Abstract
Russet formation in apples (Malus domestica Borkh.) is a superficial skin disorder that detracts from fruit appearance and therefore is a breeding target in many apple scion breeding programs. Previous studies have reported quantitative trait loci (QTLs) for russet formation, but these results have been limited to a narrow genetic background, which reduces breeding utility. In this study, 14 breeding parents were represented in 13-full sib families, nine of which were ‘Honeycrisp’-derived while the other four were derived from ‘Minneiska’, an offspring of ‘Honeycrisp.’ Two previously reported QTLs, on linkage group (LG) 2 and LG6, were confirmed and characterized for the first time in ‘Honeycrisp’-derived germplasm. Five other putative QTLs were also detected. The QTLs characterized in this study could be targeted for DNA test development to be used in marker-assisted selection. Non-additive interactions were observed at and between the LG2 and LG6 QTLs. Future work should include validation of QTL and haplotype effects in other germplasm, development of trait-predictive DNA tests for use in apple breeding programs, and exploration of genome-wide prediction models for russet formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruit appearance is an important aspect of apple (Malus domestica Borkh.) fruit quality. Fruits with poor appearance are often downgraded from fresh market to processing, which can result in a three-to-four-fold loss of wholesale income (USDA National Agricultural Statistics Service 2021). Russet formation is one contributor to poor appearance in apple fruit that can reduce grower packout and therefore is an important target in many apple scion breeding programs (Baugher et al. 1996).
Russet formation is caused by microcracking of the fruit skin and is hypothesized to be the result of the production and transportation of suberin, a cork-like substance, to the outer dermal layers of the apple fruit (Faust and Shear 1972). Microcracking can be induced by several abiotic and biotic factors such as high humidity, frost damage, fungal pathogens, and management practices (Faust and Shear 1972). Several apple cultivars (e.g. ‘Golden Russet’, ‘Chestnut Crab’, and ‘Egremont Russet’) are prone to severe russet formation while other cultivars will only develop russet under conducive environmental conditions.
Phenotyping russet formation can be challenging due to the strong influences of environmental conditions on russet formation and challenges associated with subjective visual ratings. Russet formation is strongly influenced by environmental conditions (e.g., humidity) and, therefore, can vary from year to year and even tree to tree within a given year (Faust and Shear 1972; Sugar et al. 2005; Eccher and Hajnajari 2006). Typically, breeders estimate the amount of russet formation with visual ratings. Differing fruit background colors and/or shapes can make russet formation appear more or less prominent. Previous studies have attempted to address these challenges by using binary or categorical bins to classify russet formation or using labor intensive techniques to obtain precise russet formation measurements (Scharwies et al. 2014; Falginella et al. 2015; Lashbrooke et al. 2015; Kunihisa et al. 2016; Takeuchi et al. 2021).
Several quantitative trait loci (QTLs) associated with russet formation have been previously reported for apple and pear (Pyrus), a genus having a high degree of genomic co-linearity with Malus (Celton et al. 2009). In apple, russet formation QTLs have been reported on linkage groups (LGs) 2, 4, 6, 8, 10, 11, 12, and 15 (Kunihisa et al. 2014; Falginella et al. 2015; Lashbrooke et al. 2015). Two major QTLs have been reported to play important roles in russet formation in Japanese (Pyrus pyrifolia (Burm. F.) Nakai) and Chinese (P. ussuriensis Maxim., P. bretschneideri Rehder) pear species. The R locus, a dominant locus identified on LG8 of Japanese pear, has been reported in several studies to be associated with russet formation. The I locus, a modifier genetic factor, is associated with russet severity but has not been mapped. In addition, studies have reported other small-effect QTLs associated with russet formation in Japanese pear (Wang et al. 2016; Takeuchi et al. 2021). Although russet formation is an attractive target for DNA-informed breeding (e.g., marker-assisted seedling selection), breeders are limited by the few DNA tests that are currently available.
