Abstract
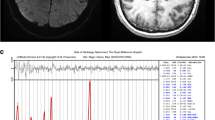
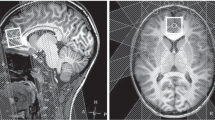
It is generally accepted that N-acetylaspartate (NAA) can be used a biochemical marker for assessing neuronal viability/integrity after cerebral ischemia. However, this view has recently been questioned based on observations showing that after a photothrombotic permanent ischemia the acute decline of NAA in the infracted regions, where massive neuronal loss persists, is reversible over time. In this study, we measured the longitudinal changes of NAA and total creatine (Cr) in ischemic rat brain after a 15-min transient middle cerebral artery occlusion (MCAO) by in vivo 1H magnetic resonance spectroscopy. The results showed that the levels of NAA and total Cr in the ischemic lesion decrease significantly at 1 day post-ichemia, followed by spontaneous recovery to the control levels by 2 weeks and remained stable thereafter up to 16 weeks. The normalization of NAA and total Cr levels was associated histologically with persisted neuronal loss up to 90 % in the ischemic core, and accompanied by marked reactive astrocytic responses occurring with a similar time course. The absolute T2 relaxation time in the ischemic lesion increased during acute phase, and declined afterwards during subacute and chronic phases of 15-min MCAO. The delayed decreases of T2 in the ischemic lesion might be associated with deposition of paramagnetic species, such as manganese and iron originated from chronic inflammation, vascular degradation and/or hemorrhagic transformation. The results of this study give further support to the hypothesis that the recovery of NAA after cerebral ischemia might have contributions from reactive glia cells, and caution the use of NAA as a specific neuronal marker during the chronic stage of cerebral ischemia.





Similar content being viewed by others
References
Baslow MH (2003) N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res 28:941–953
Demougeot C, Marie C, Giroud M, Beley A (2004) N-acetylaspartate: a literature review of animal research on brain ischaemia. J Neurochem 90:776–783
Malisza KL, Kozlowski P, Peeling J (1998) A review of in vivo 1H magnetic resonance spectroscopy of cerebral ischemia in rats. Biochem Cell Biol 76:487–496
Sager TN, Hansen AJ, Laursen H (2000) Correlation between N-acetylaspartate levels and histopathologic changes in cortical infarcts of mice after middle cerebral artery occlusion. J Cereb Blood Flow Metab 20:780–788
Sager TN, Topp S, Torup L, Hanson LG, Egestad B, Moller A (2001) Evaluation of CA1 damage using single-voxel 1H-MRS and un-biased stereology: can non-invasive measures of N-acetyl-asparate following global ischemia be used as a reliable measure of neuronal damage? Brain Res 892:166–175
Federico F, Simone IL, Lucivero V, Giannini P, Laddomada G, Mezzapesa DM, Tortorella C (1998) Prognostic value of proton magnetic resonance spectroscopy in ischemic stroke. Arch Neurol 55:489–494
Gideon P, Sperling B, Arlien-Soborg P, Olsen TS, Henriksen O (1994) Long-term follow-up of cerebral infarction patients with proton magnetic resonance spectroscopy. Stroke 25:967–973
Graham GD, Blamire AM, Howseman AM, Rothman DL, Fayad PB, Brass LM, Petroff OA, Shulman RG, Prichard JW (1992) Proton magnetic resonance spectroscopy of cerebral lactate and other metabolites in stroke patients. Stroke 23:333–340
Clark JB (1998) N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci 20:271–276
Demougeot C, Bertrand N, Prigent-Tessier A, Garnier P, Mossiat C, Giroud M, Marie C, Beley A (2003) Reversible loss of N-acetyl-aspartate in rats subjected to long-term focal cerebral ischemia. J Cereb Blood Flow Metab 23:482–489
Wang X, Qian J, He R, Wei L, Liu N, Zhang Z, Huang Y, Lei H (2006) Delayed changes in T1-weighted signal intensity in a rat model of 15-minute transient focal ischemia studied by magnetic resonance imaging/spectroscopy and synchrotron radiation X-ray fluorescence. Magn Reson Med 56:474–480
Vanhamme L, van den Boogaart A, Van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129:35–43
van der Toorn A, Dijkhuizen RM, Tulleken CA, Nicolay K (1995) T1 and T2 relaxation times of the major 1H-containing metabolites in rat brain after focal ischemia. NMR Biomed 8:245–252
