Abstract
Cells of the mononuclear phagocyte lineage fuse to form multinucleated giant cells and osteoclasts. Several lines of evidence suggest that P2 receptors, in particular P2X7, are involved in this process, although P2X7 is not absolutely required for fusion because P2X7-null mice form multinucleated osteoclasts. Extracellular ATP may be an important regulator of macrophage fusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mononuclear phagocytes fuse to form multinucleated giant cells in several different contexts. When called upon to delimit an inert object they cannot consume, macrophages fuse to become foreign body giant cells and a foreign body granuloma arises. If the object is an intracellular pathogen that cannot be completely eradicated when cell-mediated immunity is activated, mononuclear phagocytes coalesce to form Langhans giant cells which are morphologically distinct from those found in foreign body granulomas. Finally, cells of the mononuclear phagocyte lineage also form osteoclasts, cells that are responsible for bone resorption. P2 receptors are highly expressed on mononuclear phagocytes, and several investigators have published data that suggest a role for these receptors in the process of macrophage fusion.
Proteins involved in multinucleation
Macrophage fusion is poorly understood although several different molecules have been implicated in this process. A number of cytokines including interferon (IFN)-γ, interleukin (IL)-3, IL-4, IL-6, and IL-13 can induce fusion of macrophage precursors or cell lines in vitro [1]. Several plasma membrane molecules have been invoked as mediators of the fusion process, including CD44 [2, 3], SIRP-α [4], CD47 [5], CD98 [6], and ADAM9 [7]. Perhaps the protein most convincingly shown to be involved in the multinucleation process is DC-STAMP, an integral membrane protein first identified in dendritic cells [8]. DC-STAMP-null mice fail to form multinucleated giant cells or osteoclasts, and reexpression of DC-STAMP was sufficient to restore multinucleation [9].
P2X7 and giant cell formation
The first indication that P2 receptors might play a role in fusion of mononuclear phagocytes was provided by Falzoni et al., who demonstrated that fusion of human monocyte-derived macrophages induced by concanavalin A or IFN-γ could be substantially reduced by incubating the cells in 300 μM oxidized ATP (oATP), a treatment which inhibits, but is not totally specific for, P2X receptors [10]. Further data supporting a role for P2X7 in fusion of J774 mouse macrophage-like cells was obtained using J774 variant cell lines selected for either increased or decreased P2X7 activity, assessed as ATP-induced uptake of the fluorescent dye Lucifer Yellow [11]. J774 cell lines with increased P2X7 activity demonstrated spontaneous cell fusion and multinucleation, while unselected J774 cells and J774 cells with decreased ATP-induced pore formation did not. In addition, the spontaneous multinucleation seen in the cells with increased ATP-induced pore formation was again inhibited by preincubation of the cells in medium containing oATP. It also appeared that under basal conditions the amount of P2X7 activity, and the degree of spontaneous multinucleation, was diminished because of spontaneous release of ATP into the medium. In support of this conjecture, addition of hexokinase to consume extracellular ATP resulted in enhanced multinucleation in cultures of J774 cells with high P2X7 activity.
The development of a monoclonal antibody that recognizes an extracellular epitope of human P2X7 and blocks a number of different P2X7 activities [12] provided an opportunity to address the role of P2X7 in giant cell formation more specifically [13].
When concanavalin A-stimulated human monocyte-derived macrophages were incubated with this antibody, formation of multinucleated giant cells was blocked, although the cells remained capable of forming aggregates. Immunofluorescence micrographs suggested that P2X7 was more highly concentrated at sites of interaction between adjacent cells.
In sum the above experiments with primary mononuclear cells and cells of the J774 mouse macrophage-like cell line provide evidence for a role of P2X7 in fusion of mononuclear phagocytes to form multinucleated giant cells. They leave open the question of the precise role of P2X7 in this process, whether P2X7 needs to be activated to support the process of multinucleation, and if so, what the signaling molecule or counterreceptor is. What is the role of ATP itself in this process? Ambient ATP in the extracellular environment appeared actually to be inhibitory, as enzymatic hydrolysis of ATP enhanced fusion, but it is still possible that ATP may activate P2X7 in the context of a delimited space that is formed after cell-cell contact.