Current understanding of the genetic basis of russet formation in apple is insufficient and has to-date been limited to a relatively narrow set of germplasm. The important modern US cultivar and breeding parent ‘Honeycrisp’ was not represented in previous studies and russet formation has been commonly observed in its ancestors, ‘Frostbite,’ ‘Keepsake,’ and ‘Golden Delicious.’ Russet formation has also been observed in germplasm derived from ‘Honeycrisp’ in the University of Minnesota (UMN) apple breeding program, including on fruit of ‘Minneiska' (SweeTango® apple). There currently are no predictive markers available for DNA-informed breeding for russet formation on apples in this germplasm. To address this limitation, QTLs associated with russet formation must be detected and/or validated in relevant breeding germplasm, QTL alleles need to be characterized, and predictive DNA tests for russet formation QTLs need to be developed. The goal of this study was to examine the genetic basis of russet formation in a pedigree-connected germplasm set relevant to apple scion breeding programs that utilize ‘Honeycrisp’-derived germplasm. The objectives of this study were to (1) identify QTLs associated with russet formation and (2) characterize QTL allele effects. We hypothesized that multiple QTLs for russet formation would be detected.
Materials and methods
Germplasm
Thirteen pedigree-connected full-sib families (n = 1009 offspring) were evaluated in this study (Table 1). These thirteen families were grouped into two populations. The first population consisted of individuals from nine families with ‘Honeycrisp’ as a common parent. The other parents in this population were ‘Jonafree,’ ‘Pitmaston Pine Apple,’ ‘Minnewashta,’ ‘WA 2,’ and Arkansas selection AA44, which is also known as ‘MonArk’ but will be referred to as AA44 throughout, and the UMN advanced selections MN1702, MN1764, MN1836, and MN1915. The second population consisted of individuals from four families with ‘Minneiska’ (‘Honeycrisp’ × ‘Minnewashta’) as a common parent. The other parents in this population were ‘Wildung,’ ‘MN55,’ and the UMN advanced selections MN1702 and MN1965.
Phenotypic data
Individuals were evaluated for russet formation from 2017 to 2019 at the UMN Horticultural Research Center in Chanhassen, MN, USA, with each individual being evaluated in up to 3 years. Trees were grown on B9 and/or G16 rootstock. Three to 10 fruit per individual were harvested within a given year from August to October. Differences in the number of harvested individuals and families across years were in part due to biennial bearing and juvenility of certain families that had harvestable fruit in the later years (Table 1).
Individual fruit were rated visually (1–10 scale) for six russet formation categories: overall russet, and percentages of russet formation covering stem cavity, shoulders, body, lenticels, and calyx (Fig. 1). Overall russet category described the amount of russet formation across the whole apple. The stem, shoulders, body, and calyx russet categories were the amount of russet formation within the shaded regions described below in Fig. 1. The lenticel russet category was determined by the percentage of lenticels that had russet regardless of severity. Russet formation ratings were assigned using the following scale: 1 (0–10% affected), 2 (11–20%), 3 (21–30%), 4 (31–40%), 5 (41–50%), 6 (51–60%), 7 (61–70%), 8 (71–80%), 9 (89–90%), and 10 (91–100%). Ratings were averaged by individual for each trait to capture average russet severity within a given year. Best linear unbiased predictions (BLUP) for visual ratings were estimated across years and used as phenotypic values in QTL analyses. These BLUP values will be referred to as “russet ratings” throughout this paper.
Genotypic data
Individuals were genotyped via the International ROSBREED SNP Consortium 8 K Illumina Infinium® array v1 or the Illumina Infinium 20 K array (Chagné et al. 2012; Bianco et al. 2014) with DNA extracted as described by Clark et al. (2014). SNP data were filtered to include SNPs common to both arrays for a total of 2213 SNPs across 17 linkage groups (Vanderzande et al. 2018). Genetic positions from the iGL genetic map (Di Pierro et al. 2016) updated as described in Howard et al. (2021) were used for the SNPs included in this study.
