Abstract
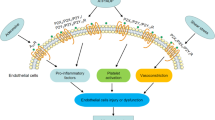
Atherosclerosis is an immunoinflammatory process that involves complex interactions between the vessel wall and blood components and is thought to be initiated by endothelial dysfunction [Ross (Nature 362:801–09, 1993); Fuster et al. (N Engl J Med 326:242–50, 1992); Davies and Woolf (Br Heart J 69:S3–S11, 1993)]. Extracellular nucleotides that are released from a variety of arterial and blood cells [Di Virgilio and Solini (Br J Pharmacol 135:831–42, 2002)] can bind to P2 receptors and modulate proliferation and migration of smooth muscle cells (SMC), which are known to be involved in intimal hyperplasia that accompanies atherosclerosis and postangioplasty restenosis [Lafont et al. (Circ Res 76:996–002, 1995)]. In addition, P2 receptors mediate many other functions including platelet aggregation, leukocyte adherence, and arterial vasomotricity. A direct pathological role of P2 receptors is reinforced by recent evidence showing that upregulation and activation of P2Y2 receptors in rabbit arteries mediates intimal hyperplasia [Seye et al. (Circulation 106:2720–726, 2002)]. In addition, upregulation of functional P2Y receptors also has been demonstrated in the basilar artery of the rat double-hemorrhage model [Carpenter et al. (Stroke 32:516–22, 2001)] and in coronary artery of diabetic dyslipidemic pigs [Hill et al. (J Vasc Res 38:432–43, 2001)]. It has been proposed that upregulation of P2Y receptors may be a potential diagnostic indicator for the early stages of atherosclerosis [Elmaleh et al. (Proc Natl Acad Sci U S A 95:691–95, 1998)]. Therefore, particular effort must be made to understand the consequences of nucleotide release from cells in the cardiovascular system and the subsequent effects of P2 nucleotide receptor activation in blood vessels, which may reveal novel therapeutic strategies for atherosclerosis and restenosis after angioplasty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a pathological phenomenon primarily affecting the large conduit arteries, for example the aorta, coronary, carotid iliac, and femoral arteries. The development of atherosclerotic lesions in arteries involves the intimal recruitment of smooth muscle cells (SMC) within the blood vessel wall, and also the infiltration of blood-derived cells [1]. This process necessitates the proliferation and migration of SMC from the underlying media and the endothelial adhesion of leukocytes and their infiltration into the subendothelium. A similar intimal accumulation of SMC also takes place during the postangioplasty restenotic process. Although the factors involved in intimal cell recruitment are not clearly identified, it is becoming evident that endothelial dysfunction is a key factor in the development of vascular disease. Experimental evidence suggests that an intact endothelium plays a central role in maintaining a low proliferative state of SMC under normal conditions [10]. In arterial injury, endothelial cells, SMC, and various blood cells can release chemotactic factors and mitogens, including ATP and other nucleotides (see [4] for review). Activation of P2 nucleotide receptors has been shown to induce not only the proliferation and migration of vascular SMC but also apoptosis, a process involved in the evolution of the atherosclerotic plaque [11]. In addition, P2 receptors mediate both vasorelaxation and vasoconstriction of arteries that may be involved in the vascular remodeling accompanying atherosclerosis and postangioplasty restenosis [5]. A better understanding of the causative agents and mechanisms of proliferation and migration of vascular SMC as well as recruitment of blood-derived cells by the endothelium could lead to prevention, attenuation, or even reversal of intimal thickening, which may dramatically reduce morbidity and mortality from vascular diseases such as atherosclerosis and restenosis after angioplasty. In this respect, a better understanding of the physiological role of P2 receptors in both normal and pathological blood vessels could potentially lead to a breakthrough in the fight against vascular disease.
P2 receptors in the cardiovascular system
Extracellular nucleotides bind to cell surface receptors known as P2 receptors, which are present in many tissues. To date, these receptors have been classified into two main families: the P2X receptors that are ligand-gated ion channels comprised of homo- or hetero-oligomers [12] and P2Y receptors that are seven membrane spanning receptors coupled via G proteins (Gq/11 or Gi/o) to phospholipase C (PLC) and/or adenylate cyclase [12–14]. In turn, PLC activation generates inositol 1,4,5-triphosphate (IP3), a mediator of Ca2+ release from intracellular stores, and diacylglycerol, an activator of protein kinase C (PKC), whereas adenylate cyclase generates cyclic adenosine monophosphate (cAMP), an activator of protein kinase A (PKA). The cloning of seven P2X (P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, P2X7) and eight P2Y (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14) receptor subtypes has made it possible to use molecular and pharmacological approaches to study the distribution and functional properties of specific P2 receptor subtypes at the tissue and cellular level.
P2 receptors in vascular cells
The normal arterial wall consists of three layers: the intima, media, and adventitia. The single layer of endothelial cells facing the vessel lumen is a very important component of the vascular wall in terms of releasing both vasodilators such as nitric oxide (NO) and prostacyclin (PGI2) and vasoconstrictors like thromboxane A2 and endothelin. The principal P2Y receptor subtypes that have been functionally characterized in endothelial cells are P2Y1 and P2Y2, but mRNAs for P2Y4 and P2Y6 receptors have also been detected [4]. Endothelium-dependent vasorelaxation has been attributed to the release of NO and PGI2 after binding of nucleotides to P2Y1 and P2Y2 receptors in endothelial cells [15], whereas vasoconstrictor effects in SMC result from the action of nucleotides on P2Y2 and P2X receptors [16, 17]. In most blood vessels, P2Y1 receptors for ADP are present on the endothelium and regulate vasodilatation by Ca2+-dependent (PLC-mediated) activation of nitric oxide synthase (NOS) and generation of endothelial-dependent relaxing factor (EDRF) (see [4] for review). Endothelial prostacyclin production is also stimulated by P2Y1 and P2Y2 receptors, but this seems to play a minimal role in vasodilatation, at least under physiological conditions (see [18] for review). Recent studies have indicated that in the aorta of P2Y2-null mice the endothelium-dependent relaxation by ATP and ATPγS was inhibited, demonstrating the role of the P2Y2 receptors, but that a relaxation by UTP and UDP was maintained, suggesting the additional involvement of P2Y6 receptors [19]. The majority of the cells in intact blood vessels are SMC, which occupy most of the media, and are involved in vasoconstriction and vasorelaxation of the vessel. P2Y2Rs in SMC mediate the induction of immediate-early and delayed-early cell cycle-dependent genes, consistent with a role for P2Y2Rs in vascular proliferation of SMC [20, 21]. A recent study demonstrated that P2Y2 is the predominant functional receptor that responds to ATP and UTP in rat aortic SMC [22]. In human cerebral arteries, P2Y6 seems to be the predominant subtype and induces vasoconstriction when activated by UDP/UTP [23, 24]. This is consistent with findings from rat pulmonary and mesenteric arteries [25, 26]. In addition, a recent study on P2X1 knockout mice further supports the prominent contractile effect of the P2Y6 subtype in mesenteric arterial trees [27]. Taken together, it seems that the principal receptor mediating UTP/UDP-induced contractile responses in blood vessels might be the P2Y6 subtype. Other studies have reported the presence of both P2Y4 and P2Y6 receptors in rat aortic SMC [21, 28, 29]. P2Y1 receptors are expressed in SMC of a number of blood vessel types and like their EC counterparts mediate vasodilatation most likely through the activation of K+ channels (see [18] for review). The presence of several P2X receptor subtypes also has been reported in human saphenous vein SMC, including P2X1, P2X2, P2X4, and P2X7 receptors [30]. The outermost layer of the blood vessel consists of connective tissue and fibroblasts, which have not been appreciated in the regulation of vascular tone. A recent study showed that fibroblasts can migrate into the neointima, suggesting their possible involvement in the development of vascular diseases such as atherosclerosis and restenosis after angioplasty [31]. Since human and rat fibroblasts are known to express P2Y1, P2Y2, P2Y4, P2Y6, P2X3, P2X4, and P2X7 receptors [32], further investigation is needed to establish the role of fibroblast P2 receptors in either physiological or pathophysiological conditions.