Monocytes harvested from patients with sarcoidosis, a disease characterized by granuloma formation, were more susceptible to the cytolytic effect of BzATP [14]. These data from a granulomatous disorder of humans may indicate a correlation between giant cell formation and P2X7 expression.
P2X7 and osteoclast formation
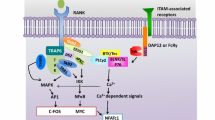
In addition to forming immune and foreign body giant cells, cells of the mononuclear phagocyte lineage fuse to form osteoclasts, multinucleated cells that resorb bone. Osteoclasts function in concert with osteoblasts, fibroblast lineage cells which deposit new bone. Osteoblasts and osteoclasts must coordinate their activity for normal bone remodeling and the maintenance of skeletal integrity, and P2 receptors may be involved in several aspects of this coordinated activity.
Osteoclast formation is perhaps the most thoroughly investigated form of macrophage fusion and multinucleation for several reasons. The generation of multinucleated osteoclasts is better defined than is generation of other monocyte-derived multinucleated giant cells. Thus, while a number of cytokines including tumor necrosis factor (TNF), IFN-γ, IL-3, IL-4, and IL-6 may play a role in giant cell formation, it is clear that in addition to m-CSF, only RANKL is necessary for osteoclast differentiation and multinucleation in vitro. While other multinucleated giant cells are formed in response to invading pathogens or foreign bodies, osteoclasts are generated in the course of normal bone homeostasis. Their function is relatively straightforward to assess in vitro and significant impairment of osteoclast function results in osteopetrosis, in which bone morphology is abnormal, and failure of bone marrow cavity formation occurs and is readily assessed in living animals by X-ray studies and by histomorphometry of explanted bone. Osteopetrosis leads to death because of failure of hematopoiesis. Striking examples of osteopetrosis due to failure of osteoclast formation have been described in mice in which m-CSF or RANKL signaling has been disrupted. Extracellular nucleotides cause calcium rises in osteoclasts, both via calcium influx and release of calcium stores [15]. P2X2, P2X4, and P2X7 have been identified in rat osteoclasts [16]; P2Y2 is expressed but does not localize to the cell surface and does not appear to be functional [17]. Gartland et al. performed an analysis of P2X7 expression in vivo and in human osteoclastic cells derived by in vitro culture of human peripheral blood mononuclear cells with m-CSF and RANKL and demonstrated P2X7 throughout at various stages in osteoclast differentiation using a blocking extracellular monoclonal antibody [18]. This antibody inhibited the formation of multinucleated osteoclasts from human peripheral blood monocytes. In the presence of this antibody, m-CSF- and RANKL-treated cells expressed tartrate-resistant acid phosphatase, a phenotypic marker for osteoclast differentiation, in spite of the fact that they did not become multinucleated. Osteoclast resorptive activity, assessed on dentine discs, was also markedly inhibited by antibody treatment.
P2X7 gene-deleted mice have been generated, are fertile, and appear relatively normal [19]. Bone formation and resorption in these mice at 2 and 9 months of age is abnormal [20]. Although the femurs are normal in length, they have a significant reduction in periosteal circumference. There is also a decrease in cortical bone content and periosteal bone formation. Furthermore, bone resorption is increased and osteoclasts appeared normal although increased in number. Thus, activity of the osteoclast compartment appears to be enhanced in the absence of P2X7.
Gartland et al. specifically examined the ability of mononuclear precursor cells to generate multinucleated osteoclasts in vivo and in vitro in the P2X7-null mice and confirmed that multinucleation of osteoclasts did indeed occur in both instances [21]. Cells from P2X7-null cells were able to undergo plasma membrane permeabilization in response to maitotoxin, which operates a pore with characteristics similar to those induced by P2X7 ligation. It had been previously proposed that P2X7 may not be itself the ATP-induced pore, but may instead activate a pore that also can be activated by maitotoxin [22]. Thus, the above findings may suggest that while P2X7 may not be an integral part of the fusion machinery, it may promote osteoclast fusion by activating a fusogenic pore-forming plasma membrane protein that is currently unidentified.