QTL analyses
QTL analyses were conducted with FlexQTL™ software (www.flexqtl.nl), a pedigree-based QTL analysis software that uses Markov Chain Monte Carlo (MCMC) simulation (Bink et al. 2002, 2008, 2012). Parameters for analyses are reported in Supplementary Table S1, and MCMC convergence was considered reached if effective chain size lengths for the estimates of trait means, numbers of QTLs, and their variances reached at least 100 (as used in Bink et al. 2014; Kostick et al. 2021). Phenotypic data were only included for unselected offspring to avoid selection bias (as in Howard et al. 2018; Verma et al. 2019; Kostick et al. 2021).
QTL significance was determined using Bayes factors (BF) and posterior intensity values. Evidence for QTLs on individual linkage groups was considered none (< 2), positive (2 to < 5), strong (5 to < 10), or decisive (10 ≤) based upon the Bayes factor (2lnBF10) range given in the parentheses (Kass and Raftery 1995). QTL regions for QTLs with strong to decisive evidence were determined by the same method reported in Howard et al. (2018). QTL intervals were defined in a series of successive 2 cM “bins,” chromosomal segments, that had a BF above 5 and the mode within a given QTL region was considered the most probable QTL position. The proportion of phenotypic variance explained by a QTL was estimated by dividing the reported variance explained by the whole phenotypic variance provided by FlexQTL™ software (Verma et al. 2019). QTL intervals were then compared to previous literature alongside the GDDH13v1.1 reference genome (Daccord et al. 2017) to determine if multiple QTLs appear to colocalize in the same region.
Haplotype characterization
QTLs were targeted for haplotype characterization if they had decisive evidence (BF > 10) for the presence of a QTL. SNPs included for haplotyping were chosen based on their proximity to QTL peaks. Proximity was determined by including SNPs that fell in the 2 cM bins on either side of the QTL peak were included with redundant markers, based upon parental haplotypes removed. Marker phasing was performed through FlexQTL™ software. Haplotypes were traced through extended pedigrees to the furthest known ancestors using techniques described in Howard et al. (2018), and employed in Luby et al. (2022), to reconstruct pedigrees in the UMN apple breeding program. Additionally, unpublished extended pedigree information for ‘WA 2’ generated from the project described by Howard et al. (2018) was used. Identical haplotypes were described as identical-by-descent (IBD) if they could be traced to a common ancestor whereas identical haplotypes that could not be traced to a common ancestor were considered identical-by-state (IBS). When necessary, imputation of missing SNP information was performed via an examination of progeny and ancestors, as described by Kostick et al. (2021). Recombinants and haplotypes that were unable to be resolved were excluded from further statistical analyses.
For each QTL, analysis of variance (ANOVA) was used to examine if parental haplotypes varied significantly for russet rating in the offspring. Using mean separation via Tukey’s honest significant difference (HSD) test, haplotypes at a given QTL were grouped into relative functional allele groups based on their relative effects (i.e., low, moderate (non-significant), and high relative levels of russet ratings). Functional haplotype effects were determined using a combination of Tukey’s HSD results and haplotype russet rating mean values and ranges.
To examine the effects of haplotypes across QTLs, individuals were grouped by functional (i.e., low, moderate, high) QTL genotypes across the targeted QTLs, and a two-way ANOVA was used to determine if compound QTL genotypes differed significantly for russet ratings.
Results
Phenotypic data
A total of 60% of individuals were harvested in at least 2 years. Raw family means, standard deviations, and ranges for BLUP-adjusted shoulder and lenticel russet data are presented in Table 2, while all other trait data by family are presented in Supplementary Table 2. Russet rating distributions were often skewed towards low russet ratings. Overall and body russet ratings were highly correlated (r ≥ 0.8 to 0.9) across years and had means ranging from 1.2 to 1.8 for overall and 1.1 to 1.6 for body russet ratings. Shoulder russet ratings were also highly to moderately correlated with overall and body russet ratings (r ≥ 0.4 to 0.7) across years and had means ranging from 1.5 to 2.9 russet ratings (Table 2, Supplementary Table S3). Year had a significant effect (P < 0.001) on shoulder and lenticel russet formation traits (Supplementary Table S4). In 2018, incidence of russet formation was low (2018 shoulder russet rating mean = 1.6, mean overall years = 2.0). Mean shoulder russet ratings for families were within two points of the population mean and were at the lower end of the russet rating scale. Despite skewed distributions, family effects were significant (p < 0.001, Supplementary Table S4). Families had standard deviations that varied from 0.46 to 2.05 and had ranges within families from 1–3.5 to 1–10 for shoulder russet formation (Table 2). Minimal phenotypic variation for stem, body, calyx, and overall russet ratings was observed within and among families. Therefore, QTL analyses were not carried out for stem, body, calyx, and overall russet ratings. Quantitative variation among and within families for shoulder russet ratings was observed although ratings were skewed towards low values. Lenticel russet ratings were evenly distributed over the entire range of the rating scale (Table 2).
