Abstract
Previously, we observed that sustained activation of P2Y1 leads to inhibition of Na+,K+,Cl− cotransport (NKCC) in C11 cells resembling intercalated cells from collecting ducts of the Madin-Darby canine kidney. This study examined the role of stress-activated protein kinases (SAPK) in NKCC inhibition triggered by purinergic receptors. Treatment of C11 cells with ATP led to sustained phosphorylation of SAPK such as JNK and p38. Activation of these kinases also occurred in anisomycin-treated cells. Surprisingly, we observed that compounds SP600125 and SB202190, known as potent inhibitors of JNK and p38 in cell-free systems, activated rather than inhibited phosphorylation of the kinases in C11 cells. Importantly, similarly to ATP, all the above-listed activators of JNK and p38 phosphorylation inhibited NKCC. Thus, our results suggest that activation of JNK and/or p38 contributes to NKCC suppression detected in intercalated-like cells from distal tubules after their exposure to P2Y1 agonists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Na+,K+,Cl− cotransporter (NKCC), providing electroneutral symport of monovalent ions in the stoichiometry of 1Na+:1K+:2Cl− and selectively inhibited by bumetanide and other high-ceiling diuretics, belongs to the superfamily of Cl−-coupled monovalent cation cotransporters. Two isoforms of this carrier have been cloned from vertebrate cDNA libraries. A ubiquitous NKCC1 isoform is expressed in all types of cells studied so far [1], including the basolateral membrane of epithelial cells derived from collecting ducts of the Madin-Darby canine kidney (MDCK) [2]. This isoform contributes to cell volume regulation, adjustment of [Cl−]i above the values predicted by Nernst equilibrium potential and transcellular movement of salt and \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} \) in the secretory epithelium [1, 3]. Unlike NKCC1, three alternatively spliced variants of the NKCC2 isoform were found exclusively on the apical membrane of renal epithelial cells from the macula densa and thick ascending limb of Henle’s loop. In the thick ascending limb, NKCC2 plays a key role in bulk salt reabsorption [3], whereas in the macula densa this carrier is involved in sensing extracellular Cl− concentration and regulating renal function via tubuloglomerular feedback [4].
Because of compensatory reactions occurring in proximal segments via tubuloglomerular feedback, collecting ducts are considered a major target for regulation of water/salt and acid-base homeostasis by hormones and neurotransmitters, including vasopressin, bradykinin, atrial natriuretic peptide, prostanoids, mineralocorticoids, and catecholamines [5]. Under certain circumstances, extracellular nucleotides, such as ATP, UTP, and ADP, can also regulate renal epithelium function by activating two receptor subtypes. P2Y receptors are coupled to heterotrimeric G proteins and expressed in all types of renal cells studied so far, whereas P2X receptors correspond to ligand-gated cation channels, and their expression in the kidney is mainly limited to mesangial and vascular smooth muscle cells [6–10].
The renal ion transport systems affected by P2Y receptors have been explored mainly in MDCK cells where extracellular ATP leads to transient Cl− secretion [11]. Two populations of cells have been isolated from commercially available stocks of MDCK cells: C7- and C11-MDCK cells [12]. C7 cells have high transepithelial electrical resistance (Rte), are peanut lectin negative, maintain pHi at 7.39, and have large K+ conductance, thus resembling principal cells from the collecting ducts. C11 cells, on the other hand, resemble intercalated cells; they have low Rte, are peanut lectin positive, maintain pHi at 7.16, and have large Cl− and H+ conductances [12]. We were the first to report that transient activation of basolateral NKCC in ATP-treated C7 cells is evoked by elevation of [Ca2+]i [13] and contributes to transepithelial Cl− secretion [14]. We also noted that in contrast to C7 and other cells studied so far, sustained application of ATP to C11 cells resulted in sharp inhibition of this carrier [15] that was absent under the addition of other modulators of transepithelial ion fluxes [16].
