Abstract
Purpose
The purpose of this study was to evaluate the safety, dosimetry, and apparent receptor occupancy (RO) of [64Cu]DOTA-patritumab, a radiolabeled monoclonal antibody directed against HER3/ERBB3 in subjects with advanced solid tumors.
Procedures
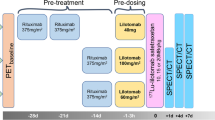
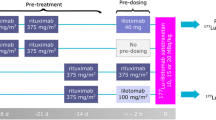
Dosimetry subjects (n = 5) received [64Cu]DOTA-patritumab and underwent positron emission tomography (PET)/X-ray computed tomography (CT) at 3, 24, and 48 h. Evaluable RO subjects (n = 3 out of 6) received [64Cu]DOTA-patritumab at day 1 and day 8 (after 9.0 mg/kg patritumab) followed by PET/CT at 24 h post-injection. Endpoints included safety, tumor uptake, and efficacy.
Results
The tumor SUVmax (± SD) was 5.6 ± 4.5, 3.3 ± 1.7, and 3.0 ± 1.1 at 3, 24, and 48 h in dosimetry subjects. The effective dose and critical organ dose (liver) averaged 0.044 ± 0.008 mSv/MBq and 0.46 ± 0.086 mGy/MBq, respectively. In RO subjects, tumor-to-blood ratio decreased from 1.00 ± 0.32 at baseline to 0.57 ± 0.17 after stable patritumab, corresponding to a RO of 42.1 ± 3.
Conclusions
[64Cu]DOTA-patritumab was safe. These limited results suggest that this PET-based method can be used to determine tumor-apparent RO.

Similar content being viewed by others
References
Gullick WJ (1996) The c-erbB3/HER3 receptor in human cancer. Cancer Surv 27:339–349
Gala K, Chandarlapaty S (2014) Molecular pathways: HER3 targeted therapy. Clin Cancer Res 20:1410–1416
Treder M, Hartmann S, Ogbagabriel S et al (2008) Fully human anti- HER3 monoclonal antibodies (mAbs) inhibit oncogenic signaling and tumor cell growth in vitro and in vivo [abstract LB-20]. Presented at American Association for Cancer Research Annual Meeting; April 12–16, 2008; San Diego, CA
Freeman D, Ogbagabriel S, Rothe M et al (2008) Fully human anti-HER3 monoclonal antibodies (mAbs) have unique in vitro and in vivo functional and antitumor activities versus other HER family inhibitors [abstract LB-21]. Presented at American Association for Cancer Research Annual Meeting; April 12,16, 2008; San Diego, CA
LoRusso P, Janne PA, Oliveira M et al (2013) Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 19:3078–3087
Sharp TL, Glaus C, Fettig H et al (2011) Pharmacological evaluation of 64CU–DOTA–AMG 888 (U3-1287) in control and tumor bearing mice using biodistribution and microPET imaging [Abstract T206]. World Molecular Imaging Congress, San Diego, CA
McCarthy DW, Shefer RE, Klinkowstein RE et al (1997) Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol 24:35–43
Lewis MR, Kao JY, Anderson AL et al (2001) An improved method for conjugating monoclonal antibodies with N-hydroxysulfosuccinimidyl DOTA. Bioconjug Chem 12:320–324
Wessels BW, Bolch WE, Bouchet LG et al (2004) Bone marrow dosimetry using blood-based models for radiolabeled antibody therapy: a multiinstitutional comparison. J Nucl Med 45(10):1725–33
Stabin MG, Sparks RB, Crowe E (2005) OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 46:1023–1027
Ishibashi H, Suzuki T, Suzuki S et al (2003) Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab 88:2309–2317
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. US Department of Health and Human Services. May 28, 2009. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf Accessed March 7, 2014
Dijkers EC, Oude Munnink TH, Kosterink JG et al (2010) Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586–592
Tamura K, Kurihara H, Yonemori K et al (2013) 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med 54:1869–1875
Wakui H, Yamamoto N, Nakamichi S et al (2014) Phase 1 and dose-finding study of patritumab (U3-1287), a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 73:511–516
Lockhart AC, Liu Y, Dehdashti F et al (2014) A phase 1 evaluation of 64Cu-DOTA-patritumab to assess dosimetry, receptor occupancy, and safety in advanced solid tumors. Cancer Res 74:5443
Rizvi SN, Visser OJ, Vosjan MJ et al (2012) Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur J Nucl Med Mol Imaging 39:512–20
Wiseman GA, White CA, Stabin M et al (2000) Phase I/II 90Y-Zevalin (yttrium-90 ibritumomab tiuxetan, IDEC-Y2B8) radioimmunotherapy dosimetry results in relapsed or refractory non-Hodgkin’s lymphoma. Eur J Nucl Med 27:766–77
Goldenberg EM (1993) Monoclonal antibodies in cancer detection and therapy. Am J Med 94:297–312
Rajendran JG, Gopal AK, Fisher DR et al (2008) Myeloablative 131I-tositumomab radioimmunotherapy in treating non-Hodgkin’s lymphoma: comparison of dosimetry based on whole-body retention and dose to critical organ receiving the highest dose. J Nucl Med 49:837–44
Buck E, Eyzaquirre A, Haley JD et al (2006) Inactivation of Akt by the epidermal growth factor receptor inhibitor erlotinib is mediated by HER-3 in pancreatic and colorectal tumor cell lines and contributes to erlotinib sensitivity. Mol Cancer Ther 5:2051–9
Acknowledgments
The authors wish to thank the subjects and their families for participating in this study, Daiichi-Sankyo for supporting this research, and Akita Biomedical for assistance in writing the manuscript. We thank Deborah Sultan, Hannah Luehmann, Tom Voller, and Stephen Moerlein for the radiopharmaceutical preparation. We also wish to recognize the important contribution of the late Dr. Michael Welch. He was the driving force behind this study and is greatly missed as a colleague and mentor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study (ClinicalTrials.gov NCT01479023) was performed under an investigator-sponsored IND (114334). The study was approved by the Washington University Institutional Review Board and Radioactive Drug Research Committee prior to patient enrollment. Written informed consent was obtained for each participant.
[64Cu]DOTA-patritumab was prepared in compliance with the Good Manufacturing Practices by previously published methods in the Biologic Therapy Core Facility of the Siteman Cancer Center at Washington University.
Conflict of Interests
Drs. Lockhart, Liu, Dehdashti, Laforest, Welch, and Siegel received research support from Daiichi-Sankyo to conduct the preclinical and clinical components of this study. Drs. Desai, Mahmood, and Mendell were Daiichi-Sankyo employees at the time that this study was conducted.
Additional information
Michael J. Welch passed away during the preparation of this study.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 537 kb)
Rights and permissions
About this article
Cite this article
Lockhart, A.C., Liu, Y., Dehdashti, F. et al. Phase 1 Evaluation of [64Cu]DOTA-Patritumab to Assess Dosimetry, Apparent Receptor Occupancy, and Safety in Subjects with Advanced Solid Tumors. Mol Imaging Biol 18, 446–453 (2016). https://doi.org/10.1007/s11307-015-0912-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-015-0912-y