QTL detection
Two QTLs on LG2 and LG6 were detected with decisive (Bayes Factor; BF; 2lnBF10 > 10) evidence and five QTLs were detected with strong (BF > 5) evidence for shoulder russet formation (Table 3). QTL intervals with decisive evidence were located at 48–54 cM and 57–69 cM for LG2 and LG6, respectively. FlexQTL™ software runs with lenticel russet rating data failed to reach convergence and, thus, failed to detect large-effect QTLs.
Haplotype analysis
Linkage group 2 haplotype analysis
Twelve parental LG2 QTL haplotypes, constructed from 11 SNPs spanning 4.3 cM (6.24 Mbp), segregated in the thirteen families (Supplementary Tables S5 and S6). Offspring that inherited haplotypes 2B (n = 28), 2G (n = 47), and 2 J (n = 282) exhibited low relative shoulder russet ratings, with adjusted BLUP means of 1.4, 1.6, and 1.6, respectively. In contrast, offspring that inherited 2A (n = 561), 2D (n = 48), 2H (n = 75), 2 L (n = 29), and 2F (n = 30) exhibited high relative shoulder russet ratings, with mean ratings of 2.3, 2.4, 2.6, 2.9, and 3.0, respectively. Offspring that inherited 2C (n = 444), 2E (n = 113), 2I (n = 68), and 2 K (n = 78) demonstrated shoulder russet ratings were similar to average russet ratings of the alternate haplotype and had moderate average shoulder russet ratings, with mean ratings of 1.9, 1.9, 1.9, and 2.0, respectively (Fig. 2).
Distributions of shoulder russet ratings for offspring haplotypes for both LG2 and LG6 QTLs. Haplotypes are in ascending order by the adjusted BLUP means for each group. Numbers of individuals with one or more copies of each haplotype are listed above the boxplot. Total number of individuals represented in the LG 2 haplotype figure (n = 984) and LG 6 haplotype figure (n = 997). Individuals with unresolved or recombinant haplotypes were not included in distributions
The low shoulder russet formation haplotype, 2 J, of ‘Minneiska,’ ‘Minnewashta,’ and the UMN selection MN1915 was likely inherited from ‘Tetofsky’, through ‘Goodland.’ Other sources for low shoulder russet formation haplotypes (2B, 2G) were ‘Aspa’ (2B) and ‘McIntosh’ (2G). The high shoulder russet formation haplotype, 2A, of ‘Honeycrisp,’ ‘MN55,’ ‘Minneiska,’ MN1764, and MN1836 was likely inherited from ‘Northern Spy.’ Other sources for high shoulder russet formation haplotypes (2D, 2F, 2H, 2L) were ‘Rome Beauty’ (2D), ‘Cox’s Orange Pippin’ (2D), ‘Reinette Franche’ (2F), PRI selection 855–102 (2H), AA44 (2H), ‘Golden Delicious’ (2H), and ‘Pitmaston Pine Apple’ (2L). All LG2 haplotypes, including the four LG2 QTL haplotypes, which had moderate effects and their ancestral origins are described in Supplementary Table S7.
Linkage group 6 haplotype analysis
Six parental LG6 QTL haplotypes, constructed from nine SNPs spanning 2.8 cM (0.59-Mbp), segregated in the thirteen families (Supplementary Table S5 and S6). Offspring that inherited haplotypes 6D (n = 446), 6E (n = 577), and 6B (n = 29) demonstrated low shoulder russet ratings, with adjusted BLUP means of 1.9, 1.8, and 1.8, respectively. In contrast, offspring that inherited 6C (n = 67), 6F (n = 627), and 6A (n = 27) demonstrated relatively high shoulder russet ratings, with mean ratings of 2.1, 2.4, and 3.5, respectively (Fig. 2).