Several hypotheses might be proposed to explain the cell type-specific impact of extracellular nucleotides on NKCC activity. First, NKCC inhibition is mediated by the P2 receptor isoform whose expression is limited to intercalated cells. However, in a recent study, we established that NKCC inhibition in ATP-treated C11 cells is caused by activation of P2Y1 receptors [13] whose expression has been detected in most of the cell types studied so far, including C7 cells [17]. Second, in intercalated cells, sustained activation of P2Y1 receptors leads to inhibition of the cell type-specific NKCC isoform. This hypothesis contradicts data showing that prolonged exposure of C11 cell monolayers to ATP abolished the increment of bumetanide-sensitive 86Rb uptake across the basolateral membrane by transfection with human NKCC1 [18]. Third, delayed NKCC1 inhibition seen in ATP-treated C11 cells is mediated by the de novo expression of intercalated cell-specific inhibitor(s) of this carrier. However, the inhibitory action of ATP on NKCC activity in C11 monolayers was preserved in the presence of inhibitors of RNA and protein synthesis [18]. Fourth, NKCC1 inhibition is caused by the intercalated cell-specific signaling cascade that couples P2Y1 activation with NKCC1 inhibition.
In renal epithelial cells, P2Y receptors trigger diverse signaling, including transient elevation of [Ca2+]i, cAMP production, activation of phospholipase C (PLC), phospholipase A2 (PLA2), prostaglandin synthase, protein kinase C (PKC), and protein kinase A (PKA) [19, 20]. We did not observe any effect of extracellular Ca2+ and intracellular Ca2+ chelator on NKCC inhibition in C11 cells [15, 21]. The inhibitory action of ATP is also preserved in the presence of inhibitors of PLC, PLA2, and prostaglandin synthase (U73122, AACOCF3, and indomethacin, respectively) as well as under activation of adenylate cyclase with forskolin and isoproterenol and inhibition of PKA with H-89 [15, 21]. In C11 cells, NKCC inhibition was also revealed in the presence of the PKC activator 4β-phorbol-12-myristate-13-acetate (PMA). However, the same action of PMA was also documented in C7 cells [15]. Moreover, suppression of NKCC in ATP-treated cells was not affected in the presence of PKC inhibitors (staurosporine and calphostin C) and after downregulation of this enzyme by chronic exposure to PMA [15, 21]. Viewed collectively, these data strongly suggest that none of the above-listed intermediates of the intracellular signaling cascade is involved in the purinergic inhibition of NKCC [15, 21].
In contrast to the above-listed signals documented in both principal- and intercalated-like cells, activation of P2Y receptors resulted in phosphorylation of members of the superfamily of mitogen-activated protein kinases (MAPK), such as the extracellular signal-activated protein kinases Erk1 and Erk2 and the Jun N-terminal kinase JNK1. Compound PD98059, a potent Erk inhibitor, strongly suppressed Erk1 and Erk2 phosphorylation in ATP-treated C11 cells, but did not affect the inhibitory action of ATP on NKCC activity [15, 21]. Considering these results, we designed the present investigation to examine the involvement of JNK and p38, a member of the stress-activated protein kinases (SAPK), in P2Y-induced NKCC inhibition.
Methods
Cell culture
C11-MDCK cells were obtained from Dr. M. Gekle (University of Würzburg, Germany) and cultured in DMEM supplemented with 2.5 g/l sodium bicarbonate, 2 g/l hydroxyethylpiperazine ethanesulfonic acid (HEPES), 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (Gibco Laboratories, Burlington, ON, Canada). The cells were passaged upon reaching subconfluent density by treatment in Ca2+- and Mg2+-free Dulbecco’s phosphate-buffered saline (PBS) with 0.1% trypsin from Sigma (St. Louis, MO, USA), then scraped from the flasks with a rubber policeman. Dispersed cells were counted and inoculated at 1.25 × 103 cells/cm2. Before experimentation, the cells were subjected to 24-h serum deprivation in the presence of 0.1% bovine serum albumin (BSA).