The bone phenotype seen in the P2X7-null mouse may or may not be due to absence of P2X7 from osteoclasts, since osteoblasts also express P2X7. Osteoblastic cell lines [23–25], rat primary osteoblasts [26], and primary cultures of human osteoblasts [24] all express P2 receptors. Several members of both P2X and P2Y classes are present on the surface of human and rat osteoblastic cells, in particular, P2Y2, P2X2, and P2X5 [27–29]. P2X7 expression and function has also been demonstrated in murine osteoblasts [30] and osteoblast P2X7 activity is required for normal skeletal response to mechanical stress [31].
Downregulation of P2X7 and inhibition of osteoclast formation in RAW cells
RAW 264.7 (RAW) is a mouse macrophage-like cell line that forms multinucleated osteoclastic cells in vitro in the presence of RANKL. This model is quite reproducible in vitro. When RANKL is added to RAW cells, one sees almost no multinucleation during days 1–, but between days 3 and 4 widespread cell fusion and multinucleation occurs. Although cells remain unfused during the first 3 days, they acquire a number of osteoclast markers, such as tartrate-resistant acid phosphatase. Thus, RAW cells cannot only be used as a model for osteoclast fusion, but allow one to distinguish between osteoclast differentiation, as assessed by acquisition of phenotypic markers, and osteoclast fusion. RAW cells have been used to explore the relationship between P2 receptors, extracellular ATP, and osteoclast fusion [32]. In some macrophage cell lines, incubation of cells expressing P2X7 in ATP for long periods of time selects cells that do not express P2X7-mediated pore formation. When RAW cells were incubated in medium containing 2 mM ATP overnight, some of the cells died but the surviving cells did not become permeable to the fluorescent dye YO-PRO when incubated in ATP (ATP-R cells). This phenotype was reversible: if the cells were subsequently incubated in medium without ATP overnight, they regained ATP-induced permeability to YO-PRO (ATP-S cells). The mechanism for this reversible ATP resistance was explored, and it was discovered that the RAW cells maintained in ATP expressed as much P2X7 protein as did untreated cells, but they expressed no P2X7 on the cell surface. Thus, in untreated RAW cells and in ATP-S cells most of the P2X7 is in an intracellular vesicular pool, but roughly 10% localized to the cell surface. In ATP-R cells, this plasma membrane pool of P2X7 vanishes.
Unlike untreated RAW cells, when ATP-R cells are incubated in medium containing RANKL to induce osteoclastogenesis, cell fusion and giant cell formation is not detected (Fig. 1). Although the ATP-R cells remain unfused, they do become positive for TRAP and other markers of osteoclast differentiation, suggesting that the defect in ATP-R cells is related to the fusion process itself and not to the ability of these cells to undergo osteoclast differentiation, and probably occurs at a late step in the process of osteoclastogenesis. Supporting this hypothesis, if ATP is removed from the medium 72 h after addition of RANKL, cell fusion occurs in the next 24 h. These experiments demonstrate that prolonged incubation of RAW cells in ATP induces internalization of P2X7 and prevents cell fusion and multinucleation. Incubation of RAW cells in BzATP, but not adenosine diphosphate (ADP), uridine diphosphate (UDP), or uridine triphosphate (UTP), yielded similar results, suggesting that P2X7 or another P2X receptor is involved in inhibition of osteoclast fusion in this model system.
Prolonged incubation in ATP prevents osteoclast formation but not acquisition of TRAP positivity. RAW cells made resistant to the permeabilizing effect of extracellular ATP by prolonged incubation in ATP (RAW ATP-R) or RAW cells that regained ATP sensitivity after removal of ATP (RAW ATP-S) were incubated in medium with or without RANKL for 4 days, fixed, and stained for tartrate-resistant acid phosphatase (TRAP). RAW ATP-S cells formed multinucleated osteoclasts in response to RANKL, but RAW ATP-R cells did not. Nevertheless, RAW ATP-R cells became TRAP positive, indicating that osteoclast differentiation was not totally inhibited in these cells
Given that P2X7-null mice form multinucleated osteoclasts, several possible interpretations of the above data arise. It is possible that P2X7 is indeed directly involved in mononuclear cell fusion, but that its role in mononuclear cell fusion can be taken over by other receptors in its absence. Perhaps other P2X receptors can fulfill this role, and preliminary data demonstrate that RAW cells express P2X4 and P2X6 in addition to P2X7. In this context, the fact that P2X7 monoclonal antibodies inhibit fusion may indicate either that these antibodies affect other P2X7 receptors, that the other receptors that mediate fusion are not present or upregulated in this situation, or that the presence of P2X7-P2X7 antibody complexes on the cell surface exerts an inhibitory effect on the process of fusion. Another possibility is that P2X7 plays a less direct role in the fusion process, perhaps by influencing a signaling cascade that is involved in the fusion process, and which may involve one or more of the other proteins that have been implicated in mononuclear cell fusion.
