Abstract
Background
Gastro-entero-pancreatic neuroendocrine carcinomas (GEP-NECs) are an aggressive subgroup of neuroendocrine neoplasms (NENs). In patients affected with NEN, there is a growing body of evidence that increased C-X-C motif chemokine receptor (CXCR4) expression is linked to decreasing overall survival (OS) in an ex-vivo setting. Thus, we aimed to determine whether the in-vivo-derived CXCR4-directed whole-body PET signal can also determine GEP-NEC patients with shorter OS.
Methods
We retrospectively included 16 patients with histologically proven GEP-NEC, who underwent CXCR4-directed PET/CT for staging and therapy planning. We assessed maximum, peak, and mean standardized uptake values as well as whole-body tumor volume (TV) and total-lesion uptake (TLU = SUVmean × TV) using a semi-automatic segmentation tool with a 50% threshold. Association of PET-based biomarkers and OS or radiographic progression-free survival (rPFS; according to RECIST 1.1 criteria) was analyzed using univariable and multivariable cox regression.
Results
Median OS and rPFS was 7.5 and 7 months, respectively. A significant correlation between TV and TLU was found for OS (TV: hazard ratio (HR) 1.007 95% confidence interval (CI) 1.000–1.014, p = 0.0309; TLU: HR 1.002 95% CI 1.000–1.003, p = 0.0350) and rPFS (TV: HR 1.010 95% CI 1.002–1.021; p = 0.0275; TLU: HR 1.002 95% CI 1.000–1.004, p = 0.0329), respectively. No significant correlation with OS or rPFS was found for non-volumetric parameters (p > 0.4). TV remained a significant predictive marker for OS and rPFS in multivariable analysis (OS: HR 1.012 95%, CI 1.003–1.022, p = 0.0084; rPFS: HR 1.009, 95% CI 0.9999–1.019, p = 0.0491), whereas TLU remained only prognostic for OS (HR 1.009, 95% CI 0.9999–1.019, p = 0.0194) but narrowly failed significance for rPFS (p = 0.0559).
Conclusion
In-vivo assessment of CXCR4 PET-derived volumetric parameters is predictive for outcome of patients with GEP-NEC and could be used as a risk stratification tool, which detects patients prone to early progression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gastro-entero-pancreatic neuroendocrine carcinomas (GEP-NECs) are highly aggressive neuroendocrine neoplasms (NENs). The differentiation of NEN is defined upon histomorphological features and proliferation index (Ki-67) [1]. NECs are dedifferentiated neoplasms that constitute only 10–20% of all NENs and are associated with a poor prognosis [2]. Whereas treatment of well-differentiated NEN is based upon targeting the somatostatin receptor (SSTR) using somatostatin analogs or SSTR-directed radioligand therapy [3], treatment possibilities of NECs are largely limited to conventional chemotherapy because of a lack of SSTR expression and a highly aggressive tumor growth [4]. Both European Neuroendocrine Tumor Society (ENETS) and European Society for Medical Oncology (ESMO) guidelines recommend platinum-based chemotherapy using cisplatin/etoposide or carboplatin/etoposide as first-line therapy for advanced NEC [3, 5]. However, there is no standardized treatment regime for second-line therapy and proposed therapies include fluorouracil-based chemotherapy together with either irinotecan or oxaliplatin as well as temozolomide in monotherapy or in combination with capecitabine. Nevertheless, progression is unavoidable [4] and further therapeutic options or personalized treatments are therefore desperately warranted.