NKCC measurement
Serum-deprived cells seeded in 24-well plates were washed twice with 2 ml of PBS and incubated for 30 min at 37°C in 1 ml of Cl−-depleted medium A containing 140 mM Na gluconate, 5 mM K gluconate, 1 mM MgSO4, 1 mM CaCl2, 5 mM D-glucose, and 20 mM HEPES-Tris (pH 7.4). After 30 min of Cl− depletion, ATP and other test compounds were added at concentrations indicated in the figure legends. In a major part of the experiments, preincubation with ATP was limited to 30 min. Then, Cl−-depleted medium was replaced with 0.25 ml of medium B containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM D-glucose, 20 mM HEPES-Tris (pH 7.4), ~1 μCi/ml 86Rb, and 50 μM ouabain ± 10 μM bumetanide. In 5 min, 86Rb uptake was terminated by the addition of 2 ml of ice-cold medium C containing 100 mM MgCl2 and 10 mM HEPES-Tris buffer (pH 7.4). The cells were then transferred on ice, washed 4 times with 2 ml of ice-cold medium C, and lysed with 1 ml of a 1% SDS/4 mM ethylenediaminetetraacetate (EDTA) mixture. The radioactivity of the cell lysate was measured with a liquid scintillation analyzer. The rate of 86Rb (K+) influx was calculated as V = A/amt where A was the radioactivity in the sample (cpm), a was the specific radioactivity of 86Rb (K+) (cpm/nmol) in the incubation medium, m was the protein content in the sample measured by modified Lowry’s method (mg), and t was the incubation time (min). NKCC activity was estimated as the rate of ouabain-resistant, bumetanide-sensitive 86Rb influx.
Western blotting
C11 cells grown in 6-well plates were incubated for 24 h in DMEM containing 0.1% BSA and stimulated with ATP in Cl−-depleted medium A. Then, the cells were washed twice with ice-cold PBS and lysed in 200 μl of lysis buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 2 mM ethyleneglycoltetraacetic acid (EGTA), 0.25% deoxycholate, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 200 μM Na-orthovanadate, and 1 mM NaF. The lysed cells were scraped and centrifuged at 13,000 rpm for 5 min, and an equal volume of clear lysates containing 20 μg of protein was treated for 5 min at 95°C and applied on 10% polyacrylamide gel, followed by electrophoresis and transfer to Immobilon-P membranes (Millipore Corp., Bedford, MA, USA). The membranes were washed with PBS containing 0.05% Tween 20 (PBS-Tween) and 0.5% skim milk, and incubated overnight at 4°C with antibodies. After incubation, the membranes were washed 3 times with PBS-Tween, incubated for 1 h with horseradish peroxidase-conjugated antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), washed with PBS-Tween, and the protein bands were visualized with an enhanced chemiluminescence detection kit (Santa Cruz Biotechnology, Santa Cruz, CA, USA) before exposure to X-ray film. Relative protein content was quantified by the NIH image program.
Chemicals
ATP, ouabain, bumetanide, forskolin, and PMA were obtained from Sigma (St. Louis, MO, USA); anisomycin, cell-permeable inhibitors of JNK and p38 kinases (compounds SP600125 and SB202190, respectively) and their negative controls [N1-methyl-1,9-pyrazoloanthrone (MPA) and compound SB202474, respectively] were purchased from Calbiochem (La Jolla, CA, USA), and 86RbCl from Dupont (Boston, MA, USA). Anti-phospho-SAPK/JNK (Thr183/Tyr185) and anti-phospho-p38 (Thr180/tyr182) antibodies were provided by Cell Signaling Technology Inc. (Hornby, ON, Canada). Salts, D-glucose, and buffers were obtained from Sigma (St. Louis, MO, USA) and Anachemia (Montreal, QC, Canada).