The above RAW cell experiments may suggest that extracellular ATP itself is an important regulator of multinucleation, and the pharmacological data imply that this occurs through activation of P2X receptors. To determine whether the inhibitory effect of prolonged incubation in extracellular ATP requires the presence of P2X7, ATP incubation experiments similar to the RAW cell experiments described above were performed with bone marrow mononuclear cells derived from P2X7-null mice (Hiken and Steinberg, manuscript in preparation). These experiments could not be performed with cells from wild-type mice, because prolonged exposure to ATP killed the cells. However, when cells from P2X7-null mice were incubated in 2 mM ATP, most cells survived. Bone marrow cells from either wild-type or P2X7-null mice incubated in medium containing m-CSF and RANK formed TRAP-positive multinucleated osteoclasts. In contrast, cells from P2X7-null mice that had been incubated in ATP did not fuse, although they did acquire TRAP positivity. Thus, prolonged exposure of osteoclast precursors to ATP inhibits osteoclast fusion by a process that does not absolutely require P2X7. Prolonged exposure of RAW cells to ATP downregulated P2X7, and it seems reasonable to speculate that other P2 receptors are similarly removed from the plasma membrane by incubation of cells in ATP.
Conclusions
Although there is considerable interest in defining the mechanism of macrophage multinucleation, this process remains poorly defined. The recent demonstration that DC-STAMP-null mice are unable to form multinucleated giant cells constitutes a major advance in this field, and the relationship between DC-STAMP and P2 receptors remains to be determined. Although much evidence suggests that P2X7 influences the multinucleation process, P2X7 is not absolutely required for osteoclast formation, and it is unclear whether other P2X receptors are involved in giant cell formation and if they can compensate for the lack of P2X7. Extracellular ATP alters giant cell formation in vitro, as consumption of extracellular ATP enhances giant cell formation and prolonged incubation in extracellular ATP inhibits osteoclast fusion. These observations suggest that extracellular ATP may be an important modulator of giant cell formation and osteoclastogenesis in vivo.
References
Anderson JM (2000) Multinucleated giant cells. Curr Opin Hematol 7(1):40–7
Cui W, Zhang KJ, Zhang Q, Ke HZ, Chalouni C, Vignery A (2005) The intracellular domain of CD44 promotes the fusion of macrophages. Blood 107(2):796–05
Sterling H, Saginario C, Vignery A (1998) CD44 occupancy prevents macrophage multinucleation. J Cell Biol 143(3):837–47
Saginario C, Sterling H, Beckers C, Kobayashi R, Solimena M, Ullu E et al (1998) MFR, a putative receptor mediating the fusion of macrophages. Mol Cell Biol 18(11):6213–223
Han X, Sterling H, Chen Y, Saginario C, Brown EJ, Frazier WA et al (2000) CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J Biol Chem 275(48):37984–7992
Ohgimoto S, Tabata N, Suga S, Nishio M, Ohta H, Tsurudome M et al (1995) Molecular characterization of fusion regulatory protein-1 (FRP-1) that induces multinucleated giant cell formation of monocytes and HIV gp160-mediated cell fusion. FRP-1 and 4F2/CD98 are identical molecules. J Immunol 155(7):3585–592
Namba K, Nishio M, Mori K, Miyamoto N, Tsurudome M, Ito M et al (2001) Involvement of ADAM9 in multinucleated giant cell formation of blood monocytes. Cell Immunol 213(2):104–13
Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K et al (2004) RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med 200(7):941–46
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N et al (2005) DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202(3):345–51
Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, Di VF (1995) The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest 95(3):1207–216