All LG6 QTL haplotypes are described with their ancestral origins in Supplementary Table S8. The low shoulder russet formation haplotype of ‘Honeycrisp,’ AA44, ‘Jonafree,’ ‘Minneiska,’ ‘Minnewashta,’ and MN1702 (haplotype 6E) was traced to multiple ancestors, including ‘Duchess of Oldenburg,’ ‘Northwest Greening,’ and ‘Malinda.’ Other sources for low shoulder russet formation haplotypes (6B, 6D) were traced to multiple sources including ‘Frostbite’ (6B), MN1764 (6D), ‘McIntosh’ (6D), ‘Northern Spy’ (6D), ‘Spasovka Kvasna’ (6D), ‘Minnewashta’ (6D) ‘Reinette Franche’ (6D), and ‘Golden Delicious’ (6D). The common high shoulder russet formation haplotype 6F of ‘Honeycrisp,’ ‘Minneiska,’ ‘MN55,’ MN1702, MN1764, and MN1965 was traced to ‘Frostbite.’ Other sources for high shoulder russet formation haplotypes (6A, 6C) were ‘Winter White Pearmain’ (6A), ‘Pitmaston Pine Apple’ (6C), and ‘Jonathan’ (6C).
Combined LG2 and LG6 QTL effects
Phenotypic variances explained by the LG2, LG6, and the two QTLs combined were 4%, 8%, and 12%, respectively (Table 3). Together, LG2 and LG6 QTLs had significant effects on shoulder russet formation (Supplementary Table S4). In general, the higher the number of high shoulder russet formation haplotypes an individual had across QTLs, the higher the shoulder russet rating (Fig. 3). However, non-additive interactions among haplotypes were observed. The presence of at least two low shoulder russet formation haplotypes, at either or both loci, resulted in lower shoulder russet ratings (Fig. 3). In the presence of zero or one low shoulder russet formation haplotype, the mean shoulder russet ratings for offspring with one (n = 74), two (n = 247), three (n = 112), or four (n = 5) high shoulder russet formation haplotypes were 1.61, 2.78, 3.17, and 4.23, respectively. In the presence of two or more low shoulder russet formation haplotypes, the mean shoulder russet ratings for offspring with zero (n = 97), one (n = 290), or two (n = 122) high shoulder russet formation haplotypes were 1.59, 1.54, and 1.80, respectively (Supplementary Table S9).
Distributions of shoulder russet ratings for offspring compound functional QTL genotypes across the two QTLs. Low (L) shoulder russet formation haplotypes were 2B, 2G, 2 J, 6B, 6D, and 6E. Moderate (M) shoulder russet formation haplotypes were 2C, 2E, 2I, and 2 K. High (H) shoulder russet formation haplotypes were 2A, 2D, 2F, 2H, 2L, 6A, 6C, and 6F. Numbers of individuals within each functional group are listed above the boxplot. Total number of individuals represented was 974
Discussion
This is the first report of QTLs associated with shoulder russet formation in ‘Honeycrisp’-derived families and should be of interest to numerous apple scion breeders now using ‘Honeycrisp’ and its descendants in their breeding programs. Two QTLs for shoulder russet formation were characterized on LG2 and LG6 in a ‘Honeycrisp’-derived germplasm set. A total of eight high shoulder russet formation haplotypes and six low shoulder russet formation haplotypes were characterized. Findings of this study could help inform breeding decisions and/or be used to develop DNA tests for breeding use.
