org/10.1111/anec.12901
Berry RB, Albertario CL, Harding SM, Uoyd RM, Plante DT, Quan SF, Troester MM, Vaughn BV (2018) The AASM Manual for the Scoring of Sleep and Association Events: Rules, Terminology and Technical Specifications, Version 2.5. American Academy of Sleep Medicine, Darien, IL
Weinreich G, Terjung S, Wang Y, Werther S, Zaffaroni A, Teschler H (2018) Validation of a non-contact screening device for the combination of sleep-disordered breathing and periodic limb movements in sleep. Sleep Breath 22(1):131–138. https://doi.org/10.1007/s11325-017-1546-x
Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG (2017) Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an american academy of sleep medicine clinical practice guideline. J Clin Sleep Med 13(3):479–504. https://doi.org/10.5664/jcsm.6506
Kobayashi M, Namba K, Tsuiki S, Nakamura M, Hayashi M, Mieno Y, Imizu H, Fujita S, Yoshikawa A, Sakakibara H, Inoue Y (2013) Validity of sheet-type portable monitoring device for screening obstructive sleep apnea syndrome. Sleep Breath 17(2):589–595. https://doi.org/10.1007/s11325-012-0725-z
Hayano J, Ueda N, Kisohara M, Yoshida Y, Tanaka H, Yuda E (2020) Non-REM sleep marker for wearable monitoring: power concentration of respiratory heart rate fluctuation. Appl Sci 10(9):3336
Edouard P, Campo D, Bartet P, Yang RY, Bruyneel M, Roisman G, Escourrou P (2021) Validation of the Withings Sleep Analyzer, an under-the-mattress device for the detection of moderate-severe sleep apnea syndrome. J Clin Sleep Med 17(6):1217–1227. https://doi.org/10.5664/jcsm.9168
Acknowledgements
The authors would like to thank the following clinical staffs of the Gifu Mates Sleep Clinic for the data collection/scoring: Rika Kajita, Takayoshi Saiki, Hiroko Terada, Miki Nishikawa, Izumi Nonaka, Ayako Yabashi, Rino Yasue, Takahiro Uchiyama, Yurie Kawamura, and Hiroyuki Kawashima.
Funding
Sumitomo Riko Company Limited, Komaki, Aichi, Japan provided financial support to Heart Beat Science Lab to fund this research, approved the design of the study, and provided the piezoelectric rubber sheet sensor used in this study. The sponsor had no role in the conduct of this research.
Author information
Authors and Affiliations
Contributions
J.H., H.T., and E.Y. conceived the study. J.H. performed the analysis and wrote the main manuscript. H.Y. contributed to data acquisition and clinical interpretation. H.T. and E.Y. critically reviewed the manuscript. All authors reviewed the manuscript and approved the submission.
Corresponding author
Ethics declarations
Ethics approval
All procedures were performed in accordance with the Regulations Concerning the Conduct of Life Science and Medical Research Involving Human Subjects at Tohoku University, Japan, the Ethical Guidelines for Medical Research Involving Human Subjects issued by the Japanese Ministry of Health, Labor and Welfare, and the 1964 Declaration of Helsinki and its subsequent amendments. The study protocol was approved by the Research Ethics Committee of the Center for Data-driven Science and Artificial Intelligence, Tohoku University, Japan (registration number 2022–7). All subjects who participated in this study gave written informed consent, including consent to the publication of this paper.
Consent to participate
All subjects who participated in this study gave written informed consent, including consent to the publication of this paper.
Competing Interests
J.H. is the President and E.Y. is the Chief Technical Officer of Heart Beat Science Lab Inc., which received funding for this study from Sumitomo Riko Company Limited. The other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Detection of respiratory events from respiratory component of micromotion
Output from the piezoelectric rubber sheet sensor included respiratory, body movement, and ballistocardiogram components, which could be separated from each other by the typical frequency bands in which they reside. We developed algorithms to estimate respiratory event index (REI) from the respiratory signal.
The algorithm for detection of respiratory events and calculation of REI is shown in Fig. 5. First, the micromotion signal was standardized to have a dynamic range of ± 223 and the respiration signal was extracted with a 0.08 Ha to 0.5 Hz bandpass filter. Second, the respiratory signal was rectified and natural-logarithmic transformed to reflect the magnitude of respiratory movement while minimizing the impacts of large changes in respiratory amplitude and of large noises. Third, the slow and fast upper envelopes of the respiratory signal were calculated as the moving 95th percentile with window widths of 60 and 6 s, respectively. The slow envelope reflects the average submaximal magnitude of respiratory motion smoothed out by the effects of sleep apnea/hypopnea, while the fast envelope faithfully reflects the changes in the magnitude of respiratory movements including those with sleep apnea/hypopnea. Fourth, a respiratory event was considered to occur when the dip of the fast envelope, compared to the slow envelope, surpassed a threshold value (ln (1.43)), indicating a 30% decline in magnitude, continuously for a duration ranging from 10 to 70 s. Finally, the hourly frequency of respiratory events during total recording time (TRT) of the polysomnogram was calculated as the REI.
The threshold values for the dips of fast envelope and the duration of dips were determined in the training group using a grid search method. This involved repeated analyses with small parameter adjustments until the closest correlation between REI and AHI was achieved (see main text).
Measurement of heartbeat interval from ballistocardiogram component of micromotion
The algorithm for measuring heartbeat intervals from the micromotion signal is shown in Fig. 6. First, since the micromotion signal was digitalized by a 24-bit converter, the offset was removed by subtracting 223 and dividing by 210 to standardize the dynamic range to ± 213 (± 8192). Second, the data were divided into segments of 50-s duration with 20-s overlap at both ends. Second, ballistocardiogram was extracted with bandpass filters of 4–11 Hz. The signal was rectified and natural-logarithmic transformed, and pulse wave signals were extracted as the upper envelope of each signal. Finally, the heartbeat intervals were measured as the intervals between successive foot points of the pulse wave signal.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hayano, J., Yamamoto, H., Tanaka, H. et al. Piezoelectric rubber sheet sensor: a promising tool for home sleep apnea testing. Sleep Breath 28, 1273–1283 (2024). https://doi.org/10.1007/s11325-024-02991-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-024-02991-9