A factor that might influence outcome of patients affected with NECs is the expression of C-X-C motif chemokine receptor (CXCR4). CXCR4 is a G-protein-coupled receptor which is overexpressed in multiple hematological malignancies and solid cancers and a possible target for theranostic approaches [6]. Our study group has shown that CXCR4-targeted imaging using the radiopharmaceutical [68Ga]Ga-PentixaFor is an effective image tool in staging a variety of neoplasms, especially multiple myeloma, mantle cell lymphoma, small cell lung cancer, and adrenocortical neoplasms [7]. Of note, the therapeutic counterpart [177Lu]Lu/[90Y]Y-PentixaTher can be used for CXCR4-directed radioligand therapy and has proven substantial anti-lymphoma effect in selected malignancies [8,9,10]. In an earlier work of our study group, we assessed the diagnostic value of CXCR4-directed PET/CT compared to the performance to reference standard [18F]fluorodesoxyglucose (FDG) PET/CT in patients with NEC. In this analysis of 11 treatment-naive patients, the diagnostic performance of CXCR4 PET/CT was inferior to those of FDG PET/CT and in-vivo-assessed CXCR4 expression was not sufficient for possible CXCR4-directed radioligand therapy. However, increased CXCR4 expression in GEP-NEN in an ex-vivo setting was linked to decreasing overall survival (OS) [11]. Of note, an ex-vivo sampling only displays tumor biology and receptor expression in the respective lesion, while assessment of the in-vivo CXCR4 expression offers a non-invasive whole-body evaluation, which reflects also possible intraindividual tumor heterogeneity. Thus, in-vivo-assessed CXCR4 expression might be a prognostic biomarker for response to therapy and outcome prediction.
The aim of this study was to investigate the prognostic impact of CXCR4 PET-derived biomarker in patients with NEC and their potential impact on OS and radiographic progression-free survival (rPFS).
Methods
Patient Population
Searching our institutional PET/CT database we retrospectively identified 16 patients with neuroendocrine carcinomas of the gastrointestinal tract who underwent [68 Ga]Ga-PentixaFor PET/CT between July, 2015, and July, 2022. Patients were also included in case of unknown primary if imaging and histopathological results indicated gastrointestinal origin. Patients were excluded if neither follow-up imaging nor survival data was available. Patients were referred to our institution by the treating physicians to evaluate potential theranostic considerations after first-line therapy using the therapeutic equivalent [177Lu]Lu- or [90Y]Y-PentixaTher [12]. In 15 of 16 patients (94%) a recent FDG PET/CT was available as part of clinical routine diagnostic. Due to the retrospective character of this study, the local institutional review board waived the requirement for additional approval (No. 20230721 01). All patients provided written informed consent. Parts of this cohort have been examined in previous studies [7, 13,14,15,16,17,18]; however, without comparing PET-based parameters to patient outcome.
Imaging Procedure
All PET/CTs were performed on Siemens Biograph mCT 64 or 128 scanners (Siemens Healthineers, Erlangen, Germany). PET/CTs were acquired approximately 60 min after the administration of 115 ± 29 MBq [68 Ga]Ga-PentixaFor or 292 ± 30 MBq [18F]FDG from vertex to mid-thigh. The acquisition parameters were 2–3 min/bed position (mCT 64)/continuous bed motion at 1.1 mm/s (mCT 128); 3 iterations, subsets 24 (mCT 64)/21 (mCT 128); matrix, 200 * 200; Gaussian filter, 2.0 mm. A contrast-enhanced diagnostic CT scan or a low dose CT scan was acquired for attenuation correction and anatomical coregistration (tube voltage, 100–120 kV; tube current modulation; reconstructed axial slice thickness 3.0–5.0 mm) [15, 18].
Image Evaluation
Images were analyzed by one reader (KM). Fiji [19] and the Beth Israel plugin [20] were used for autosegmentation of PET-positive lesions, i.e., volumes of interest (VOIs). VOIs including tissue with physiological radioligand uptake were removed manually, while VOIs for pathological lesions not detected by autosegmentation were added manually by the reader. In analogy to whole-body tumor segmentation on prostate-specific membrane antigen-targeted PET/CT [21, 22], we used a lesion-specific threshold of 50% of the local SUVmax for CXCR4 PET as well as for FDG PET according to the guideline of the European Association of Nuclear Medicine (EANM) [23]. The summed volumes of all lesions represent the whole-body tumor volume (= TV, measured in milliliters). In addition, we assessed maximum, peak, and mean standardized uptake values as well as total-lesion uptake (= SUVmean × TV). Radiographic PFS was assessed by two board-certified radiologists in consensus (AK and WS) using RECIST 1.1 criteria on CT follow-up imaging [24].