P2 receptors in blood cells
G protein-coupled P2Y receptors whose activation leads to intracellular calcium mobilization have been observed in neutrophils [4, 33, 34], and turkey erythrocytes express both P2Y1 and P2X7 receptors [35, 36]. ATP and UTP act as secretagogues by binding to P2Y2Rs to enhance exocytosis of primary granules in neutrophils [37]. In macrophages, ATP activates a P2X7 receptor that differs from other ligand-gated ion channel P2X receptors by its ability to generate plasma membrane pores when activated [38]. Human T lymphocytes also have been shown to express P2X7 receptors [39]. Human monocytes and macrophages coexpress P2X1, P2X4, and P2X7 receptors, whereas granulocytes only express P2X7 receptors [40]. P2Y1, P2Y2, and P2Y6 receptors also are expressed in monocytes, B lymphocytes, and polymorphonuclear granulocytes [41]. Human platelets express P2Y1, P2Y12, and P2X1 receptors [42–44]. Thus, the diversity of P2 receptor expression in blood cells, as well as endothelial cells, SMC, and fibroblasts, suggests that this receptor superfamily plays a significant role in the regulation of cardiovascular functions.
P2 receptors regulate nucleotide-induced vascular smooth muscle cell proliferation
Proliferation of SMC is a hallmark of vascular diseases such as atherosclerosis and restenosis following angioplasty. ATP and other nucleotides are released by aggregating platelets and damaged vascular cells, such as endothelial cells and SMC in pathological or stress conditions [45]. Extracellular nucleotides can act via P2X and P2Y receptors to induce acute responses such as the regulation of vascular tone [46]. However, it is generally believed that the ionotropic P2X receptors do not mediate the chronic responses of nucleotides such as cell proliferation. The mitogenic effect of extracellular nucleotides on vascular SMC (VSMC) has been known for years [47]. However, a potent antiproliferative effect of UTP on VSMC also has been reported in human arterial and venous SMC [48]. In either case, the P2 receptor subtype(s) responsible for these effects on the proliferation of VSMC has not been determined. Earlier studies have shown that ATP or UTP increased DNA and protein synthesis in subcultured rat aortic VSMC [49, 50]. In the same cell culture model, however, Malam-Souley et al. [51] were unable to detect increases in DNA synthesis after ATP/UTP stimulation, although ATP or UTP upregulated the expression of mRNA to several cell cycle progression-related genes. Because P2X receptor agonists were essentially inactive, it was concluded that a P2U-like receptor (now termed P2Y2) was responsible for the mitogenic effects of ATP/UTP. However, the role of a P2Y4 receptor cannot be excluded, because the nucleotide agonist profile between rat P2Y2 and P2Y4 receptors is essentially indistinguishable [52]. Indeed, Harper et al. [53] suggested that the P2Y4 receptor mediated ATP/UTP-induced proliferation of rat aortic VSMC. Recent studies indicated that ATP, UTP, or ITP, three agonists of the cloned porcine P2Y2 receptor, increased DNA and protein synthesis and cell number in coronary artery SMC [54]. Indeed, treatment of pig coronary artery SMC with UTP, ATP, or ITP caused a concentration-dependent increase in DNA and protein synthesis and cell number, whereas UDP only caused a small increase in protein synthesis. Intriguingly, ATP was much more potent and efficacious than UTP, ITP, and UDP in increasing DNA synthesis and expression of PCNA, a protein marker of cell proliferation, suggesting that another receptor may contribute to the proliferative response [54]. In vivo experiments have shown that intimal thickening of collared rabbit carotid arteries was greatly enhanced by in situ UTP application and was closely associated with osteopontin expression in medial SMC [6]. Osteopontin is chemotactic for SMC and is associated with arterial smooth muscle cell proliferation [55]. Moreover, both UTP and ATP increased osteopontin expression in cultured SMC, whereas ADP, UDP, and 2-MeSATP were ineffective, which suggests a role for the P2Y2R in which ATP and UTP are equipotent. Direct evidence for involvement of the P2Y2R was provided by inhibition of UTP-induced osteopontin expression in cultured SMC by P2Y2 antisense oligonucleotides [6]. P2Y6 receptors also have been shown to mediate proliferation of SMC in rat aorta [56].