Statistics
The data were analyzed by Student’s t-test or the t-test for dependent samples, as appropriate. Significance was defined as p < 0.05.
Results
In a previous study, we observed that 1-h preincubation of C11 cells in Cl−-depleted medium abolished the transient activation of NKCC by ATP but sharply increased baseline NKCC activity, allowing more precise estimation of the inhibitory action of P2Y-induced signaling [13]. Considering these results, Cl−-depleted cells were used in the present experiments. Figure 1 shows that NKCC inhibition in Cl−-depleted cells occurred after a 10-min lag phase with maximal attenuation of the carrier’s activity in 30–40 min of ATP addition.
Incubation for 2 min with ATP was sufficient to induce full-scale phosphorylation of JNK1 (p46) and p38 kinases (Fig. 2). Phosphorylation of JNK2 in ATP-treated cells was observed after a 5-min lag phase, and its time course was negatively correlated with NKCC inhibition. In contrast to sustained JNK phosphorylation, p38 phosphorylation was partially normalized in 40 min of ATP addition. Activation of adenylate cyclase with forskolin did not affect SAPK phosphorylation. Very modest p38 and JNK phosphorylation was detected in 20 min of PMA addition. These results show that neither PKA nor PKC is involved in SAPK phosphorylation by ATP, which is consistent with the lack of involvement of these serine-threonine kinases in inhibition of NKCC in ATP-treated C11 cells [15, 16, 21].
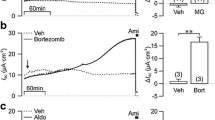
Consistent with numerous previous observations, anisomycin sharply elevated SAPK phosphorylation (Fig. 3). Figure 4a reveals that the action of anisomycin on JNK phosphorylation was lower than that of ATP; in contrast, anisomycin was a more potent activator of p38 phosphorylation than ATP. However, these differences were not statistically significant. We observed that 40-min exposure to anisomycin resulted in the inhibition of NKCC to about the same level as was detected in ATP-treated cells (Fig. 4b).
Representative blots revealing the effects of SP600125 (a), MPA (b), SB202190 (c), and SB202474 (d) on JNK and p38 phosphorylation in C11 cells under control conditions and in the presence of 100 μM ATP or 0.1 μM anisomycin. ATP and anisomycin were added during the last 40 min of preincubation of cells in Cl−-depleted medium. Compounds SP600125, MPA, SB202190, and SB202474 were added at the indicated concentrations 30 min before ATP or anisomycin
Effect of ATP and anisomycin on JNK and p38 phosphorylation (a) and NKCC (b) in C11 cells. 100 μM ATP and 0.1 μM anisomycin were added during the last 40 min of preincubation of cells in Cl−-depleted medium. Phosphoprotein content and NKCC activity in the absence of ATP and anisomycin were taken as 1.0 and 100%, respectively. Mean values from three (a) or four (b) independent experiments are shown
With cell-free systems, it was demonstrated that several newly synthesized cell-permeable compounds inhibited purified or recombinant JNK and p38 without significant action on other protein kinases. Thus, compound SP600125 inhibited recombinant JNK1-3 with an IC50 of 0.05–0.20 μM, whereas half-maximal inhibition of other members of the MAPK superfamily, such as Erk and p38 as well as PKA and cyclin-dependent kinases, was observed at concentrations higher than 5 μM [22, 23]. It should be underlined that SP600125 was a much less potent inhibitor of JNK in intact cells. Indeed, inhibition of JNK in the anisomycin-treated KB-3 carcinoma cell line was detected after 1-h preincubation with this compound at concentrations higher than 3 μM [23]. To decrease JNK phosphorylation by two- to threefold in PMA-treated Jurkat cells, SP600125 should be added at a concentration of 50 μM [22]. Surprisingly, we observed that at a concentration of 20 μM SP600125 and SB202190, i.e., potent inhibitors of p38 SAPK in cell-free systems, increased JNK and p38 phosphorylation to the level comparable with their phosphorylation in the presence of ATP and anisomycin (Fig. 3a and c). Moreover, like other potent activators of JNK and p38 phosphorylation, such as ATP and anisomycin, both SP600125 and SB202190 sharply decreased NKCC activity in C11 cells (Fig. 5). In contrast, neither JNK/p38 phosphorylation (Fig. 3b and d) nor NKCC activity in the presence and absence of ATP (data not shown) was affected by the inactive structural analogues of SP600125 and SB202190, compounds MPA and SB202474, respectively.