Statistical Analysis
Statistical analyses were performed using GraphPad Prism Version 9.3.1 (GraphPad Prism Software, La Jolla, CA, USA). Descriptive data are presented as mean ± standard deviation or median and range. A paired t-test was used to assess differences between the two PET tracers. Outcome data is analyzed using univariable and multivariable cox regression including age, Ki67-score, presence of liver metastases, and pretreatment (yes vs. no). OS and rPFS are calculated from the day of CXCR4 PET/CT and are presented as median with the 95% confidence interval in square brackets. A p value less than 0.05 was considered statistically significant.
Results
Patients’ Characteristics
Mean patient age was 65 ± 10 (range 44–76) years (Table 1). Most patients were male (13 of 16; 81%). Median Ki67-score was 80 (range 45–90)%. Most patients were treatment naïve (12 of 16; 75%) at the time of CXCR4 PET/CT. Mean time between initial diagnosis and CXCR4 PET was 9 ± 9 (range 2–23) months for those patients (4 of 16) who were pretreated. Median time interval between CXCR4 and FDG PET (15 of 16 patients; 94%) was 6 days (range 1–53 days). Assessment of rPFS was possible in 14 of 16 patients (88%), because follow-up imaging was not available in 2 of 16 patients (12%).
CXCR4 PET-Derived Volumetric Parameters Are Prognostic for OS and rPFS
Mean parameters for TV, TLU, SUVmax, SUVpeak, and SUVmean were 83.0 ± 112.1 ml (range 0–476.6 ml), 391.3 ± 443.7 (range 0–2215.5), 9.6 ± 5.0 (range 0–18.5), 7.3 ± 4.3 (range 0–18.5), and 4.5 ± 2.3 (range 0–8.2), respectively. Of note, 2 of 16 patients (13%) did not show any pathological uptake on CXCR4 PET, despite liver and lymph node metastases seen on corresponding FDG PET/CT. Disease progression occurred in all patients. Thirteen of 16 patients (81%) died during follow-up. Median OS and rPFS of the whole patient cohort was 7.5 (n = 16) and 7 (n = 14) months, respectively. A significant correlation between TV and TLU was found for OS (TV: hazard ratio (HR) 1.007, 95% confidence interval (CI) 1.000–1.014, p = 0.0309; TLU: HR 1.002, 95% CI 1.000–1.003, p = 0.0350) and rPFS (TV: HR 1.010, 95% CI 1.002–1.021; p = 0.0275; TLU: HR 1.002, 95% CI 1.000–1.004, p = 0.0329), respectively. No significant correlation with neither OS nor rPFS was found for non-volumetric parameters (p > 0.4, respectively).
FDG PET-Derived Parameters Are Not Prognostic for OS and rPFS
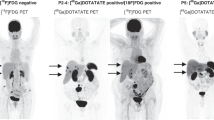
Mean parameters for TV, TLU, SUVmax, SUVpeak, and SUVmean were 243.7 ± 229.0 ml (range 1.8–785.9 ml), 2422.4 ± 2510.7 (range 8.7–7758.4), 18.6 ± 10.7 (range 6.5–43.3), 14.0 ± 7.9 (range 4.5–29.7), and 9.0 ± 4.8 (range 3.5–20.8), respectively. The two patients with negative CXCR4 PET showed intense uptake on FDG PET (TV: 18.4 ml, TLU: 164.1, SUVmax: 14.7, SUVpeak: 10.3, SUVmean: 8.9 and TV: 433.9 ml, TLU: 7341.6, SUVmax: 37.1, SUVpeak: 29.7, SUVmean: 16.9). All PET-based parameter were significantly higher FDG PET compared to CXCR4 PET (p-values: TV: 0.0467, TLU: 0.0097, SUVmax: 0.0075, SUVpeak: 0.0082, SUVmean: 0.0046; Fig. 1). Median OS and rPFS of those patients who underwent an additional FDG PET/CT was 6 (n = 15) and 5.5 (n = 13) months, respectively. None of the volumetric or non-volumetric parameters were prognostic for OS or rPFS (p > 0.06). Table 2 summarizes the outcome data of CXCR4 and FDG PET-derived parameters. Figure 2 depicts different patterns of tracer uptake on CXCR4 and FDG PET.
