Abstract
Purpose
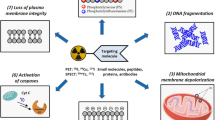
Externalization of phosphatidylethanolamine (PE) in dying cells makes the phospholipid an attractive target for apoptosis imaging. However, no ideal PE-targeted positron emission tomography (PET) radiotracer was developed. The goal of the study was to develop a novel PE-targeted radiopharmaceutical to imaging apoptosis.
Procedure
In this study, we have radiolabeled PE-binding polypeptide duramycin with fluorine-18 for PET imaging of apoptosis. Al[18F]F-NOTA-PEG3-duramycin was synthesized via chelation reaction of NOTA-PEG3-duramycin with Al[18F]F. PE-binding capacity of Al[18F]F-NOTA-PEG3-duramycin was determined in a competitive radiometric PE-binding assay. The pharmacokinetic profile was evaluated in Kunming mice. The apoptosis imaging capacity of Al[18F]F-NOTA-PEG3-duramycin was evaluated using in vitro cell uptake assay with camptothecin-treated Jurkat cells, along with in vivo PET imaging using erlotinib-treated nude mice.
Results
The total synthesis procedure lasted for 30 min, with a decay-uncorrected radiochemical yield of 21.3 ± 2.6 % (n = 10). Compared with the control cells, the binding of Al[18F]F-NOTA-PEG3-duramycin with camptothecin-induced apoptotic cells resulted in a tripling increase. A competitive radiometric PE-binding assay strongly confirmed the binding of Al[18F]F-NOTA-PEG3-duramycin to PE. The biodistribution study showed rapid blood clearance, prominent kidney retention, and low liver uptake. In the in vivo PET/CT imaging, Al[18F]F-NOTA-PEG3-duramycin demonstrated 2-fold increase in erlotinib-treated HCC827 tumors in nude mice.
Conclusion
Considering the facile preparation and improved biological properties, Al[18F]F-NOTA-PEG3-duramycin seems to be a promising PET tracer candidate for imaging apoptosis in the monitoring of cancer treatment.






Similar content being viewed by others
References
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629
Meiler J, Schuler M (2006) Therapeutic targeting of apoptotic pathways in cancer. Curr Drug Targets 7:1361–1369
Allen AM, Ben-Ami M, Reshef A et al (2012) Assessment of response of brain metastases to radiotherapy by PET imaging of apoptosis with 18F-ML-10. Eur J Nucl Med Mol Imaging 39:1400–1408
Blankenberg FG, Kalinyak J, Liu L et al (2006) 99mTc-HYNIC-Annexin V SPECT imaging of acute stroke and its response to neuroprotective therapy with anti Fas ligand antibody. Eur J Nucl Med Mol Imaging 33:566–574
Kartachova M, van Zandwijk N, Burgers S et al (2007) Prognostic significance of 99mTc-Hynic-rh-Annexin V scintigraphy during platinum based chemotherapy in advanced lung cancer. J Clin Oncol 25:2534–2539
Oborski MJ, Laymon CM, Lieberman FS et al (2014) First use of 18F-labeled ML-10 PET to assess apoptosis change in a newly diagnosed glioblastoma multiforme patient before and early after therapy. Brain Behav 4:312–315
Zeng W, Wang X, Xu P, Liu G, Eden HS, Chen X (2015) Molecular imaging of apoptosis: from micro to macro. Theranostics 5:559–582
Challapalli A, Kenny LM, Hallett WA et al (2013) 18F-ICMT-11, a caspase-3-specific PET tracer for apoptosis: biodistribution and radiation dosimetry. J Nucl Med 54:1551–1556
Dubash SR, Merchant S, Heinzmann K et al (2018) Clinical translation of [18F]ICMT-11 for measuring chemotherapy-induce caspase 3/7 activation in breast and lung cancer. Eur J Nucl Med Mol Imaging 45:2285–2299