Quantitative variation for russet formation observed within germplasm set
Most russet formation occurred within the shoulder region or on the lenticels, and therefore these two russet ratings were examined in this study. Shoulder russet ratings were highly correlated with overall russet ratings, a common russet formation metric used in other studies (Kunihisa et al. 2014; Falginella et al. 2015; Lashbrooke et al. 2015). Therefore, use of a single russet formation metric is likely sufficient for russet formation QTL detection. Previous studies that investigated the genetic architecture of russet formation captured higher levels of russet formation within their populations but relied upon biparental populations having at least one parent being considered susceptible to russet formation (Falginella et al. 2015; Lashbrooke et al. 2015). In contrast, most of the parents of families evaluated in the present study are not known for high levels of russet formation, but high shoulder russet formation of individual offspring has been observed within the UMN breeding program. The variation of shoulder russet ratings observed in this study, both within and among families, and the identification of two large effect QTLs as well as the multiple additional putative QTLs suggested multi-genic control for shoulder russet formation in this germplasm.
Identities of linkage group 2 and 6 QTLs
Both LG2 and LG6 QTLs colocalized with previously reported russet formation QTLs in apple. The LG2 QTL (17.48 – 27.04 Mbp) detected in this study colocalized with the LG2 russet and percent russet QTLs (~ 17.5 Mbp) previously reported by Lashbrooke et al. (2015), the LG2 ‘Akane’ russet-calyx QTL (~ 25.5-Mbp) reported by Kunihisa et al. (2014), and possibly with the LG2 russet QTL (~ 36.9-Mbp) reported by Falginella et al. (2015). The LG6 QTL (32.31 – 34.76 Mbp) detected in this study could potentially be the same as the ‘Akane’ russet-pedicel QTL (~ 28.9 Mbp) reported in Kunihisa et al. (2014).
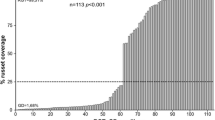
Of the other putative russet formation QTLs detected in this study, both the LG15 QTL (13.46–15.49 Mbp) and the LG12 QTL (25.94–31.30 Mbp) had previously reported QTLs near the QTL region. Lashbrooke et al. (2015) reported a QTL for tensile strength of the fruit skin (~ 12 Mbp) which was used to indirectly measure susceptibility to form russet. Falginella et al. (2015) reported a major QTL for increased levels (> 25% overall coverage) of russet formation with a QTL peak at ~ 28.9 Mbp.
Linkage groups 2 and 6 shoulder russet formation QTLs colocalized with important disease resistance QTLs
The LG2 and LG6 shoulder russet formation QTLs detected in this study colocalized with disease resistance QTLs. The LG2 apple scab resistance QTL (~ 31.1 Mbp) reported by Calenge et al. (2004), colocalized with the LG2 russet formation QTL detected in this study (Calenge et al. 2004; Galli et al. 2010; Bus et al. 2011). The LG6 russet formation QTL in this study colocalized with a fire blight resistance/susceptibility QTL (31.22–36.72 Mbp) reported by Kostick et al. (2021). To our knowledge, there is no established relationship in the literature between russet formation and either of these diseases. The association between disease resistance traits and the russet formation QTLs could be examined in future studies. Colocalization of multiple trait loci demonstrates the importance of considering these regions when making breeding decisions. Breeders could leverage knowledge of the different allele effects at these trait loci to develop high quality apple cultivars more efficiently.
Unique high and low shoulder russet formation haplotypes at linkage group 2 QTL in ‘Minneiska’
This is the first report of a QTL associated with shoulder russet formation in ‘Minneiska’ derived families. Haplotype analysis indicated that ‘Minneiska’ was heterozygous for the LG2 QTL. Found in ‘Minneiska’ and its progenitors, haplotype 2 J was significantly associated with reduced levels of shoulder russet ratings. Haplotype 2 J traced back to ‘Tetofsky,’ an old Russian cultivar that is described as having thicker, waxy skin, although russet formation is not mentioned (Beach et al. 1905). ‘Minneiska’ is described as having variable levels of russet formation, starting at the stem end with the potential to have extensive netting spreading out and down the shoulder area (Bedford and Luby 2008). Offspring of ‘Minneiska’ crosses also varied in their range of shoulder russet ratings depending on the other parent. In general, when 2 J was inherited from ‘Minneiska,’ or its parent ‘Minnewashta,’ and paired with other low shoulder russet formation haplotypes, offspring had low shoulder russet ratings. The other haplotype of ‘Minneiska’ (2A) was represented in all families and could be traced back to ‘Northern Spy’; all 2A haplotypes, except from MN1764, were inherited through ‘Honeycrisp’ directly or via ‘Minneiska.’ Haplotype 2A was significantly associated with increased levels of shoulder russet ratings.
