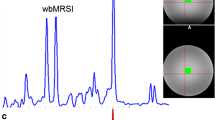
Abstract
Introduction
In recent years multivariate projection techniques of data analysis (PCA, PLS-DA) have been increasingly used for detection of complex 1H MRS derived metabolic signatures in pathologic conditions. However, these techniques have not been applied in the studies of metabolic heterogeneity of the normal human brain.
Objective
In this work we extended current knowledge about regional distribution of metabolites by multivariate analysis of metabolite levels obtained from various cortical and subcortical regions.
Methods
The studied group consisted of 71 volunteers with no neurological disorders. The metabolite levels obtained from short echo time 1H MRS in vivo spectra were subjected to univariate and multivariate analysis.
Results
The major variance direction in the dataset was dominated by glutamine + glutamate, creatine, myo-inositol and was successful in differentiation of the cortical grey matter and cerebellar vermis from the cortical white matter, pons, basal ganglia, hippocampus and thalamus. The projection plane formed by the second and third variance directions was dominated by N-acetylaspartate + N-acetylaspartylglutamate, choline and glutamine + glutamate variation not explained by the first direction. This plane revealed a huge metabolic contrast between the pons and basal ganglia, differentiation between the cortical grey matter regions and cerebellar vermis as well as biochemical heterogeneity between the regions such as: thalamus, basal ganglia and hippocampus.
Conclusion
Multivariate approach to 1H MRS data analysis provides an insight into the normal brain biochemistry and is helpful in understanding the regional heterogeneity of the normal brain. Such knowledge is crucial for a proper interpretation of altered metabolic pathways in diseases.




Similar content being viewed by others
References
Arnold, S. E., & Trojanowski, J. Q. (1996). Human fetal hippocampal development: I. cytoarchitecture, myeloarchitecture, and neuronal morphologic features. Journal of Comparative Neurology, 376, 274–292.
Baker, E. H., Basso, G., Barker, P. B., Smith, M. A., Bonekamp, D., & Horská, A. (2008). Regional apparent metabolite concentrations in young adult brain measured by (1)H MR spectroscopy at 3 T. Journal of Magnetic Resonance Imaging, 27, 489–499.
Bracken, B. K., Jensen, J. E., Prescot, A. P., Cohen, B. M., Renshaw, P. F., & Ongür, D. (2011). Brain metabolite concentrations across cortical regions in healthy adults. Brain Research, 1369, 89–94.
Brand, A., Richter-Landsberg, C., & Leibfritz, D. (1993). Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Developmental Neuroscience, 15, 289–298.
Choi, C. G., & Frahm, J. (1999). Localized proton MRS of the human hippocampus: metabolite concentrations and relaxation times. Magnetic Resonance in Medicine, 41(1), 204–207.
Emir, U. E., Auerbach, E. J., Van De Moortele, P. F., Marjañska, M., Uğurbil, K., Terpstra, M., et al. (2012). Regional neurochemical profiles in the human brain measured by ¹H MRS at 7 T using local B1 shimming. NMR in Biomedicine, 25, 152–160.
Fauvelle, F., Boccard, J., Cavarec, F., Depaulis, A., & Deransart, C. (2015) Assessing susceptibility to epilepsy in three rat strains using brain metabolic profiling based on HRMAS NMR spectroscopy and chemometrics. Journal of Proteome Research, 14, 2177–2189.
Ganji, S. K., An, Z., Banerjee, A., Madan, A., Hulsey, K. M., & Choi, C. (2014). Measurement of regional variation of GABA in the human brain by optimized point-resolved spectroscopy at 7 T in vivo. NMR in Biomedicine, 27, 1167–1175.
Gasparovic, C., Bedrick, E. J., Mayer, A. R., Yeo, R. A., Chen, H., Damaraju, E., et al. (2011). Test-retest reliability and reproducibility of short-echo-time spectroscopic imaging of human brain at 3 T. Magnetic Resonance in Medicine, 66, 324–332.
Gasparovic, C., Song, T., Devier, D., Bockholt, H. J., Caprihan, A., Mullins, P. G., et al. (2006). Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Medicine, 55, 1219–1226.