Comparison of PET-based biomarker of corresponding maximum intensity projections of CXCR4 (upper row) and FDG (lower row) PET. Tumor is delineated using Fiji [19] and the Beth Israel Plugin [20] and marked in blue. SUV window ranging from 0 to 5. A, B A 75-year-old patient with pretreated gastric neuroendocrine carcinoma (NEC) and a negative CXCR4 PET scan with missing tracer uptake of the liver metastases. C, D A 55-year old patient with initial diagnosis of an esophageal NEC and metastases in lymph nodes, liver, bones, and the lungs. E, F A 68-year-old patient with initial diagnosis of a pancreatic NEC and local lymph node metastases only seen on CXCR4 PET. G, H A 76-year-old patient with initial diagnosis of a gastric NEC and metastases in lymph nodes, liver, bones, and the lungs
CXCR4 PET-Derived Tumor Volume Remains a Prognostic Marker in Multivariable Cox Regression
Multivariable cox regression including age, Ki67-score, presence of liver metastases, and pretreatment (yes vs. no) for CXCR-derived TV and TLU confirmed TV as a significant predictive marker as well as for OS and for rPFS (OS: HR 1.012 95% CI 1.003–1.022, p = 0.0084; rPFS: HR 1.009 95% CI 0.9999–1.019, p = 0.0491), whereas TLU remained only predictive for OS (HR 1.009 95% CI 0.9999–1.019, p = 0.0194) but narrowly failed significance for rPFS (p = 0.0559). The other parameters were not significantly associated with OS or rPFS (p > 0.07, respectively).
Discussion
This is the first study to evaluate the prognostic impact of whole-body in-vivo assessment of CXCR4 expression using CXCR4 PET/CT in patients with GEP-NEC. CXCR4-based TV and TLU were significantly correlated to OS and rPFS as well as in univariable and for TV also in multivariable analysis. Interestingly, corresponding FDG PET-derived biomarkers were not associated with outcome. In contrast, Langen Stokmo et al. found TV, TLU, and SUVmax on FDG PET to be significant predictors for OS and rPFS in 14 patients with gastroenteropancreatic neuroendocrine tumors G3 and 52 patients with gastroenteropancreatic NEC [25]. Jiang et al. showed a significant association between NECs of the uterine cervix in 22 patients and the volume as well as the lesion uptake solely of the primary tumor on FDG PET and PFS, whereas SUVmax of the primary tumor was not a prognostic marker [26]. Hou et al. also described a significant prognostic value of TV and TLU on FDG PET in 28 patients with esophageal NECs [27]. The fact that FDG PET was not a prognostic tool in our cohort could be due to the small sample size in which a statistical significant effect does not break through. CXCR4 PET-derived parameters appear to be more robust predictors for rPFS and OS with significant results even in a small study group.
Volumetric parameters (TV and TLU) on CXCR4 PET were the only predictive biomarker. This was not due to mere tumor extent since the “real” tumor volume on FDG PET significantly diverged. A possible reason might be tumor heterogeneity, which cannot be depicted by SUVmax/peak or SUVmean. Hence, CXCR4 PET might serve as a risk stratification tool which is able to detect patients prone to early progression. Consequently, CXCR4 PET/CT might help the treating physician to identify candidates in risk of early progression under first-line therapy or even single out high-risk lesions that can be targeted by a local procedure such as surgery, radiofrequency ablation, or chemoembolisation.
