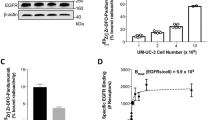
Abstract
Purpose
New generation of receptor tyrosine kinase inhibitors (RTKIs) have shown to improve survival in many solid tumors. However, an imaging biomarker is needed for patient selection and prediction of treatment response. This study evaluates the use of quantitative changes of HER3 on 68 Ga-NOTA-HER3P1 PET/MRI for prediction of early response to pan-RTKIs in gastric cancer (GCa).
Procedures
GCa cell lines were evaluated for expression of RTKs, and downstream signaling pathways (AKT and MAPK). Cell viability was assessed following 24–72 h of treatment with 0.01–1 µmol/L of afatinib, a pan-RTKI. HER3-expressing afatinib-sensitive (NCI-N87) and resistant cells (SNU16) were selected for evaluation of changes in RTKs expression and downstream pathways, with 24–72 h of 0.1 µmol/L afatinib treatment. 68 Ga-NOTA-HER3P1 PET/MRI was performed in subcutaneous NCI-N87 and SNU16 xenografts (nu:nu, n = 12/group) at baseline and 4 days after afatinib treatment (10 mg/kg, PO, daily). Temporal changes in PET measures were correlated to HER3 expression in tumors, tumor growth rate, and treatment response.
Results
With afatinib therapy, NCI-N87 cells showed increased total HER3 expression, and reduction of other RTKs and downstream nodes within 72 h, while SNU16 cells showed no significant change in total HER3 and downstream nodes. 68 Ga-HER3P1 PET/MRI showed increased uptake in NCI-N87 and no significant change in SNU16 tumors (day 4 vs. baseline SUVmean: 3.8 ± 0.7 vs. 1.6 ± 0.6, p < 0.05 in NCI-N87, and 1.5 ± 0.7 vs. 1.7 ± 0.7, p > 0.05 in SNU16). These findings were in concordance with HER3 expression in histopathological analyses and tumor growth over 3 weeks of treatment (mean tumor volume in treated vs. control: 11 ± 17 mm3 vs. 293 ± 79 mm3, p < 0.001 in NCI-N87, and 238 ± 91 mm3 vs. 282 ± 35 mm3, p > 0.05 in SNU16).
Conclusions
Quantitative changes in HER3 PET could be used to predict response to pan-RTKI within few days after initiation of treatment and can help with personalizing GCa management.






Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M (1993) Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 72:37–41
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE (2010) Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev (3):CD004064. https://doi.org/10.1002/14651858.CD004064.pub3
Matsuoka T, Yashiro M (2015) Recent advances in the HER2 targeted therapy of gastric cancer. World J Clin Cases 3:42–51
Zhang Z, Wang J, Ji D et al (2014) Functional genetic approach identifies MET, HER3, IGF1R, INSR pathways as determinants of lapatinib unresponsiveness in HER2-positive gastric cancer. Clin Cancer Res 20:4559–4573
Abrahao-Machado LF, Scapulatempo-Neto C (2016) HER2 testing in gastric cancer: an update. World J Gastroenterol 22:4619–4625
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Hecht JR, Bang YJ, Qin SK et al (2016) Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J Clin Oncol 34:443–451
Yonesaka K, Kudo K, Nishida S et al (2015) The pan-HER family tyrosine kinase inhibitor afatinib overcomes HER3 ligand heregulin-mediated resistance to EGFR inhibitors in non-small cell lung cancer. Oncotarget 6:33602–33611
O’Neill F, Madden SF, Clynes M et al (2013) A gene expression profile indicative of early stage HER2 targeted therapy response. Mol Cancer 12:69
Yang Z, Hackshaw A, Feng Q et al (2017) Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer 140:2805–2819
Janjigian YY, Viola-Villegas N, Holland JP et al (2013) Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET. J Nucl Med 54:936–943
Keller S, Zwingenberger G, Ebert K et al (2018) Effects of trastuzumab and afatinib on kinase activity in gastric cancer cell lines. Mol Oncol 12:441–462
Collins DM, Conlon NT, Kannan S, Verma CS, Eli LD, Lalani AS, Crown J (2019) Preclinical characteristics of the irreversible pan-HER kinase inhibitor neratinib compared with lapatinib: implications for the treatment of HER2-positive and HER2-mutated breast cancer. Cancers (Basel) 11(6):737. https://doi.org/10.3390/cancers11060737
Yang JC, Sequist LV, Geater SL et al (2015) Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 16:830–838