Role of P2 receptors in the migration of vascular SMC
Recent studies indicate that the extracellular nucleotides ATP, ADP, UTP, and UDP serve as directional cues for the migration of rat aortic SMC [57]. At identical concentrations, the most powerful migratory response induced by these nucleotides was elicited by UTP. Nucleotide-induced migration of SMC is the consequence of both chemotaxis and chemokinesis and may result either from the activation of one particular P2 nucleotide receptor subtype or from activation of several P2 receptor subtypes. The ability of UTP at submicromolar levels to stimulate migration of SMC supports the hypothesis that this response could have physiological consequences and is essentially mediated by P2Y2 receptor activation without excluding participation of other P2Y receptor subtypes. The difference in the capacity of UTP and ATP to elicit migration of SMC could be due to the inhibition of nucleotide-induced cell migration by adenosine generated from ATP catabolism by cell surface ectonucleotidases. Indeed, ATP and UTP were equally effective in causing migration of SMC when ATP was prevented from degradation by addition of the ectonucleotidase inhibitor α,β-methylene-ATP [57]. Several P2Y receptor subtypes could be involved in nucleotide-induced migration of SMC. It has been shown in rat aortic SMC that the P2Y2 receptor is the predominant P2Y receptor subtype [28, 58], whereas lower levels of P2Y4 and P2Y1 receptor mRNA were detected [58]. The very low level of P2Y1 receptor mRNA expression was consistent with the absence of ADP-induced migration of cultured rat aortic SMC, demonstrating that P2Y1 is not involved in this process [58]. In addition, the same study showed that a commercially available solution of hexokinase-treated UDP (UTP-free) induced cell migration equally well as untreated UDP, thereby demonstrating that UDP is chemotactic for aortic SMC by activation of the P2Y6 receptor. Conversely, migration of rat aortic SMC induced by UTP occurred even when UTP degradation by nucleoside diphosphate kinase was inhibited, demonstrating the involvement of P2Y2 and/or P2Y4 receptor(s) [58].
The increased migration of SMC in response to extracellular nucleotides could be related to increases in extracellular matrix (ECM) protein expression. Indeed, previous studies have shown that UTP induces osteopontin expression in rat and rabbit aortic SMC [51, 6]. Increased expression of osteopontin, an RGD-containing ECM protein, is associated with the activation of rat arterial SMC in vitro and in vivo [57]. The increase in osteopontin expression plays a key role in UTP-induced migration of rat aortic SMC, since a monoclonal antibody against osteopontin fully abolished UTP-induced migration [57], whereas an antibody against vitronectin, another ECM protein also involved in migration of human SMC [59], had no effect on the migration of rat aortic SMC [57]. UTP induces increases in osteopontin mRNA expression by increasing both osteopontin mRNA stabilization and osteopontin promoter activity [60]. Recent studies have shown that activation of an AP-1 binding site located 76 bp upstream of the transcription start in the rat osteopontin promoter is involved in UTP-induced osteopontin expression. Using a luciferase promoter deletion assay, Renault et al. [61] identified a new region of the rat osteopontin promoter (-1837 to -1757) that is responsive to UTP. This region contains an NFκB site located at -1800 and an Ebox located at -1768. Supershift electrophoretic mobility shift and chromatin immunoprecipitation assays identified NFκB and USF-1/USF-2 as DNA binding proteins induced by UTP. Using dominant negative mutants of IκB kinase and USF transcription factors, it was confirmed that NFκB and USF-1/USF-2 are involved in the UTP-induced expression of osteopontin.
This ability of nucleotides to act as chemoattractants for rat arterial SMC in a concentration range potentially found in pathological vessels [62] and the findings of previous studies demonstrating the mitogenic activity of extracellular nucleotides for these cells suggest that nucleotides released from mechanically stretched vascular or damaged cells during the angioplasty process may participate in arterial wall remodeling.
Role of P2 receptors in nucleotide-induced vascular inflammation
In addition to their mitogenic effects, extracellular nucleotides may also cause cell recruitment by inducing lymphocyte and macrophage adhesion to human pulmonary artery endothelial cells as demonstrated in vitro [63]. Nucleotides can also modulate rat aortic smooth muscle cell adhesion and migration by increasing the expression of osteopontin [51, 57], a protein involved in both processes. Moreover, extracellular nucleotides may play a role in intra-arterial attraction of monocytes by inducing an increased expression of monocyte-chemoattractant protein-1 by arterial SMC [21]. Stimulation of P2 receptors is coupled to the release of the proinflammatory cytokines interleukin (IL)-1β, IL-1α, IL-8, and tumor necrosis factor (TNF)-α (see [4] for review) that are of obvious relevance to inflammation in atherosclerosis. Activation of P2X7 receptors on monocytes/macrophages enhances release of proinflammatory cytokines that modulate NO production and expression of inducible nitric oxide synthase (iNOS) (see [64] for review), mediators of immune cell activation that is an early step in atherosclerotic lesion development.
Monocyte recruitment into the vessel wall is a complex process that includes cell rolling, firm attachment, and directed migration. It is now becoming evident that adhesion molecules such as VCAM-1 play an important role in leukocyte adherence to vascular endothelial cells [65, 66]. VCAM-1 expression is induced or upregulated by proinflammatory cytokines such as TNF-α and IL-1β in cellular components of the arterial wall including endothelial cells, smooth muscle cells, and fibroblasts [67-69]. ATP and UTP have been shown to induce cell-cell adhesion in a human monocyte/macrophage lineage and neutrophil adherence to human endothelial cell monolayers [63, 70].
Recent studies have shown that local UTP delivery via an osmotic pump to collared rabbit carotid arteries induced intimal accumulation of macrophages, similar to oxidized low-density lipoprotein (LDL), a response that was mediated by activation of P2Y2 receptors [6]. Leukocyte migration depends on the activities of adhesion proteins (e.g., selectins and integrins) on leukocytes and vascular endothelial cells. We have demonstrated that activation of P2Y2 receptors in endothelial cells causes the expression of VCAM-1 that mediates the adherence of monocytes to vascular endothelium [71], leading to their penetration into the vessel wall to promote arterial inflammation associated with atherosclerosis.
Recent studies have revealed that a Src homology-3 (SH3) binding domain in the C-terminal tail of the P2Y2 receptor promotes the nucleotide-induced association of Src with the P2Y2 receptor, leading to the transactivation of growth factor receptors, such as the epithelial growth factor (EGF) and vascular endothelial growth factor (VEGF) receptors, and nucleotide-induced upregulation of VCAM-1 [72, 73]. Since leukocyte infiltration and migration are key processes involved in atherosclerosis, these findings suggest that P2Y2 receptors represent a novel target for reducing arterial inflammation associated with cardiovascular disease.
P2 receptors in vascular apoptosis
Apoptosis (programmed cell death) has been reported to occur in various vascular diseases such as atherosclerosis, restenosis, and hypertension [74, 75]. The major cell types undergoing apoptosis in human atherosclerotic lesions are arterial SMC [11, 76–78] and macrophages [79]. In restenosis following balloon angioplasty, there is a peak in the proliferation and apoptosis of rat vascular SMC 14 days postangioplasty [76]. Furthermore, apoptosis of arterial SMC has been described in animal models of intimal thickenings [80] and probably takes part in the normal process involved in the control of hyperplasia. In contrast, apoptosis of SMC in advanced human atherosclerotic plaques may destabilize the fibrous lesion to promote plaque rupture and its clinical consequences.