Effect of ATP, SP600125, and SB202190 on NKCC activity in C11 cells; 100 μM ATP was added during the last 40 min of preincubation of cells in Cl−-depleted medium. Compounds SP600125 and SB202190 were added at 20 μM concentration 30 min before ATP. NKCC activity in control cells was taken as 100%. Mean ± SE values from experiments performed in quadruplicate are shown
Increased phospho-JNK and phospho-p38 levels in SP600125- and SB202190-treated C11 cells, revealed in our study, are in contrast with the inhibitory action on these SAPK in cell-free systems [22, 23]. Indeed, the Calbiochem catalogue notes that SP600125 and SB202190 inhibit purified JNK and p38 with IC50 of 50 and 16 nM, respectively. It should be underlined that both compounds are ATP-competitive kinase inhibitors, and the data reported were obtained at an ATP concentration of 5 μM, i.e., 1,000-fold lower than the intracellular ATP concentration. For this reason, in experiments with intact cells, these inhibitors are used at much higher concentrations where diverse side effects cannot be excluded. For more details, see [24].
Discussion
The data obtained in our study show that NKCC inhibition in ATP-treated C11 cells is accompanied by activation of JNK and/or p38. We also report here that activation of these SAPK with three distinct stimuli, such as actinomycin, SP600125, and SB202190, results in NKCC inhibition. Additional experiments should be performed to establish the causal relationship between SAPK activation and NKCC inhibition. It should also be noted that the modest inhibitory action of ATP on NKCC was preserved in the presence of the above-listed activators of SAPK. This observation suggests that the signaling cascade is not limited to the activation of these kinases. Recent studies have demonstrated that the with no lysine (K) kinase member WNK4 downregulates NKCC1 via the STE20-related kinase PASK interacting with the conserved domain in the NKCC1 N-terminus [25–27]. The role of WNK kinases in ATP-induced NKCC1 inhibition limited to intercalated cells deserves further investigation.
Two questions should be answered to evaluate the role of purinergic-dependent ion transporters in the regulation of renal function in vivo. First, are concentrations of ATP and other extracellular nucleosides sufficient for activation of P2Y receptors on the apical and basolateral surfaces of collecting duct epithelia? Second, what is the physiological consequence of purinergic regulation of NKCC and other ion transporters in principal and intercalated cells comprising collecting ducts?
In peripheral blood, ectonucleotidases maintain circulating levels of ATP < 10 nM [28], a concentration at which renal P2 receptors with ID50(ATP) > 1 μM [29] cannot be activated. However, nucleosides can act in paracrine and autocrine ways, reaching high extracellular concentrations after sympathetic [30] and cholinergic [31] stimulation or exposure to shear [32, 33], osmotic [34–36], and ischemic stresses [33, 37–39]. Sympathetic innervation is probably the major source of nucleosides for basolateral purinergic receptors [38] whereas shear and osmotic stresses, occurring in proximal tubules and the juxtaglomerular apparatus, contribute to ATP release through the apical membrane in tubular fluid [20, 40]. Using chimeric Staphylococcus aureus protein A-luciferase bound to endogenous antigens on the human airway epithelium, it was shown that hypotonic shock leads to elevation of ATP concentration in surface fluid from ~0.001 to 1 μM [41]. Released ATP is rapidly metabolized by ectoenzymes to adenosine. Importantly, modest elevation of UTP [33] and adenosine [42] sharply increased ATP release from MDCK cells, suggesting a positive feedback loop. It is well documented that osmolality of tubular fluid in collecting ducts varies from ~400 to 1,500 mOsm [43]. Baseline concentrations of ATP and other P2Y agonists in collecting duct tubular fluid and their modulation by osmotic perturbations remain unknown.