Nakata S, Fujita M, Nakanishi H (2019) Efficacy of afatinib and lapatinib against HER2 gene-amplified trastuzumab-sensitive and -resistant human gastric cancer cells. Anticancer Res 39:5927–5932
Martin N, Isambert N, Gomez-Roca C et al (2018) Phase I trial of afatinib and 3-weekly trastuzumab with optimal anti-diarrheal management in patients with HER2-positive metastatic cancer. Cancer Chemother Pharmacol 82:979–986
Wehrenberg-Klee E, Sinevici N, Nesti S et al (2021) HER3 PET imaging identifies dynamic changes in HER3 in response to HER2 inhibition with lapatinib. Mol Imaging Biol 23:930–940
Cao GD, Chen K, Xiong MM, Chen B (2016) HER3, but not HER4, plays an essential role in the clinicopathology and prognosis of gastric cancer: a meta-analysis. PLoS ONE 11:e0161219
Wehrenberg-Klee E, Turker NS, Heidari P et al (2016) Differential receptor tyrosine kinase PET imaging for therapeutic guidance. J Nucl Med 57:1413–1419
Leto SM, Sassi F, Catalano I et al (2015) Sustained inhibition of HER3 and EGFR is necessary to induce regression of HER2-amplified gastrointestinal carcinomas. Clin Cancer Res 21:5519–5531
Sergina NV, Rausch M, Wang D et al (2007) Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445:437–441
Sinevici N, Ataeinia B, Zehnder V et al (2020) HER3 differentiates basal from claudin type triple negative breast cancer and contributes to drug and microenvironmental induced resistance. Front Oncol 10:554704
Larimer BM, Phelan N, Wehrenberg-Klee E, Mahmood U (2017) Phage display selection, in vitro characterization, and correlative PET imaging of a novel HER3 peptide. Mol Imaging Biol 20(2):300–308. https://doi.org/10.1007/s11307-017-1106-6
Leece AK, Heidari P, Yokell DL, Mahmood U (2013) A container closure system that allows for greater recovery of radiolabeled peptide compared to the standard borosilicate glass system. Appl Radiat Isot 80:99–102
Esfahani SA, Callahan C, Rotile NJ, Heidari P, Mahmood U, Caravan PD, Grant AK, Yen YF (2022) Hyperpolarized [1-13C]pyruvate magnetic resonance spectroscopic imaging for evaluation of early response to tyrosine kinase inhibition therapy in gastric cancer. Mol Imaging Biol. https://doi.org/10.1007/s11307-022-01727-z
Heidari P, Esfahani SA, Turker NS et al (2015) Imaging of secreted extracellular periostin, an important marker of invasion in the tumor microenvironment in esophageal cancer. J Nucl Med 56:1246–1251
Heidari P, Deng F, Esfahani SA et al (2015) Pharmacodynamic imaging guides dosing of a selective estrogen receptor degrader. Clin Cancer Res 21:1340–1347
Amin DN, Sergina N, Ahuja D et al (2010) Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med 2:16ra17
Montero-Conde C, Ruiz-Llorente S, Dominguez JM et al (2013) Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov 3:520–533
der Houven M-V, van Oordt CW, McGeoch A, Bergstrom M et al (2019) Immuno-PET imaging to assess target engagement: experience from (89)Zr-anti-HER3 mAb (GSK2849330) in patients with solid tumors. J Nucl Med 60:902–909
Lockhart AC, Liu Y, Dehdashti F et al (2016) Phase 1 evaluation of [(64)Cu]DOTA-patritumab to assess dosimetry, apparent receptor occupancy, and safety in subjects with advanced solid tumors. Mol Imaging Biol 18:446–453
Bensch F, Lamberts LE, Smeenk MM et al (2017) (89)Zr-lumretuzumab PET imaging before and during HER3 antibody lumretuzumab treatment in patients with solid tumors. Clin Cancer Res 23:6128–6137
Pool M, Kol A, de Jong S, de Vries EGE, Lub-de Hooge MN, Terwisscha van Scheltinga AGT (2017) (89)Zr-mAb3481 PET for HER3 tumor status assessment during lapatinib treatment. MAbs 9:1370–1378
Park JG, Frucht H, LaRocca RV et al (1990) Characteristics of cell lines established from human gastric carcinoma. Cancer Res 50:2773–2780
Ebert K, Mattes J, Kunzke T, Zwingenberger G, Luber B (2019) MET as resistance factor for afatinib therapy and motility driver in gastric cancer cells. PLoS ONE 14:e0223225
Huang L, Cai M, Zhang X et al (2017) Combinational therapy of crizotinib and afatinib for malignant pleural mesothelioma. Am J Cancer Res 7:203–217
Torigoe H, Shien K, Takeda T et al (2018) Therapeutic strategies for afatinib-resistant lung cancer harboring HER2 alterations. Cancer Sci 109:1493–1502
Acknowledgements
Authors are grateful to Chris Farrar, PhD for his help with developing PET/MR imaging acquisition techniques.