As a mediator of cell-to-cell communication, ATP can trigger a variety of biological responses after being rapidly released in large amounts from various sources, including activated platelets, endothelial cells, nerve terminals, antigen-stimulated T cells, and other cell types following hypoxia, stress, and tissue damage. For example, in human umbilical cord vein endothelial cells (HUVEC), a substantial release of ATP (and UTP) is induced by shear stress [81], which may lead to alterations in the balance between proliferation and apoptosis regulated by P1 adenosine and P2 (particularly P2X7) nucleotide receptors [82]. P2X7 and P1 receptors have been previously linked to apoptosis in other cell types, including immune cells, astrocytes, and thymocytes [83–85]. The P2X7R also has been shown to mediate ATP-induced cell death in human embryonic kidney cells [86], human cervical epithelial cells [87], and primary rat cortical neurons [88]. In human arterial SMC, adenosine-induced apoptosis is essentially mediated via the A2b-adenosine receptor subtype and involves a cAMP-dependent pathway [89].
As an important constituent of atherosclerotic plaques, fibroblasts share several features with smooth muscle cells. In human fibroblasts, P2X7 was identified as the main nucleotide receptor involved in the high glucose concentration-dependent responses modulated by ATP, including morphological changes, enhanced apoptosis, caspase-3 activation, and IL-6 release [90]. In the immune system, ATP also plays important roles through nucleotide receptors in leukocyte functions. P2X7 receptor-mediated apoptosis has been demonstrated in various types of leukocytes, including a lymphocytic cell line, murine thymocytes, murine peritoneal macrophages, human macrophages, mesangial cells, dendritic cells, and microglial cells [83, 91–96]. Extracellular ATP acting via the P2X7 receptor activates the transcription factor NFκB by selectively targeting NFκB p65 (Rel A) in the N9 mouse microglial cell line [97]. It also has been reported that the P2X7 receptor modulates macrophage production of TNF-α, IL-1β, and nitric oxide (NO) following lipopolysaccharide (LPS) exposure [98], consistent with a role for the P2X7 receptor in inflammation. In HUVEC, TNF-α markedly increases apoptotic cell death via the activation of caspase-3 [74]. Recent reports indicate that ATP/ADP activate NFκB and induce apoptosis probably through P2X7 receptors in porcine aortic endothelial cells [99]. These studies have provided compelling evidence suggesting a role for P2 and P1 receptor-mediated apoptosis in vascular diseases; however, further studies are needed to determine the precise pathways involved and to accumulate direct evidence that these pathways contribute significantly to the development of atherosclerosis, hypertension, and restenosis.
Modulation of P2 receptors in vascular injury
Experimental arterial intimal hyperplasia can be induced by balloon angioplasty or by the perivascular placement of a silicone collar around an artery. An influx of leukocytes precedes the migration and proliferation of vascular SMC into the intima in both these models [100]. In normal adult rat aorta, P2Y2 mRNA was found in the endothelial cell lining, while a sustained expression of P2Y2 mRNA was detected in few medial SMC [20]. In contrast, P2Y2 mRNA was detected in all medial SMC of rat fetal aortas and in most of the aortic SMC of intimal lesions after balloon angioplasty, with an overexpression in cells lining the lumen both 1 and 3 weeks after injury.
In the collar model, neointimal formation appears to be triphasic [100]. The first phase is characterized by vascular infiltration of leukocytes beginning 2 h after collar placement around a rabbit carotid artery. The second phase begins within 12 h of collar placement and is characterized by medial replication of SMC. The third phase is characterized by the appearance, beginning at day 3 after collar placement, of subendothelial SMC. In situ hybridization with sham-operated rabbit carotid arteries indicated that P2Y2 mRNA expression was localized to CD31-positive aortic endothelial cells and not medial SMC [6]. High levels of P2Y2 mRNA were detected in medial SMC 3 days after collar placement, before appearance of neointima. At day 14, all intimal and medial SMC were P2Y2 positive. Fura-2 digital imaging of single SMC, used to measure changes in myoplasmic calcium concentration in response to P2Y receptor agonists, confirmed an increase in P2Y2 receptor activity. However, the same study showed that P2Y4 mRNA was equivalently expressed in sham-operated and collared arteries or cultured rabbit carotid SMC, whereas P2Y6 mRNA was not detected in carotid arteries or cultured SMC. In a more recent study, it was shown that P2Y2 receptor upregulation occurs in stented porcine coronary artery, a clinically relevant model of arterial injury [54]. P2Y2 receptor mRNA levels were significantly increased in coronary SMC dispersed from stented segments of coronary arteries 3 weeks after stent angioplasty compared with SMC from unstented segments. There was no significant difference observed in levels of P2Y6 mRNA in the stented and unstented artery segments, whereas P2Y4 receptor mRNA was undetectable.
Upregulation of functional P2Y receptors also occurs in the basilar artery of the rat double-hemorrhage model [7], in the coronary artery of diabetic dyslipidemic pigs [8], and in human atherosclerotic lesions (Seye and Desgranges, unpublished data). It has been proposed that upregulation of P2Y receptors could be a potential diagnostic indicator for the early stages of atherosclerosis [9]. Interestingly, a more recent study showed that high shear stress, associated with vascular diseases, can selectively upregulate P2Y2 and P2Y6 receptors in perfused arterial SMC [101]. P2X1 and P2X4 receptors have been shown to be upregulated in rabbit intimal thickenings [102]. Taken together, these findings strongly suggest that at least some P2 receptor subtypes (most notably P2Y2) are implicated in the development of vascular disease.