Zhao M (2011) Lantibiotics as probes for phosphatidylethanolamine. Amino Acids 41:1071–1079
Johnson SE, Li Z, Liu Y et al (2013) Whole-body imaging of high-dose ionizing irradiation induced tissue injuries using 99m Tc-duramycin. J Nucl Med 54:1397–1403
Elvas F, Vangestel C, Pak K, Vermeulen P, Gray B, Stroobants S, Staelens S, Wyffels L (2016) Early prediction of tumor response to treatment: preclinical validation of 99mTc-duramycin. J Nucl Med 57:805–811
Liu Z, Larsen BT, Lerman LO, Gray BD, Barber C, Hedayat AF, Zhao M, Furenlid LR, Pak KY, Woolfenden JM (2016) Detection of atherosclerotic plaques in ApoE-deficient mice using 99mTc-duramycin. Nucl Med Biol 43:496–505
Audi SH, Jacobs ER, Zhang X et al (2017) Protection by inhaled hydrogen therapy in a rat model of acute lung injury can be tracked in vivo using molecular imaging. Shock 48(4):467–476
Medhora M, Haworth S, Liu Y et al (2016) Biomarkers for radiation pneumonitis using noninvasive molecular imaging. J Nucl Med 57:1296–1301
Li Y, Liu C, Xu X, Lu X, Luo J, Gray B, Pak KY, Cheng J, Zhang Y (2018) [99mTc]duramycin, a potential molecular probe for early prediction of tumor response after chemotherapy. Nucl Med Biol 66:18–25
Zhao M, Li Z (2012) A single-step kit formulation for the 99mTc-labeling of HYNIC-duramycin. Nucl Med Biol 39:1006–1011
Wang L, Wang F, Fang W et al (2015) The feasibility of imaging myocardial ischemic/reperfusion injury using 99mTc-labeled duramycin in a porcine model. Nucl Med Biol 42:198–204
Yao S, Hu K, Tang G et al (2015) Positron emission tomography imaging of cell death with [18F]FPDuramycin. Apoptosis 19:841–850
McBride WJ, Sharkey RM, Karacay H et al (2009) A novel method of 18F radiolabeling for PET. J Nucl Med 50:991–998
Luan X, Huang Y, Gao S, Sun X, Wang S, Ma L, Teng X, Lu H, Yu J, Yuan S (2016) 18F-alfatide PET/CT may predict short-term outcome of concurrent chemoradiotherapy in patients with advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging 43:2336–2342
Wu J, Wang S, Zhang X et al (2018) 18F-Alfatide II PET/CT for identification of breast cancer: a preliminary clinical study. J Nucl Med 59:1809–1816
Liu T, Liu C, Xu X et al (2019) Preclinical evaluation and pilot clinical of Al18F-PSMA-BCH for prostate cancer imaging. J Nucl Med. https://doi.org/10.2967/jnumed.118.221671
Lieberman BP, Ploessl K, Wang L et al (2011) PET imaging of glutaminolysis in tumors by 18F-(2S, 4R)4-fluoroglutamine. J Nucl Med 52:1947–1955
Perreault A, Richter S, Bergman C et al (2016) Targeting phosphatidylserine with a 64Cu-labeled peptide for molecular imaging of apoptosis. Mol Pharm 13:3564–3577
Zhao M, Li Z, Bugenhagen S (2008) 99mTc-labeled duramycin as a novel phosphatidylethanolamine-binding molecular probe. J Nucl Med 49:1345–1352
Yao S, Hu K, Tang G et al (2015) Molecular PET imaging of cyclophosphamide induced apoptosis with 18F-ML-8. Biomed Res Int 317403. https://doi.org/10.1155/2015/317403
Rix A, Drude NI, Mrugalia A et al (2019) Assessment of chemotherapy-induced organ damage with Ga-68 labeled duramycin. Mol Imaging Biol:1–11. https://doi.org/10.1007/s11307-019-01417-3