Both C7 and C11 cells, resembling principal and intercalated cells, are highly abundant with P2Y1 and P2Y2 receptors [13, 17]. In these cells, P2Y1 and P2Y2 receptors have been shown to mainly reside on the basolateral and apical membranes, respectively [44]. Using monolayers of C7 cells, it was noted that basolateral P2Y1 receptors activate Cl− secretion via PLA2-PKA-mediated activation of Cl− channels [17]. Our studies demonstrated that P2Y2 receptors contribute to Cl− secretion via Ca2+-calmodulin-mediated activation of NKCC1 [14] (Fig. 6a). Glanville and coworkers reported that in mouse collecting ducts, basolateral NKCC possesses the same affinity for K+ and \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} \) (K0.5 ~1.5 mM) [45]. Because bumetanide markedly decreased the acidification rate in NH4Cl-treated cells, they proposed a key role for NKCC1 in H+ secretion and \( {\text{NH}}^{{\text{ + }}}_{{\text{4}}} \) handling by intercalated cells. With intercalated-like C11 cells, we observed that apical P2Y2 receptors transiently activated NKCC1 via Ca2+-calmodulin-mediated signaling, whereas sustained activation of basolateral P2Y1 receptors inhibited this carrier [13]. The present data suggest that NKCC1 inhibition in intercalated cells evoked by P2Y1 agonists is caused by activation of SAPK (Fig. 6b). Further studies should be performed to examine the relative contribution of purinergic signaling in the final adjustment of salt reabsorption and acid-base balance by principal and intercalated cells in vivo as well as the pathophysiological implications of these regulatory pathways.
Possible mechanisms of purinergic signaling in the regulation of Cl− secretion and acid-base homeostasis by principal (a) and intercalated (b) cells from collecting ducts in vivo. 1 Cl− channels, 2 H+-ATPase, 3 anion exchange, PLC phospholipase C, IP 3 inositol 1,4,5-triphosphate, COX cyclooxygenase, PGE 1 prostaglandin E1, CAMK (Ca2++CaM)-dependent protein kinase, CA carbonic anhydrase, a.m. and b.m. apical and basolateral membranes, respectively. For other abbreviations and more details, see text
References
Russell JM (2000) Sodium-potassium-chloride cotransport. Physiol Rev 80:212–276
Delpire E, Rauchman MI, Beier DR, Hebert SC, Gullans SR (1994) Molecular cloning and chromosome localization of a putative basolateral Na+-K-2Cl- cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J Biol Chem 269:25677–25682
Gamba G (2005) Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85:423–493
Bell PD, Lapointe J-Y, Peti-Peterdi J (2003) Macula densa cell signaling. Annu Rev Physiol 65:481–500
Breyer MD, Ando Y (1994) Hormonal signaling and regulation of salt and water transport in the collecting duct. Annu Rev Physiol 56:711–739
Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Strai K, Burnstock G, Unwin RJ (2000) Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58:1893–1901
Rice WR, Burton FM, Fiedeldey DT (1995) Cloning and expression of the alveolar type II cell P2U-purinergic receptor. Am J Respir Cell Mol Biol 12:27–32
Schwiebert EM, Wallace DP, Braunstein CM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP et al (2002) Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol 282:F763–F775
Takeda M, Kobayashi M, Endou H (1998) Establishment of a mouse clonal early proximal tubule cell line and outer medullary collecting duct cells expressing P2 purinoceptors. Biochem Mol Biol Int 44:657–664