In the present study, we did not analyze the diagnostic performance or the theranostic potential of CXCR4 PET/CT compared to the reference standard. However, two patients showed false-negative CXCR4 PET scans in our patient cohort and the diagnostic inferiority of CXCR4 PET in patients with (neuroendocrine) gastrointestinal neoplasms has been shown before by our study group [14, 28]. Hence, other theranostic radiopharmaceuticals in this tumor entity would be desirable. One potential option is fibroblast activation protein (FAP)-targeting radioligands. FAP-directed PET/CT showed promising diagnostic performance compared to FDG PET/CT in a subcohort of seven patients with GEP-NEC in an analysis of our own study group [18]. These results encourage the development of a theranostic FAP-targeted twin [29, 30] in order to provide additional therapeutic strategies.
This study suffers from several limitations. Apart from the retrospective study design and the single-center approach, we only included a small sample size due to the rare incidence of NECs. We assessed rPFS based upon the routine follow-up imaging including conventional CT scan and/or FDG PET/CT. Imaging follow-up was thus not standardized performed but routinely undertaken after 3 months. We also assessed PET-based parameters of CXCR4 PET compared to the reference standard FDG, but corresponding FDG PET was not available in one patient. However, this is to our knowledge the only analysis, which includes a substantial sample size of GEP-NEC with dual tracer imaging.
Conclusion
In-vivo assessment of CXCR4 PET-derived volumetric parameters is prognostic for patient outcome with GEP-NECs and might reflect tumor heterogeneity and aggressiveness. These parameters could be used for risk stratification in order to detect patients prone to early progression and support treatment decisions.
References
Klimstra DS, Modlin IR, Coppola D et al (2010) The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 39:707–712
Pavel M, Oberg K, Falconi M et al (2020) Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:844–860
Delle Fave G, O’Toole D, Sundin A et al (2016) ENETS consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology 103:119–124
Mollazadegan K, Welin S, Crona J (2021) Systemic treatment of gastroenteropancreatic neuroendocrine carcinoma. Curr Treat Options Oncol 22:68
Parker C, Castro E, Fizazi K et al (2020) Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:1119–1134
Chatterjee S, Behnam Azad B, Nimmagadda S (2014) The intricate role of CXCR4 in cancer. Adv Cancer Res 124:31–82
Buck AK, Haug A, Dreher N et al (2022) Imaging of C-X-C motif chemokine receptor 4 expression in 690 patients with solid or hematologic neoplasms using (68)Ga-pentixafor PET. J Nucl Med 63:1687–1692
Habringer S, Lapa C, Herhaus P et al (2018) Dual targeting of acute leukemia and supporting niche by CXCR4-directed theranostics. Theranostics 8:369–383
Lapa C, Hanscheid H, Kircher M et al (2019) Feasibility of CXCR4-directed radioligand therapy in advanced diffuse large B-cell lymphoma. J Nucl Med 60:60–64
Buck AK, Grigoleit GU, Kraus S et al (2023) C-X-C motif chemokine receptor 4-targeted radioligand therapy in patients with advanced T-cell lymphoma. J Nucl Med 64:34–39
Kaemmerer D, Trager T, Hoffmeister M et al (2015) Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget 6:27566–27579
Buck AK, Serfling SE, Lindner T et al (2022) CXCR4-targeted theranostics in oncology. Eur J Nucl Med Mol Imaging 49:4133–4144
Werner RA, Weich A, Higuchi T et al (2017) Imaging of chemokine receptor 4 expression in neuroendocrine tumors - a triple tracer comparative approach. Theranostics 7:1489–1498
Weich A, Werner RA, Buck AK, Hartrampf PE, Serfling SE, Scheurlen M et al (2021) CXCR4-directed PET/CT in patients with newly diagnosed neuroendocrine carcinomas. Diagnostics 11(4):605