Bernard-Gauthier V, Lepage ML, Waengler B, Bailey JJ, Liang SH, Perrin DM, Vasdev N, Schirrmacher R (2018) Recent advances in 18F radiochemistry: a focus on B - 18F, Si - 18F, Al -18F, and C - 18F radiofluorination via spirocyclic iodonium ylides. J Nucl Med 59:568–572
Palmieri L, Elvas F, Vangestel C et al (2018) [99mTc]duramycin for cell death imaging: impact of kit formulation, purification and species difference. Nucl Med Biol 56:1–9
Elvas F, Vangestel C, Rapic S, Verhaeghe J, Gray B, Pak K, Stroobants S, Staelens S, Wyffels L (2015) Characterization of [99mTc]duramycin as a SPECT imaging agent for early assessment of tumor apoptosis. Mol Imaging Biol 17:838–847
Da Pieve C, Allot L, Martins CD et al (2016) Efficient 18F-AlF radiolabeling of ZHER3:8683 affibody molecule for imaging of HER3 positive tumors. Bioconjug Chem 27:1839–1849
González Trotter DE, Meng X, McQuade P, Rubins D, Klimas M, Zeng Z, Connolly BM, Miller PJ, O'Malley SS, Lin SA, Getty KL, Fayadat-Dilman L, Liang L, Wahlberg E, Widmark O, Ekblad C, Frejd FY, Hostetler ED, Evelhoch JL (2017) In vivo imaging of the programmed death ligand 1 by 18F PET. J Nucl Med 58(11):1852–1857
Piotrowska Z, Sequist LV (2016) Treatment of EGFR-mutant lung cancers after progression in patients receiving first-line EGFR tyrosine kinase inhibitors: a review. JAMA Oncol 2:948–954
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomized phase 3 trial. Lancet Oncol 13:239–246
Zhou C, Wu YL, Chen G et al (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12:735–742
Hu S, Kiesewetter DO, Zhu L, Guo N, Gao H, Liu G, Hida N, Lang L, Niu G, Chen X (2012) Longitudinal PET imaging of doxorubicin-induced cell death with 18F-Annexin V. Mol Imaging Biol 14:762–770
Wang MW, Wang F, Zheng YJ, Zhang YJ, Zhang YP, Zhao Q, Shen CK, Wang Y, Sun SH (2013) An in vivo molecular imaging probe (18)F-Annexin B1 for apoptosis detection by PET/CT: preparation and preliminary evaluation. Apoptosis 18:238–247
Belhocine T, Steinmetz N, Hustinx R et al (2002) Increase uptake of the apoptosis-imaging agent 99mTc recombinant human Annexin V in human tumors after one course of chemotherapy as a predictor of tumor response and patient prognosis. Clin Cancer Res 8(9):2766–2774
Mandl SJ, Man C, Edinger M et al (2004) Multi-modality imaging identifies key times for annexin V imaging as an early predictor of therapeutic outcome. Mol Imaging 3(1):1–8
Vance JE (2008) Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J Lipid Res 49:1377–1387
Funding
This work was funded by the National Natural Science Foundation (Nos. 81571704, 81671719, 81701723), the Science and Technology Foundation of Guangdong Province (Nos. 2016B090920087, 2018B030337001), and the Science and Technology Planning Project Foundation of Guangzhou (Nos. 201604020169, 201510010145).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The experiments were approved by the Institutional animal care and utilization committee (IACUU) of the first affiliated hospital, Sun Yat-sen University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 594 kb)
Rights and permissions
About this article
Cite this article
Yuan, G., Liu, S., Ma, H. et al. Targeting Phosphatidylethanolamine with Fluorine-18 Labeled Small Molecule Probe for Apoptosis Imaging. Mol Imaging Biol 22, 914–923 (2020). https://doi.org/10.1007/s11307-019-01460-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-019-01460-0