Pathophysiological significance of P2 receptor modulation in vascular injury
Migrating and proliferating SMC in the arterial media, together with infiltrating macrophages and T lymphocytes, are the main cell types that comprise atherosclerotic and restenotic lesions [1]. In the early stages of intimal hyperplasia, SMC are modified from a differentiated, contractile phenotype to an immature, synthetic phenotype, and this enables them to migrate into the intima, to proliferate, and to secrete extracellular matrix components. In many respects, this shift in phenotype is a reversal of the normal differentiation pattern of vascular SMC during fetal and early postnatal life [103, 104]. However, there is still a lack of knowledge concerning the phenotypic regulation of SMC during vasculogenesis and vascular disease. A high level of P2 receptor expression could be related to the altered phenotype of SMC in intimal thickenings. Partially dedifferentiated SMC are found in rat arterial intimal lesions after balloon angioplasty [105, 106], and their phenotype has been compared with that of newborn rat aortic SMC [107]. Interestingly, it has been reported that medial SMC of rat embryonic aorta also exhibit high P2Y2 expression, similar to intimal thickenings [20]. Other studies have shown that P2Y1 and P2Y2 receptor transcripts are strongly upregulated with phenotypic changes in rat SMC, whereas P2X1 mRNA is completely downregulated, and P2Y4 and P2Y6 mRNA levels are unchanged [58, 28]. Taken together, these and other results suggest that P2Y2 receptor expression is upregulated in the entire cell population of intimal thickenings and is closely associated with a poorly differentiated phenotype of SMC. The dramatic increase in P2Y2 mRNA expression observed in balloon angioplasty-induced intimal lesions would suggest increased activity of extracellular nucleotides with consequent enhancement of cell proliferation and vasoreactivity. Indeed, extracellular nucleotides, particularly ATP and UTP, have been shown to induce cell cycle progression and proliferation of cultured arterial SMC [21, 49, 50, 51] and vasoconstriction in the absence of endothelial cells [17, 108, 109]. Since both neointimal hyperplasia and vasoconstrictive remodeling have been found to be involved in postangioplasty restenosis [1, 5, 107, 110], these findings suggest that extracellular nucleotides may play a significant role in this process, at least as long as functional endothelial cells, which regulate intimal thickening [111, 112] and nucleotide-induced vasorelaxation [113, 114], are not regenerated.
Increased P2Y2 receptor expression in the neointima may by itself be sufficient to enhance the local effects of extracellular nucleotides on the proliferation of SMC. Although the expression of other P2 receptors has been described in arterial SMC [21, 29, 115], the P2Y2 receptor seems to be more specifically involved in the response of SMC to ATP and UTP [116], particularly in the potentiation of proliferation [21, 50]. The effects of extracellular nucleotides are not only dependent on the nature and number of P2 receptors present on target cells, but also on the local concentrations of nucleotide agonists. Although in vivo concentrations of extracellular nucleotides are difficult to measure, various in vitro experiments suggest that extracellular nucleotides are released from blood and vascular cells exposed to various physicochemical conditions, e.g., stress, hypoxia, and other factors [117, 118, 119] associated with the angioplasty process.
P2Y2 receptors in SMC are involved in nucleotide-induced constriction of normal arteries [17, 108, 109]. Long lasting alterations in vasomotricity after endothelial cell denudation, resulting in increased sensitivity to vasoconstrictive substances, have previously been demonstrated [113, 114]. It appears that like other receptors for vasoconstrictive factors such as angiotensin II [120], endothelin [121], and platelet-derived growth factor (PDGF) [122], which are overexpressed in neointima, P2Y2 receptors may play an important role in controlling the vasoactive properties of pathological arteries, particularly in chronic constriction at the lesion site that is postulated to be one of the processes leading to postangioplasty restenosis [5, 110].
Conclusion
P2 receptor subtypes, including P2Y2, P2X1, and P2X4, appear to play a role in responses to endothelial injury that are thought to be key events in the initiation of atherosclerosis and restenosis after angioplasty. P2Y2R upregulation and activation in endothelial cells and SMC promote leukocyte transmigration and intimal thickening in arteries of animal models of vascular injury, suggesting a possible regulatory role for this receptor in the mechanisms leading to neointimal hyperplasia after angioplasty. Although there are no selective P2Y2 receptor antagonists yet available, recent progress in siRNA technology has made it possible to design small RNA interference molecules that can selectively inhibit P2 receptor subtype expression. Such molecules can be efficiently delivered into the vessel wall using adenoviral vectors. In addition, P2 receptor transgenic mice (i.e., mice in which the relevant receptor subtype has been deleted or overexpressed) will be valuable tools to substantiate the role that nucleotides and P2 receptors play in the etiology of cardiovascular disease. Further delineation of the signaling pathways involved in these P2 receptor-mediated processes may help limit or prevent vascular diseases such as atherosclerosis and restenosis after angioplasty.
References
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362(6423):801–09
Fuster V, Badimon L, Badimon JJ, Chesebro JH (1992) The pathogenesis of coronary artery disease and the acute coronary syndromes (1). N Engl J Med 326(4):242–50
Davies MJ, Woolf N (1993) Atherosclerosis: what is it and why does it occur? Br Heart J 69(1):S3–S11
Di Virgilio F, Solini A (2002) P2 receptors: new potential players in atherosclerosis. Br J Pharmacol 135(4):831–42
Lafont A, Guzman LA, Whitlow PL, Goormastic M, Cornhill JF, Chisolm GM (1995) Restenosis after experimental angioplasty: intimal, medial, and adventitial changes associated with constrictive remodeling. Circ Res 76(6):996–002
Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, Gonzalez FA, Weisman GA (2002) Functional P2Y2 nucleotide receptors mediate uridine 5–triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation 106(21):2720–726
Carpenter RC, Miao L, Miyagi Y, Bengten E, Zhang JH (2001) Altered expression of P2 receptor mRNAs in the basilar artery in a rat double hemorrhage model. Stroke 32(2):516–22