Werner RA, Kircher S, Higuchi T et al (2019) CXCR4-directed imaging in solid tumors. Front Oncol 9:770
Serfling SE, Lapa C, Dreher N et al (2022) Impact of tumor burden on normal organ distribution in patients imaged with CXCR4-targeted [(68)Ga]Ga-pentixafor PET/CT. Mol Imaging Biol 24:659–665
Lewis R, Habringer S, Kircher M et al (2021) Investigation of spleen CXCR4 expression by [(68)Ga]Pentixafor PET in a cohort of 145 solid cancer patients. EJNMMI Res 11:77
Kosmala A, Serfling SE, Schlotelburg W et al (2023) Impact of 68 Ga-FAPI-04 PET/CT on staging and therapeutic management in patients with digestive system tumors. Clin Nucl Med 48:35–42
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Kanoun S, Tal I, Vrigneaud J et al (2016) Beth Israel Plugin for FIJI: a free and open source software tool for PET/CT processing in research. Eur J Nucl Med Mol I(43):S389–S390
Seifert R, Herrmann K, Kleesiek J et al (2020) Semiautomatically quantified tumor volume using (68)Ga-PSMA-11 PET as a biomarker for survival in patients with advanced prostate cancer. J Nucl Med 61:1786–1792
Kind F, Eder AC, Jilg CA et al (2023) Prognostic value of tumor volume assessment on PSMA PET after (177)Lu-PSMA radioligand therapy evaluated by PSMA PET/CT consensus statement and RECIP 1.0. J Nucl Med 64(4):605–610
Boellaard R, Delgado-Bolton R, Oyen WJ et al (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 42:328–354
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247
Langen Stokmo H, Aly M, Bowitz Lothe IM et al (2022) Volumetric parameters from [(18) F]FDG PET/CT predicts survival in patients with high-grade gastroenteropancreatic neuroendocrine neoplasms. J Neuroendocrinol 34:e13170
Jiang Y, Hou G, Li F et al (2021) Prognostic value of FDG PET/CT in patients with neuroendocrine carcinoma of the uterine cervix. Clin Nucl Med 46:971–976
Hou G, Zhao N, Li F et al (2023) Prognostic value of pretreatment (18)F-FDG PET/CT metabolic parameters in esophageal high-grade neuroendocrine carcinoma: a bicenter retrospective study. Front Oncol 13:1145557
Weich A, Serfling SE, Schlotelburg W et al (2023) Impact of CXCR4-directed PET/CT on staging and proposed oncologic management in patients with digestive system tumors. Clin Nucl Med 48:586–593
Calais J (2020) FAP: the next billion dollar nuclear theranostics target? J Nucl Med 61:163–165
Liu Y, Watabe T, Kaneda-Nakashima K et al (2022) Fibroblast activation protein targeted therapy using [(177)Lu]FAPI-46 compared with [(225)Ac]FAPI-46 in a pancreatic cancer model. Eur J Nucl Med Mol Imaging 49:871–880
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by Bavarian Cancer Research Center (personal grant to Kerstin Michalski) and by the Interdisciplinary Center of Clinical Research (IZKF), University Hospital of Wuerzburg (grant Z-2/91 to Wiebke Schlötelburg).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kerstin Michalski has received speaker honoraria from Novartis. Rudolf A. Werner has received speaker honoraria from PentixaPharm, and is involved in [68 Ga]Ga-Pentixafor PET Imaging in PAN Cancer (FORPAN), sponsored and planned by PentixaPharm. Andreas K. Buck has received speaker honoraria from Novartis/AAA and PentixaPharm and is a member of the advisory board of PentixaPharm. All other authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aleksander Kosmala and Alexander Weich contributed equally.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Michalski, K., Schlötelburg, W., Hartrampf, P. et al. Volumetric Parameters Derived from CXCR4-Directed PET/CT Predict Outcome in Patients with Gastrointestinal Neuroendocrine Carcinomas. Mol Imaging Biol 26, 344–350 (2024). https://doi.org/10.1007/s11307-024-01899-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-024-01899-w