Chiozzi P, Sanz JM, Ferrari D, Falzoni S, Aleotti A, Buell GN et al (1997) Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol 138(3):697–06
Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, Herren S et al (1998) Blockade of human P2X7 receptor function with a monoclonal antibody. Blood 92(10):3521–528
Falzoni S, Chiozzi P, Ferrari D, Buell G, Di VF (2000) P2X(7) receptor and polykarion formation. Mol Biol Cell 11(9):3169–176
Mizuno K, Okamoto H, Horio T (2001) Heightened ability of monocytes from sarcoidosis patients to form multi-nucleated giant cells in vitro by supernatants of concanavalin A-stimulated mononuclear cells. Clin Exp Immunol 126(1):151–56
Yu H, Ferrier J (1994) Mechanisms of ATP-induced Ca2+ signaling in osteoclasts. Cell Signal 6:905–14
Hoebertz A, Townsend-Nicholson A, Glass R, Burnstock G, Arnett TR (2000) Expression of P2 receptors in bone and cultured bone cells. Bone 27(4):503–10
Bowler WB, Birch MA, Gallagher JA, Bilbe G (1995) Identification and cloning of human P2U purinoceptor present in osteoclastoma, bone, and osteoblasts. J Bone Miner Res 10:1137–145
Gartland A, Buckley KA, Bowler WB, Gallagher JA (2003) Blockade of the pore-forming P2X7 receptor inhibits formation of multinucleated human osteoclasts in vitro. Calcif Tissue Int 73(4):361–69
Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH et al (2001) Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem 276(1):125–32
Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT et al (2003) Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol Endocrinol 17:1356–367
Gartland A, Buckley KA, Hipskind RA, Perry MJ, Tobias JH, Buell G et al (2003) Multinucleated osteoclast formation in vivo and in vitro by P2X7 receptor-deficient mice. Crit Rev Eukaryot Gene Expr 13(2–):243–53
Schilling WP, Wasylyna T, Dubyak GR, Humphreys BD, Sinkins WG (1999) Maitotoxin and P2Z/P2X(7) purinergic receptor stimulation activate a common cytolytic pore. Am J Physiol 277(4 Pt 1):C766–C776
Reimer WJ, Dixon SJ (1992) Extracellular nucleotides elevate [Ca2+]i in rat osteoblastic cells by interaction with two receptor subtypes. Am J Physiol 263(5 Pt 1):C1040–C1048
Schofl C, Cuthbertson KSR, Walsh CA, Mayne C, Cobbold P, von zur Muhlen A et al (1994) Evidence for P2-purinoceptors on human osteoblast-like cells. J Bone Miner Res 7:485–91
Yu H, Ferrier J (1993) Osteoblast-like cells have a variable mixed population of purino/nucleotide receptors. FEBS Lett 328:209–14
Gallinaro BJ, Reimer WJ, Dixon SJ (1995) Activation of protein kinase C inhibits ATP-induced [Ca2+]i elevation in rat osteoblastic cells: selective effects on P2Y and P2U signaling pathways. J Cell Physiol 162(3):305–14
Bowler WB, Birch MA, Gallagher JA, Bilbe G (1995) Identification and cloning of human P2U purinoceptor present in osteoclastoma, bone, and osteoblasts. J Bone Miner Res 10:1137–145
Hoebertz A, Townsend-Nicholson A, Glass R, Burnstock G, Arnett TR (2000) Expression of P2 receptors in bone and cultured bone cells. Bone 27(4):503–10
Jørgensen NR, Geist ST, Civitelli R, Steinberg TH (1997) ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol 139:497–06
Gartland A, Hipskind RA, Gallagher JA, Bowler WB (2001) Expression of a P2X7 receptor by a subpopulation of human osteoblasts. J Bone Miner Res 16(5):846–56
Li J, Liu D, Ke HZ, Duncan RL, Turner CH (2005) The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem 280(52):42952–2959
Hiken JF, Steinberg TH (2004) ATP downregulates P2X7 and inhibits osteoclast formation in RAW cells. Am J Physiol Cell Physiol 287(2):C403–C412
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Steinberg, T.H., Hiken, J.F. P2 receptors in macrophage fusion and osteoclast formation. Purinergic Signalling 3, 53–57 (2007). https://doi.org/10.1007/s11302-006-9036-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-006-9036-9