Irie, M., Fujimura, Y., Yamato, M., Miura, D., & Wariishi, H. (2014). Integrated MALDI-MS imaging and LC-MS techniques for visualizing spatiotemporal metabolomic dynamics in a rat stroke model. Metabolomics, 10, 473–483.
Ivanisevic, J., Epstein, A. A., Kurczy, M. E., Benton, P. H., Uritboonthai, W., Fox, H. S., et al. (2014). Brain region mapping using global metabolomics. Chemistry & Biology, 21, 1575–1584.
Ivanisevic, J., & Siuzdak, G. (2015). The Role of Metabolomics in Brain Metabolism Research. Journal of NeuroImmune Pharmacology, 10, 391–395.
Iyo, M., Namba, H., Fukushi, K., Shinotoh, H., Nagatsuka, S., Suhara, T., et al. (1997). Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer’s disease. Lancet, 349, 1805–1809.
Jaeger, C., Glaab, E., Michelucci, A., Binz, T. M., Koeglsberger, S., Garcia, P., et al. (2015). The mouse brain metabolome: Region-specific signatures and response to excitotoxic neuronal injury. The American Journal of Pathology, 185, 1699–1712.
Jolliffe, I.T. Principal Component Analysis. (2002). 2nd Edn., New York: Springer.
Kaldis, P., Hemmer, W., Zanolla, E., Holtzman, D., & Wallimann, T. (1996). ‘Hot spots’ of creatine kinase localization in brain: Cerebellum, hippocampus and choroid plexus. Developmental Neuroscience, 18, 542–554.
Keevil, S. F., Barbiroli, B., Brooks, J. C., Cady, E. B., Canese, R., Carlier, P., et al. (1998). Absolute metabolite quantification by in vivo NMR spectroscopy: II. A multicentre trial of protocols for in vivo localised proton studies of human brain. Magnetic Resonance Imaging, 16, 1093–1106.
Kreis, R., Boesch, C. (2003) Bad spectra can be better than good spectra. In: Proc 11th Annual Meeting ISMRM, Toronto.
McLean, M. A., Woermann, F. G., Barker, G. J., & Duncan, J. S. (2000). Quantitative analysis of short echo time (1)H-MRSI of cerebral gray and white matter. Magnetic Resonance in Medicine, 44, 401–411.
Minati, L., Aquino, D., Bruzzone, M. G., & Erbetta, A. (2010). Quantitation of normal metabolite concentrations in six brain regions by in vivo 1 H MR spectroscopy. Journal of Medical Physics, 35, 154–163.
Natt, O., Bezkorovaynyy, V., Michaelis, T., & Frahm, J. (2005). Use of phased array coils for a determination of absolute metabolite concentrations. Magnetic Resonance in Medicine, 53, 3–8.
Novak, J. E., Turner, R. S., Agranoff, B. W., & Fisher, S. K. (1999). Differentiated human NT2-N neurons possess a high intracellular content of myo-inositol. Journal of Neurochemistry, 72, 1431–1440.
Oberg, J., Spenger, C., Wang, F. H., Andersson, A., Westman, E., Skoglund, P., et al. (2008). Age related changes in brain metabolites observed by 1H MRS in APP/PS1 mice. Neurobiology of Aging, 29, 1423–1433.
Petroff, O.A.C., Spencer, D. D., Alger, J. R., & Prichard, J. W. (1989). High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology, 39, 1197–1202.
Pouwels, P. J., & Frahm, J. (1998). Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magnetic Resonance in Medicine, 39, 53–60.
Provencher, S. W. (2001). Automatic quantitation of localized in vivo 1 H spectra with LCModel. NMR in Biomedicine, 14, 260–264.
Qian, J., Qian, B., & Lei, H. (2013). Reversible loss of N-acetylaspartate after 15-min transient middle cerebral artery occlusion in rat: A longitudinal study with in vivo proton magnetic resonance spectroscopy. Neurochemical Research, 38, 208–217.
Rae, C. D. (2014). A guide to the metabolic pathways and function of metabolites observed in human brain 1 H magnetic resonance spectra. Neurochemical Research, 39, 1–36.
Raininko, R., & Mattsson, P. (2010). Metabolite concentrations in supraventricular white matter from teenage to early old age: A short echo time 1 H magnetic resonance spectroscopy (MRS) study. (2010). Acta Radiologica, 51, 309–315.