Dreher W, Busch E, Leibfritz D (2001) Changes in apparent diffusion coefficients of metabolites in rat brain after middle cerebral artery occlusion measured by proton magnetic resonance spectroscopy. Magn Reson Med 45:383–389
Fujioka M, Taoka T, Matsuo Y, Mishima K, Ogoshi K, Kondo Y, Tsuda M, Fujiwara M, Asano T, Sakaki T, Miyasaki A, Park D, Siesjo BK (2003) Magnetic resonance imaging shows delayed ischemic striatal neurodegeneration. Ann Neurol 54:732–747
Fujioka M, Taoka T, Hiramatsu KI, Sakaguchi S, Sakaki T (1999) Novel brain ischemic change on MRI. Delayed ischemic hyperintensity on T1-weighted images and selective neuronal death in the caudoputamen of rats after brief focal ischemia. Stroke 30:1038–1042
Igarashi H, Kwee IL, Nakada T, Katayama Y, Terashi A (2001) 1H magnetic resonance spectroscopic imaging of permanent focal cerebral ischemia in rat: longitudinal metabolic changes in ischemic core and rim. Brain Res 907:208–221
Lei H, Berthet C, Hirt L, Gruetter R (2009) Evolution of the neurochemical profile after transient focal cerebral ischemia in the mouse brain. J Cereb Blood Flow Metab 29:811–819
van der Zijden JP, van Eijsden P, de Graaf RA, Dijkhuizen RM (2008) 1H/13C MR spectroscopic imaging of regionally specific metabolic alterations after experimental stroke. Brain 131:2209–2219
Konaka K, Ueda H, Li JY, Matsumoto M, Sakoda S, Yanagihara T (2003) N-Acetylaspartate to total creatine ratio in the hippocampal CA1 sector after transient cerebral ischemia in gerbils: influence of neuronal elements, reactive gliosis, and tissue atrophy. J Cereb Blood Flow Metab 23:700–708
Taylor DL, Davies SE, Obrenovitch TP, Doheny MH, Patsalos PN, Clark JB, Symon L (1995) Investigation into the role of N-acetylaspartate in cerebral osmoregulation. J Neurochem 65:275–281
Duarte JM, Lei H, Mlynarik V, Gruetter R (2012) The neurochemical profile quantified by in vivo 1H NMR spectroscopy. Neuroimage 61:342–362
Jiang W, Gu W, Brannstrom T, Rosqvist R, Wester P (2001) Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke 32:1201–1207
Lin RC, Matesic DF (1994) Immunohistochemical demonstration of neuron-specific enolase and microtubule-associated protein 2 in reactive astrocytes after injury in the adult forebrain. Neuroscience 60:11–16
Lin RC, Polsky K, Matesic DF (1993) Expression of gamma-aminobutyric acid immunoreactivity in reactive astrocytes after ischemia-induced injury in the adult forebrain. Brain Res 600:1–8
Passani L, Elkabes S, Coyle JT (1998) Evidence for the presence of N-acetylaspartylglutamate in cultured oligodendrocytes and LPS activated microglia. Brain Res 794:143–145
Urenjak J, Williams SR, Gadian DG, Noble M (1992) Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem 59:55–61
Urenjak J, Williams SR, Gadian DG, Noble M (1993) Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 13:981–989
Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, Hori M, Matsumoto M (2000) Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke 31:1735–1743
Mandai K, Matsumoto M, Kitagawa K, Matsushita K, Ohtsuki T, Mabuchi T, Colman DR, Kamada T, Yanagihara T (1997) Ischemic damage and subsequent proliferation of oligodendrocytes in focal cerebral ischemia. Neuroscience 77:849–861
Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A (2003) Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res 989:172–179
Acknowledgments
Supported by Natural Science Foundation of China grants 81171302 and 20921004, Chinese Academy of Science key project KJCX2-SW-H12-03 and a 973 grant from the Chinese Ministry of Science and Technology (2011CB707802).
Conflict of interest
No potential conflicts of interest are disclosed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Junchao Qian and Baojin Qian contributed equally to this work.
Rights and permissions
About this article
Cite this article
Qian, J., Qian, B. & Lei, H. Reversible Loss of N-Acetylaspartate After 15-Min Transient Middle Cerebral Artery Occlusion in Rat: A Longitudinal Study with In Vivo Proton Magnetic Resonance Spectroscopy. Neurochem Res 38, 208–217 (2013). https://doi.org/10.1007/s11064-012-0910-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0910-2