Funding
This study was supported by RSNA R&E Foundation Grant and Ralph Schlaeger Research Fellowship Grant from Massachusetts General Hospital (PI: S.A.E), R01CA211223 (PI: U.M.), S10-OD023503 (PI: P.C.), and R21GM137227 (PI: Y.Y.) from NIH. S.K. is supported by the NIH CaNCURE Nanomedicine Research program 5R25CA174650. P.H. is supported by the NCI K08CA249047. S.A.E. is supported by the NCI K08CA259626-A1.
Author information
Authors and Affiliations
Contributions
All authors contributed to the concept and design of the study. S.A.E., C.A.F., Y.Y., N.J.R., P.H., B.A., S.K., and O.C. contributed to the acquisition of the data. S.A.E., P.H., and C.A.F. drafted the manuscript. All authors contributed to the analysis and interpretation of the data, and they read, critically revised, and approved the final version of the manuscript and all of them agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
U.M. is a cofounder and consultant of CytoSite Biopharma, a company focused on development of PET probes for immuno-oncology and has received support (materials) from Daiichi Sankyo related to HER3, but not used in the current study. All other authors do not have relevant conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11307_2022_1763_MOESM1_ESM.jpg
Supplementary file1 (JPG 327 KB) Supplemental Fig.1. Western blot analyses of NCI-N87 and SNU16 cells before and after 72 hours of treatment with afatinib (0.1 µmol/L) in the presence of recombinant human neuregulin 1 (NRG, 50 ng/mL) in the cell media. Total HER3 level significantly increases in afatinib-sensitive NCI-N87 cells and slightly decreases in afatinib-resistant SNU16 cells at 72 hours after treatment although not statistically significant. There is a significant decrease and inhibition of phosphorylated forms of other RTKs and downstream AKT and MAPK in the NCI-N87 cells. In SNU16 cells, there is inhibition of phosphorylated forms of RTKs but sustained levels of activated AKT and MAPK. Results are shown with three to four replicates. ns: not statistically significant, *: p < 0.05
11307_2022_1763_MOESM2_ESM.jpg
Supplementary file2 (JPG 385 KB) Supplemental Fig.2. Evaluation of the changes in MET and pMET in NCI-N87 and SNU16 cells before and after treatment with afatinib (0.1 µmol/L) in the presence and absence of human recombinant neuregulin 1 (NRG, 50 ng/mL). Western blot analyses show expression of MET and pMET in the untreated cells regardless of the presence or absence of the NRG. In NCI-N87 cells, total MET significantly decreases while pMET level does not significantly change in response to afatinib. In SNU16 cells, total MET does not significantly change, while the pMET level significantly increases after treatment with afatinib. Addition of NRG to the cell media does not show a significant difference in the changes of MET and pMET in either cells line. Results are shown with three to four replicates. ns: not statistically significant, *: p < 0.05, **: p < 0.001
11307_2022_1763_MOESM3_ESM.jpg
Supplementary file3 (JPG 3343 KB) Supplemental Fig.3. Representative hematoxylin and eosin (H&E), and Ki-67 (as marker of cell proliferation) and caspase-3 (as marker of apoptosis) immunohistochemical staining of extracted tumor tissues at baseline, 4 and 21 days after treatment with afatinib 10 mg/kg/day. a) Afatinib treatment of NCI-N87 tumors results in temporal increase in tumor cell density on H&E, decrease in Ki67 cell proliferation marker, and increase in caspase-3 apoptosis marker. b) Afatinib-resistant SNU16 tumors show continuous increase in tumor cell density on H&E, increase in proliferation marker Ki67 and no significant increase in tumor apoptosis on caspase-3 staining. (X10 magnification)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Esfahani, S.A., de Aguiar Ferreira, C., Rotile, N.J. et al. HER3 PET Imaging Predicts Response to Pan Receptor Tyrosine Kinase Inhibition Therapy in Gastric Cancer. Mol Imaging Biol 25, 353–362 (2023). https://doi.org/10.1007/s11307-022-01763-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-022-01763-9