Righi, V., Roda, J. M., Paz, J., Mucci, A., Tugnoli, V., Rodriguez-Tarduchy, G., et al. (2009). 1 H HR-MAS and genomic analysis of human tumor biopsies discriminate between high and low grade astrocytomas. NMR in Biomedicine, 22(6), 629–637.
Sabati, M., Sheriff, S., Gu, M., Wei, J., Zhu, H., Barker, P. B., et al. (2015). Multivendor implementation and comparison of volumetric whole-brain echo-planar MR spectroscopic imaging. Magnetic Resonance in Medicine, 74, 1209–1220.
Savchenko, V. L., Nikonenko, I. R., Skibo, G. G., & McKanna, J. A. (1997). Distribution of microglia and astrocytes in different regions of the normal adult rat brain. Neurophysiology, 29, 431–441.
Skorupa, A., Jamroz, E., Paprocka, J., Sokół, M., Wicher, M., & Kiełtyka, A. (2013). Bridging the gap between metabolic profile determination and visualization in neurometabolic disorders: A multivariate analysis of proton magnetic resonance in vivo spectra. Journal of Chemometrics, 27, 76–90.
Smith, I.C.P., & Somorjai, R. L. (2011). Deriving biomedical diagnostics from NMR spectroscopic data. Biophysical Reviews, 3, 47–52.
Soares, D. P., & Law, M. (2009). Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clinical Radiology, 64, 12–21.
Tsang, T. M., Griffin, J. L., Haselden, J., Fish, C., & Holmes, E. (2005). Metabolic characterization of distinct neuroanatomical regions in rats by magic angle spinning 1 H nuclear magnetic resonance spectroscopy. Magnetic Resonance in Medicine, 53, 1018–1024.
Urenjak, J., Williams, S. R., Gadian, D. G., & Noble, M. (1993). Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. Journal of Neuroscience, 13, 981–989.
van der Veen, J. W., & Shen, J. (2013). Regional difference in GABA levels between medial prefrontal and occipital cortices. Journal of Magnetic Resonance Imaging, 38, 745–750.
Vingara, L. K., Yu, H. J., Wagshul, M. E., Serafin, D., Christodoulou, C., Pelczer, I., et al. (2013). Metabolomic approach to human brain spectroscopy identifies associations between clinical features and the frontal lobe metabolome in multiple sclerosis. NeuroImage, 82, 586–594.
Westman, E., Spenger, C., Oberg, J., Reyer, H., Pahnke, J., & Wahlund, L. O. (2009). In vivo 1 H-magnetic resonance spectroscopy can detect metabolic changes in APP/PS1 mice after donepezil treatment. BMC Neuroscience, 10, 33.
Wiedermann, D., Schuff, N., Matson, G. B., Soher, B. J., Du, A. T., Maudsley, A. A., & Weiner, M. W. (2001). Short echo time multislice proton magnetic resonance spectroscopic imaging in human brain: Metabolite distributions and reliability. Magnetic Resonance Imaging, 19, 1073–1080.
Wright, A. J., Fellows, G. A., Griffiths, J. R., Wilson, M., Bell, B. A., & Howe, F. A. (2010). Ex-vivo HRMAS of adult brain tumours: Metabolite quantification and assignment of tumour biomarkers. Molecular Cancer, 23, 9–66.
Zhang, Y., & Shen, J. (2015). Regional and tissue-specific differences in brain glutamate concentration measured by in vivo single voxel MRS. Journal of Neuroscience Methods, 239, 94–99.
Acknowledgements
The authors would like to thank Dr. Nia Goulden and Dr. Paul Mullins (Bangor University, United Kingdom) for making Partial Volume Correction Tool available for use in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Agnieszka Skorupa, Łukasz Boguszewicz, Marek Kijonka, Maria Sokół declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Skorupa, A., Boguszewicz, Ł., Kijonka, M. et al. Metabolic heterogeneity of the normal human brain: multivariate analysis of 1H MRS in vivo spectra acquired at 3T. Metabolomics 13, 34 (2017). https://doi.org/10.1007/s11306-017-1171-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1171-5