High shoulder russet formation haplotypes at linkage group 2 QTL in ‘Pitmaston Pine Apple’
The presence of multiple putative high shoulder russet formation haplotypes, 2F and 2L, in ‘Pitmaston Pine Apple’ was not surprising, as ‘Pitmaston Pine Apple’ is a full russet-type apple cultivar (Bussey 2016). Offspring of ‘Pitmaston Pine Apple’ had an average shoulder russet rating of 2.9, the highest family average for shoulder russet rating along with ‘Honeycrisp’ × ‘WA 2.’ Due to limited pedigree information, only one haplotype (2F) could be traced back through an extended pedigree to ‘Reinette Franche,’ an ancestor that is prone to russet formation on the stem and calyx end of its fruit (Bussey 2016). ‘Reinette Franche’ is an important ancestor in the background of many cultivars today (Muranty et al. 2020); therefore, the high shoulder russet formation haplotype 2F might segregate in other breeding germplasm. The effects of haplotypes 2F and 2L should be considered putative and targeted for validation in future studies due to relatively low representation in this study (n = 60).
Unique high shoulder russet formation haplotype at linkage group 6 QTL that colocalizes with a fire blight QTL in ‘Honeycrisp’
‘Honeycrisp’ was heterozygous at the LG6 russet formation QTL based on haplotype analysis. High shoulder russet formation haplotype 6F, found in ‘Honeycrisp,’ ‘Minneiska,’ ‘MN55,’ MN1702, MN1764, and MN1965, could be traced back to ‘Frostbite,’ a cultivar that has been reported to be prone to russet formation (Bussey 2016). This QTL region was reported by Kostick et al. (2021) to be associated with fire blight susceptibility. The ‘Honeycrisp’ fire blight allele that traced back to ‘Frostbite’ was associated with a reduced susceptibility to fire blight. Knowledge of this potential association might be important for breeders who are breeding for apples with low shoulder russet formation that are also fire blight resistant. To our knowledge, the 6F high shoulder russet formation haplotype is unique to germplasm derived from ‘Frostbite.’
Very high shoulder russet formation haplotype at linkage group 6 QTL in ‘WA 2’
The presence of a putative very high shoulder russet formation haplotype, 6A, only found in the ‘WA 2’ cross, was not surprising. While ‘WA 2’ is described as not developing russet in commercial growing areas of Washington State (Evans et al. 2010), shoulder russet formation has been noted when ‘WA 2’ is grown in Europe (Evans, personal communication). Offspring of ‘WA 2’ (n = 98) had an average shoulder russet rating of 2.9, and those having a 6A haplotype (n = 27) had an average rating of 3.5. Although based on mean separation analyses, haplotype 6A was significantly different from the other high shoulder russet formation haplotypes (Supplementary Table S8), due to the lack of representation in multiple families, we categorized it within the same group as other high haplotypes. Haplotype 6A was traced back to ‘Winter White Pearmain,’ an old cultivar of unknown origin that is reported to sometimes have lenticel russet formation and russet formation within the stem cavity (Bussey 2016). Other potentially important and breeding relevant ancestors that have inherited high shoulder russet formation haplotype 6A from ‘Winter White Pearmain’ are as follows: ‘Delicious,’ ‘Gala,’ ‘Kidd’s Orange Red,’ and ‘Splendour.’
Interactions at and among shoulder russet formation QTLs were non-additive
Unequal representation of haplotypes at and across QTLs made assessment of dominance and/or epistasis complicated. By using an approach of assigning haplotype functional effects and reducing groups into “HIGH,” “MOD” (moderate), and “LOW,” statistical power was increased.