Tokuyama Y, Hara M, Jones EM, Fan Z, Bell GI (1995) Cloning of rat and mouse P2Y purinoceptors. Biochem Biophys Res Commun 211:211–218
Simmons NL (1981) Stimulation of Cl− secretion by exogenous ATP in cultured epithelial monolayers. Biochim Biophys Acta 646:231–242
Gekle M, Wunsch S, Oberleithner H, Silbernagl S (1994) Characterization of two MDCK-cell subtypes as a model system to study principal cell and intercalated cell properties. Pflugers Arch 428:157–162
Akimova OA, Grygorzcyk A, Bundey RA, Bourcier N, Gekle M, Insel PA, Orlov SN. (2006) Transient activation and delayed inhibition of Na+,K+,Cl- cotransport in ATP-treated C11-MDCK cells involve distinct P2Y receptor subtypes and signaling mechanisms. J Biol Chem 281:31317–31325
Bourcier N, Grygorczyk R, Gekle M, Berthiaume Y, Orlov SN (2002) Purinergic-induced ion current in monolayers of C7-MDCK cells: role of basolateral and apical ion transporters. J Membr Biol 186:131–143
Orlov SN, Dulin NO, Gagnon F, Gekle M, Douglas JG, Schwartz JH, Hamet P (1999) Purinergic regulation of Na+,K+,Cl− cotransport and MAP kinases is limited to C11-MDCK cells resembling intercalated cells from collecting ducts. J Membr Biol 172:225–234
Gagnon F, Orlov SN, Tremblay J, Hamet P (1998) Complete inhibition of Na+,K+,Cl− cotransport in Madin-Darby canine kidney cells by PMA-sensitive protein kinase C. Biochim Biophys Acta 1369:233–239
Akimova OA, Bourcier N, Taurin S, Bundey RA, Grygorczyk K, Gekle M, Insel PA, Dulin NO, Orlov SN (2005) Cl- secretion in ATP-treated renal epithelial C7-MDCK cells is mediated by activation of P2Y1 receptors, phospholipase A2 and protein kinase A. J Physiol 568:789–801
Brindikova TA, Bourcier N, Torres B, Pchejetski D, Gekle M, Maximov GV, Montminy V, Insel PA, Orlov SN, Isenring P (2003) Purinergic-induced signaling in C11-MDCK cells inhibits the secretory Na-K-Cl cotransporter. Am J Physiol Cell Physiol 285:C1445–C1453
Insel PA, Ostrom RS, Zambon AC, Hughes RJ, Balboa MA, Shehnaz D, Gregorian C, Torres B, Firestein BL, Xing M et al (2001) P2Y receptors of MDCK cells: epithelial cell regulation by extracellular nucleotides. Clin Exp Pharmacol Physiol 28:351–354
Schwiebert EM, Zsembery A (2003) Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 1615:7–32
Gagnon F, Dulin NO, Tremblay J, Hamet P, Orlov SN (1999) ATP-induced inhibition of Na+,K+,Cl− cotransport in Madin-Darby canine kidney cells: lack of involvement of known purinoceptor-coupled signaling pathways. J Membr Biol 167:193–204
Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leistein JC, Motiwala A, Pierce S, Satoh Y et al (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A 98:13681–13686
Du L, Lyle CS, Obey TB, Gaarde WA, Muir JA, Bennett BL, Chambers TC (2004) Inhibition of cell proliferation and cell cycle progression by specific inhibition of basal JNK kinase. J Biol Chem 279:11957–11966
Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105
Dowd BF, Forbush B (2003) PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCK1). J Biol Chem 278:27347–27353
Gagnon KBE, England R, Delpire E (2006) Characterization of SAP and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol 26:689–698
Gagnon KB, England R, Delpire E (2006) Volume sensitivity of cation-Cl- cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine rich kinase and WNK4. Am J Physiol Cell Physiol 290:C134–C142
Lazarosski EL, Boucher RC (2001) UTP as an extracellular signaling molecule. News Physiol Sci 16:1–5
Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jackobson KA, Leff P, Williams M (1994) Nomenclature and classification of purinoceptors. Pharmacol Rev 46:143–156
Bankston LA, Guidotti G (1996) Characterization of ATP transport into chromaffin granule ghosts. Synergy of ATP and serotonin accumulation in chromaffin granule ghosts. J Biol Chem 271:17132–17138
Unsworth CD, Johnson RG (1990) Acetylcholine and ATP are coreleased from the electromotor nerve terminals of Nacrine brasiliensis by an exocytosis mechanism. Proc Natl Acad Sci U S A 87:553–557
Grygorczyk R, Hanrahan JW (1997) Cystic fibrosis transmembrane conductance regulator and adenosine triphosphate. Science 275:1325–1326
Ostrom RS, Gregorian C, Insel PA (2000) Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem 275:11735–11739
Hazama A, Shimizu T, Ando-Akatsuka Y, Hayashi S, Tanaka S, Maeno E, Okada Y (1999) Swelling-induced, CFTR-dependent ATP-release from human epithelial cell line: lack of correlation with volume-sensitive Cl— channels. J Gen Physiol 114:525–533
Boudreault F, Grygorczyk R (2002) Cell swelling-induced ATP release and gadolinium-sensitive channels. Am J Physiol 282:C219–C226
Tatur S, Kreda S, Lazarowski E, Grygorczyk R (2007) Calcium-dependent release of adenosine and uridine nucleotides from A549 cells. Purinergic Signalling (in press)
Sponsel HT, Breckon R, Anderson RJ (1995) Adenine nucleotide and protein kinase C regulation of renal tubule epithelial cell wound healing. Kidney Int 48:85–92
Bohmann C, Rump LC, Schaible U, von Kugelgen I (1995) α-Adrenoceptor modulation of norepinephrine and ATP release in isolated kidneys of spontaneously hypertensive rats. Hypertension 25:1224–1231
Motte S, Communi D, Pirotton S, Boeynaems JM (1995) Involvement of multiple receptors in the actions of extracellular ATP: the examples of vascular endothelial cells. Int J Biochem Cell Biol 27:1–7
Komlosi P, Fintha A, Bell PD (2005) Renal cell-to-cell communication via extracellular ATP. Physiology 20:86–90
Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC (2006) Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281:22992–23002
Migita K, Lu L, Zhao Y, Honda K, Iwamoto T, Kita S, Katsuragi T (2005) Adenosine induces ATP release via inositol 1,4,5-triphosphate signaling pathway in MDCK cells. Biochem Biophys Res Commun 328:1211–1215
Garcia-Perez A, Burg MB (1991) Role of organic osmolytes in adaptation of renal cells to high osmolality. J Membr Biol 119:1–13
Wolff SC, Qi A-D, Harden TK, Nicholas RA (2005) Polarized expression of human P2Y receptors in epithelial cells from kidney, lung, and colon. Am J Physiol Cell Physiol 288:C624–C632
Glanville M, Kingscote S, Thwaites DT, Simmons NL (2001) Expression and role of sodium, potassium, chloride cotransport (NKCC1) in mouse inner medullary collecting duct (mIMCD-K2) epithelial cells. Pflugers Arch 443:123–131
Acknowledgements
This work was supported by grants from the Kidney Foundation of Canada. The editorial help of Ovid Da Silva, Research Support Office, Centre de recherche, CHUM, is appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Akimova, O.A., Taurin, S., Dulin, N.O. et al. Purinergic inhibition of Na+,K+,Cl− cotransport in C11-MDCK cells: Role of stress-activated protein kinases. Purinergic Signalling 4, 183–191 (2008). https://doi.org/10.1007/s11302-007-9057-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-007-9057-z