Hill BJ, Wamhoff BR, Sturek M (2001) Functional nucleotide receptor expression and sarcoplasmic reticulum morphology in dedifferentiated porcine coronary smooth muscle cells. J Vasc Res 38(5):432–43
Elmaleh DR, Narula J, Babich JW, Petrov A, Fischman AJ, Khaw BA, Rapaport E, Zamecnik PC (1998) Rapid noninvasive detection of experimental atherosclerotic lesions with novel 99mTc-labeled diadenosine tetraphosphates. Proc Natl Acad Sci U S A 95(2):691–95
Casscells W (1992) Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation 86(3):723–29
Isner JM, Kearney M, Bortman S, Passeri J (1995) Apoptosis in human atherosclerosis and restenosis. Circulation 91(11):2703–711
North RA, Surprenant A (2000) Pharmacology of cloned P2X receptors. Ann Rev Pharmacol Toxicol 40:563–80
Cooper DM, Rodbell M (1979) ADP is a potent inhibitor of human platelet plasma membrane adenylate cyclase. Nature 282(5738):517–18
Communi D, Gonzales NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems J-M (2001) Identification of a novel human ADP receptor coupled to G1. J Biol Chem 276(44):41479–1485
Ralevic V, Burnstock G (1991) Roles of P2-receptors in the cardiovascular system. Circulation 84(1):1–4
Saiag B, Milon D, Shacoori V, Allain H, Rault B, van den Driessche J (1992) Newly evidenced pyrimidinoceptors and the P2X receptors are present on the vascular smooth muscle and respectively mediate the UTP- and ATP-induced contractions of the dog maxillary internal vein. Res Commun Chem Pathol Pharmacol 76(1):89–4
von Kügelgen I, Starke K (1990) Evidence for two separate vasoconstriction-mediating nucleotide receptors, both distinct from the P2X-receptor, in rabbit basilar artery: A receptor for pyrimidine nucleotides and a receptor for purine nucleotides. Naunyn Schmiedebergs Arch Pharmacol 341(6):538–46
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidine. Pharmacol Rev 50(3):413–92
Guns PJ, Van Assche T, Fransen P, Robaye B, Boeynaems JM, Bult H (2006) Endothelium-dependent relaxation evoked by ATP and UTP in the aorta of P2Y2-deficient mice. Br J Pharmacol 147(5):569–74
Seye CI, Gadeau AP, Daret D, Dupuch F, Alzieu P, Capron L, Desgranges C (1997) Overexpression of P2Y2 receptor in intimal lesions of the rat aorta. Arterioscler Thromb Vasc Biol 17(12):3602–610
Malam-Souley R, Seye CI, Gadeau AP, Loirand G, Pillois X, Campan M, Pacaud P, Desgranges C (1996) Nucleotide receptor P2u partially mediates ATP-induced cell cycle progression of aortic smooth muscle cells. J Cell Physiol 166(1):57–5
Kumari R, Goh G, Ng LL, Boarder MR (2003) ATP and UTP responses of cultured rat aortic smooth muscle cells revisited: dominance of P2Y2 receptors. Br J Pharmacol 140(7):1169–176
Malmsjo M, Hou M, Pendergast W, Erlinge D, Edvinsson L (2003) The stable pyrimidines UDPβS and UTPγS discriminate between contractile cerebrovascular P2 receptors. Eur J Pharmacol 458(3):305–11
Malmsjo M, Hou M, Pendergast W, Erlinge D, Edvinsson L (2003) Potent P2Y6 receptor mediated contractions in human cerebral arteries. BMC Pharmacol 3:4
Hartley SA, Kato K, Salter KJ, Kozlowski RZ (1998) Functional evidence for a novel suramin-insensitive pyrimidine receptor in rat small pulmonary arteries. Circ Res 83(9):940–46
Malmsjo M, Adner M, Harden TK, Pendergast W, Edvinsson L, Erlinge D (2000) The stable pyrimidines UDPβS and UTPγS discriminate between the P2 receptors that mediate vascular contraction and relaxation of the rat mesenteric artery. Br J Pharmacol 131(1):51–6
Vial C, Evans RJ (2002) P2X1 receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol 62(6):1438–445
Erlinge D, Hou M, Webb TE, Barnard EA, Moller S (1998) Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochem Biophys Res Commun 248(3):864–70
Chang K, Hanaoka K, Kumada M, Takuwa Y (1995) Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem 270(44):26152–6158
Cario-Toumaniantz C, Loirand G, Ladoux A, Pacaud P (1998) P2X7 receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circ Res 83(2):196–03
Zalewski A, Shi Y, Johnson AG (2002) Diverse origin of intimal cells: smooth muscle cells, myofibroblasts, fibroblasts, and beyond? Circ Res 91(8):652–55
von Kugelgen I, Wetter A (2000) Molecular pharmacology of P2Y receptors. Naunyn Schmiedebergs Arch Pharmacol 362(4–):310–23
Kuhns DB, Wright DG, Nath J, Kaplan SS, Basford RE (1988) ATP induces transient elevations of [Ca2+]i in human neutrophils and primes these cells for enhanced O2 − generation. Lab Invest 58(4):448–53
Cowen DS, Lazarus HM, Shurin SB, Stoll SE, Dubyak GR (1989) Extracellular adenosine triphosphate activates calcium mobilization in human phagocytic leukocytes and neutrophil/monocyte progenitor cells. J Clin Invest 83(5):1651–660
Parker JC, Snow RL (1972) Influence of external ATP on permeability and metabolism of dog red blood cells. Am J Physiol 223(4):888–93
Boyer JL, Downes CP, Harden TK (1989) Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J Biol Chem 264(2):884–90
Cockcroft S, Stutchfield J (1989) ATP stimulates secretion in human neutrophils and HL60 cells via a pertussis toxin-sensitive guanine nucleotide-binding protein coupled to phospholipase C. FEBS Lett 245(1–):25–9
Steinberg TH, Newman AS, Swanson JA, Silverstein SC (1987) ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem 262(18):8884–888
Baricordi OR, Ferrari D, Melchiorri L, Chiozzi P, Hanau S, Chiari E, Rubini M, Di Virgilio F (1996) An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood 87(2):682–90
Suh BC, Kim JS, Namgung U, Ha H, Kim KT (2001) P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J Immunol 166(11):6754–763
Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, Torboli M, Bolognesi G, Baricordi OR (2001) Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97(3):587–00
Sun B, Li J, Okahara K, Kambayashi J (1998) P2X1 receptor in human platelets. J Biol Chem 273(19):11544–1547
Leon C, Hechler B, Vial C, Leray C, Cazenave JP, Gachet C (1997) The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett 403(1):26–0
Hollopeter G, Jantzen H-M, Vincent D, Li G, England L, Ramakrishnan V, Yang R-B, Nurden P, Nurden A, Julius D, Conley PB (2001) Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409(6817):202–07
Hamada K, Takuwa N, Yokoyama K, Takuwa Y (1998) Stretch activates Jun N-terminal kinase/stress-activated protein kinase in vascular smooth muscle cells through mechanisms involving autocrine ATP stimulation of receptors. J Biol Chem 273(11):6334–340
Dubyak GR, El-Moatassim C (1993) Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol 265(3 Pt 1):C577–C606
Wang DJ, Huang NN, Heppel LA (1992) Extracellular ATP and ADP stimulate proliferation of porcine aortic smooth muscle cells. J Cell Physiol 153(2):221–33
White PJ, Kumari R, Porter KE, London NJ, Ng LL, Boarder MR (2000) Antiproliferative effect of UTP on human arterial and venous smooth muscle cells. Am J Physiol Heart Circ Physiol 279(6):H2735–H2742
Erlinge D, Yoo H, Edvinsson L, Reis DJ, Wahlestedt C (1993) Mitogenic effects of ATP on vascular smooth muscle cells vs. other growth factors and sympathetic cotransmitters. Am J Physiol 265(4 Pt 2):H1089–H1097
Erlinge D, You J, Wahlestedt C, Edvinsson L (1995) Characterisation of an ATP receptor mediating mitogenesis in vascular smooth muscle cells. Eur J Pharmacol 289:135–49
Malam-Souley R, Campan M, Gadeau AP, Desgranges C (1993) Exogenous ATP induces a limited cell cycle progression of arterial smooth muscle cells. Am J Physiol 264(4 Pt 1):C783–C788
Wildman SS, Unwin RJ, King BF (2003) Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol 140(7):1177–186
Harper S, Webb TE, Charlton SJ, Ng LL, Boarder MR (1998) Evidence that P2Y4 nucleotide receptors are involved in the regulation of rat aortic smooth muscle cells by UTP and ATP. Br J Pharmacol 124(4):703–10
Shen J, Seye CI, Wang M, Weisman GA, Wilden PA, Sturek M (2004) Cloning, up-regulation, and mitogenic role of porcine P2Y2 receptor in coronary artery smooth muscle cells. Mol Pharmacol 66(5):1265–274
Gadeau AP, Campan M, Millet D, Candresse T, Desgranges C (1993) Osteopontin overexpression is associated with arterial smooth muscle cell proliferation in vitro. Arterioscler Thromb 13(1):120–25
Hou M, Harden TK, Kuhn CM, Baldetorp B, Lazarowski E, Pendergast W, Moller S, Edvinsson L, Erlinge D (2002) UDP acts as a growth factor for vascular smooth muscle cells by activation of P2Y6 receptors. Am J Physiol Heart Circ Physiol 282(2):H784–H792
Chaulet H, Desgranges C, Renault MA, Dupuch F, Ezan G, Peiretti F, Loirand G, Pacaud P, Gadeau AP (2001) Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ Res 89(9):772–78
Pillois X, Chaulet H, Belloc I, Dupuch F, Desgranges C, Gadeau AP (2002) Nucleotide receptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ Res 90(6):678–81
Dufourcq P, Louis H, Moreau C, Daret D, Boisseau MR, Lamaziere JM, Bonnet J (1998) Vitronectin expression and interaction with receptors in smooth muscle cells from human atheromatous plaque. Arterioscler Thromb Vasc Biol 18(2):168–76
Renault MA, Jalvy S, Belloc I, Pasquet S, Sena S, Olive M, Desgranges C, Gadeau AP (2003) AP-1 is involved in UTP-induced osteopontin expression in arterial smooth muscle cells. Circ Res 93(7):674–81
Renault MA, Jalvy S, Potier M, Belloc I, Genot E, Dekker LV, Desgranges C, Gadeau AP (2005) UTP induces osteopontin expression through a coordinate action of NFκB, activator protein-1, and upstream stimulatory factor in arterial smooth muscle cells. J Biol Chem 280(4):2708–713
Communi D, Janssens R, Suarez-Huerta N, Robaye B, Boeynaems JM (2000) Advances in signalling by extracellular nucleotides. The role and transduction mechanisms of P2Y receptors. Cell Signal 12(6):351–60
Parker AL, Likar LL, Dawicki DD, Rounds S (1996) Mechanism of ATP-induced leukocyte adherence to cultured pulmonary artery endothelial cells. Am J Physiol 270:L695–L703
Weisman GA, Yu N, Liao Z, Gonzalez F, Erb L, Seye CI (2005) P2 receptors in health and disease. Biotechnol Genet 22:171–95
Faruqi RM, DiCorleto PE (1993) Mechanisms of monocyte recruitment and accumulation. Br Heart J 69:S19–S29
Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS (2001) A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 107(10):1255–262
Rice GE, Munro JM, Bevilacqua MP (1990) Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med 171(4):1369–374
Iademarco MF, Barks JL, Dean DC (1995) Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-alpha in cultured endothelial cells. J Clin Invest 95(1):264–71
Braun M, Pietsch P, Felix SB, Baumann G (1995) Modulation of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 on human coronary smooth muscle cells by cytokines. J Mol Cell Cardiol 27(12):2571–579
Ventura MA, Thomopoulos P (1995) ADP and ATP activate distinct signaling pathways in human promonocytic U-937 cells differentiated with 1,25-dihydroxy-vitamin D3. Mol Pharmacol 47(1):104–07
Seye CI, Yu N, Jain R, Kong Q, Minor T, Newton J, Erb L, Gonzalez FA, Weisman GA (2003) The P2Y2 nucleotide receptor mediates UTP-induced vascular cell adhesion molecule-1 expression in coronary artery endothelial cells. J Biol Chem 278(27):24960–4965
Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI, Gonzalez FA, Seye CI, Weisman GA, Erb L (2004) Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem 279(9):8212–218
Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA (2004) The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J Biol Chem 279(34):35679–5686
Mallat Z, Tedgui A (2000) Apoptosis in the vasculature: mechanisms and functional importance. Br J Pharmacol 130(5):947–62
Thomas WA, Reiner JM, Florentin FA, Lee KT, Lee WM (1976) Population dynamics of arterial smooth muscle cells, V: cell proliferation and cell death during initial 3 months in atherosclerotic lesions induced in swine by hypercholesterolemic diet and intimal trauma. Exp Mol Pathol 24(3):360–74
Han DK, Haudenschild CC, Hong MK, Tinkle BT, Leon MB, Liau G (1995) Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am J Pathol 147(2):267–77
Geng YJ, Libby P (1995) Evidence for apoptosis in advanced human atheroma: colocalization with interleukin-1β-converting enzyme. Am J Pathol 147(2):251–66
Crisby M, Kallin B, Thyberg J, Zhivotovsky B, Orrenius S, Kostulas V, Nilsson J (1997) Cell death in human atherosclerotic plaques involves both oncosis and apoptosis. Atherosclerosis 130(1–):17–7
Björkerud S, Björkerud B (1996) Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol 149(2):367–80
Bochaton-Piallat ML, Gabbiani F, Redard M, Desmoulière A, Gabbiani G (1995) Apoptosis participates in cellularity regulation during rat aortic intimal thickening. Am J Pathol 146(5):1059–064
Burnstock G (1999) Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194:335–42
Kaiser D, Freyberg MA, Friedl P (1997) Lack of hemodynamic forces triggers apoptosis in vascular endothelial cells. Biochem Biophys Res Commun 231(3):586–90
Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD (1991) Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol 112(2):279–88
Di Virgilio F, Zanovello P, Zambon A, Bronte V, Pizzo P, Murgia M (1995) Cell membrane receptors for extracellular ATP: a new family of apoptosis-signalling molecules. Fundam Clin Immunol 3:80–1
Jacobson KA, Hoffmann C, Cattabeni F, Abbracchio MP (1999) Adenosine-induced cell death: evidence for receptor-mediated signalling. Apoptosis 4(3):197–11
Wen LT, Caldwell CC, Knowles AF (2003) Poly (ADP-ribose) polymerase activation and changes in Bax protein expression associated with extracellular ATP-mediated apoptosis in human embryonic kidney 293-P2X7 cells. Mol Pharmacol 63(3):706–13
Wang Q, Wang L, Feng YH, Li X, Zeng R, Gorodeski GI (2004) P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol Cell Physiol 287(5):C1349–C1358
Kong Q, Wang M, Liao Z, Camden JM, Yu S, Simonyi A, Sun GY, Gonzalez FA, Erb L, Seye CI, Weisman GA (2005) P2X7 nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons. Purinergic Signalling 1(4):337–47
Peyot M, Gadeau A, Dandré F, Belloc I, Dupuch F, Desgranges C (2000) Extracellular adenosine induces apoptosis of human arterial smooth muscle cells via A2b-receptor. Circ Res 86(1):76–5
Solini A, Chiozzi P, Falzoni S, Morelli A, Fellin R, Di Virgilio F (2000) High glucose modulates P2X7 receptor-mediated function in human primary fibroblasts. Diabetologia 43(10):1248–256
Zanovello P, Bronte V, Rosato A, Pizzo P, Di Virgilio F (1990) Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. J Immunol 145(5):1545–550
Hogquist KA, Nett MA, Unanue ER, Chaplin DD (1991) Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci U S A 88(19):8485–491
Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS (1997) ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity 7(3):433–44
Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brune B, Sterzel RB (1998) Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol 275:F962–F971
Coutinho-Silva R, Persechini PM, Da Cunha Bisaggio R, Perfettini J, Torres de Santo AC, Kanellopoulos JM, Mottaly I, Dautry-Varsat A, Ojcius DM (1999) P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol 276:C1139–C1147
Ferrari D, Los M, Bauer MK, Vandenabeele P, Wesselborg S, Schulze-Osthoff K (1999) P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett 447(1):71–5
Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K (1997) Extracellular ATP activates transcription factor NF-κB through the P2Z purinoreceptor by selectively targeting NF-κB p65. J Cell Biol 139(7):1635–643
Hu Y, Fisette PL, Denlinger LC, Guadarrama AG, Sommer JA, Proctor RA, Bertics PJ (1998) Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J Biol Chem 273(42):27170–7175
Von Albertini M, Palmetshofer A, Kaczmarek E, Koziak K, Stroka D, Grey ST, Stuhlmeier KM, Robson SC (1998) Extracellular ATP and ADP activate transcription factor NF-κB and induce endothelial cell apoptosis. Biochem Biophys Res Commun 248(3):822–29
De Meyer GR, Van Put DJ, Kockx MM, Van Schil P, Bosmans R, Bult H, Buyssens N, Vanmaele R, Herman AG (1997) Possible mechanisms of collar-induced intimal thickening. Arterioscler Thromb Vasc Biol 17(10):1924–930
Wang L, Andersson M, Karlsson L, Watson MA, Cousens DJ, Jern S, Erlinge D (2003) Increased mitogenic and decreased contractile P2 receptors in smooth muscle cells by shear stress in human vessels with intact endothelium. Arterioscler Thromb Vasc Biol 23(8):1370–376
Pulvirenti TJ, Yin JL, Chaufour X, McLachlan C, Hambly BD, Bennett MR, Barden JA (2000) P2X (purinergic) receptor redistribution in rabbit aorta following injury to endothelial cells and cholesterol feeding. J Neurocytol 29(9):623–31
Katoh Y, Periasamy M (1996) Growth and differentiation of smooth muscle cells during vascular development. Trends Cardiovasc Med 6:100–06
Owens GK (1995) Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75(3):487–17
Gabbiani G, Kocher O, Bloom WS, Vandekerckhove J, Weber K (1984) Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest 73(1):148–52
Kocher O, Gabbiani F, Gabbiani G, Reidy MA, Cokay M, Petters H, Huttner I (1991) Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening: biochemical and morphologic studies. Lab Invest 65(4):459–70
Schwartz SM, deBlois D, O’Brien ER (1995) The intima: soil for atherosclerosis and restenosis. Circ Res 77(3):445–65
Ralevic V, Burnstock G (1991) Effects of purines and pyrimidines on the rat mesenteric arterial bed. Circ Res 69(6):1583–590
Saïag B, Milon D, Allain H, Rault B, Van Den Driessche J (1990) Constriction of the smooth muscle of rat tail and femoral arteries and dog saphenous vein is induced by uridine triphosphate via ‘pyrimidinoceptors– and by adenosine triphosphate via P2X receptors. Blood Vessels 27(6):352–54
Andersen HR, Mæng M, Thorwest M, Falk E (1996) Remodeling rather than neointimal formation explains luminal narrowing after deep vessel wall injury: insights from a porcine coronary (re)stenosis model. Circulation 93(9):1716–724
Clowes AW, Clowes MM, Reidy MA (1986) Kinetics of cellular proliferation after arterial injury. III. Endothelial and smooth muscle growth in chronically denuded vessels. Lab Invest 54(3):295–03
Haudenschild CC, Schwartz SM (1979) Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest 41(5):407–18
Shimokawa H, Flavahan NA, Vanhoutte PM (1989) Natural course of the impairment of endothelium-dependent relaxations after balloon endothelium removal in porcine coronary arteries: possible dysfunction of a pertussis toxin-sensitive G protein. Circ Res 65(3):740–53
Weidinger FF, McLenachan JM, Cybulsky MI, Gordon JB, Rennke HG, Hollenberg NK, Fallon JT, Ganz P, Cooke JP (1990) Persistent dysfunction of regenerated endothelium after balloon angioplasty of rabbit iliac artery. Circulation 81(5):1667–679
Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G (1994) A new class of ligand gated ion channel defined by P2X receptor for extracellular ATP. Nature 371(6497):516–19
Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden TK (1996) Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol 50(2):224–29
Gordon JL (1986) Extracellular ATP: effects, sources and fate. Biochem J 233(2):309–19
Bergfeld GR, Forrester T (1992) Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26(1):40–7
Bodin P, Burnstock G (1995) Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia 51(3):256–59
Viswanathan M, Strömberg C, Seltzer A, Saavedra JM (1992) Balloon angioplasty enhances the expression of angiotensin II AT1 receptors in neointima of rat aorta. J Clin Invest 90(5):1707–712
Winkles JA, Alberts GF, Brogi E, Libby P (1993) Endothelin-1 and endothelin receptor mRNA expression in normal and atherosclerotic human arteries. Biochem Biophys Res Commun 191(3):1081–088
Majesky MW, Reidy MA, Bowen-Pope DF, Hart CE, Wilcox JN (1990) PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol 111:2149–158
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Seye, C.I., Kong, Q., Yu, N. et al. P2 receptors in atherosclerosis and postangioplasty restenosis. Purinergic Signalling 3, 153–162 (2007). https://doi.org/10.1007/s11302-006-9047-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-006-9047-6