Effects across multiple QTLs have not been previously reported for russet formation in apple. Non-additive effects have been reported within QTL regions (Lashbrooke et al. 2015) and were also seen in this study (Fig. 3). When two low shoulder russet formation haplotypes were present, either within or across QTLs, shoulder russet formation was relatively low. However, further investigation is needed to examine non-additive QTL interactions.
Study limitations
A limitation of this study was the reliance on natural conditions for russet formation development. Russet formation is largely dependent on spring environmental conditions (Barceló-Vidal et al. 2013). In this study, substantial year-to-year variation in spring and summer weather was potentially reflected in the russet rating data. In 2017, a relatively wet spring and early summer might have resulted in more variation among and within families for russet formation and summer hail events reduced the number of harvestable fruit available. In 2018, little variation for russet formation within and among families was observed, which was likely due to dry environmental conditions. In 2019, a relatively wet spring but dry early summer with no severe hail events resulted in a large number of harvestable fruit with an increased incidence of russet formation. By harvesting individuals across years and using adjusted average russet rating data for QTL analysis, a large (n = 1009 individuals) representative germplasm set was used in the analyses.
Small family sizes likely limited the detection of QTLs, characterization of certain haplotypes, and examination of QTL interactions. Uneven representation of some parent genomes, a common challenge in pedigree-based QTL analyses (e.g., Bink et al. 2014; Verma et al. 2019; Kostick et al. 2021; Kostick and Luby 2022), might have led to limited or no representation of russet formation QTLs haplotypes and compound QTL genotypes. However, even with limited representation of certain haplotypes, the haplotypes characterized in this study could enable targeted crosses to be made for future validation studies.
An assumption of this study is that IBS haplotypes have the same functional effects. By looking at haplotype effects within each family, this assumption appears to be justified for most haplotypes represented in this study. Two exceptions were haplotypes 2D and 6C. Haplotype 2D was assigned a high functional effect and was represented in two families, ‘Honeycrisp’ × ‘WA 2’ and ‘Honeycrisp’ × ‘Jonafree.’ In ‘Honeycrisp’ × ‘WA 2,’ the 2D haplotype appeared to act as a high shoulder russet formation haplotype but in the ‘Honeycrisp’ × ‘Jonafree’ family, the 2D haplotype had the lowest mean shoulder russet rating for LG2 haplotypes. A similar inconsistency was seen with haplotype 6C assigned as a high shoulder russet formation haplotype and present in ‘Honeycrisp’ × ‘Pitmaston Pine Apple’ and ‘Minneiska’ × ‘Wildung.’ In ‘Honeycrisp’ × ‘Pitmaston Pine Apple,’ 6C had the highest mean shoulder russet rating for LG6 haplotypes within this family, while in ‘Minneiska’ × ‘Wildung,’ 6C had the lowest mean shoulder russet rating for LG6 haplotypes. In both cases, IBS haplotypes 2D and 6C could not be traced back to a common ancestor and extended haplotypes did not match outside the QTL range. One possible explanation for the differences in mean haplotype values could be the presence of low or high shoulder russet formation alleles at the other QTLs or differences in genomic background. Another explanation is that these IBS haplotypes could be functionally different. Validation of the effects of these haplotypes within a wider germplasm set could clarify if they are functionally different.
Breeding implications
The complexity of the genetic control of russet formation will continue to make development of cultivars with low levels of russet formation challenging. The QTLs characterized in this study accounted for a moderate proportion of variation in the ‘Honeycrisp’-derived germplasm. The LG2 and LG6 QTL allele effects should be validated in other germplasm before targeting these loci for development and deployment of trait-predictive DNA tests.
Low and high shoulder russet formation haplotype information from this study can be used to enable selection for low shoulder russet formation levels in breeding. In the short-term, haplotype information on the individuals characterized in this study could be used for parent selection by selecting for parents with multiple low shoulder russet formation alleles. Shoulder russet formation QTL haplotype information could also be used to develop trait-predictive DNA tests for one or both QTLs. Breeders will need to consider the colocalization of other trait loci (i.e., apple scab resistance/susceptibility; fire blight resistance/susceptibility) when targeting for russet formation. For example, the ‘Honeycrisp’ 6F, a high shoulder russet formation haplotype, derived from ‘Frostbite’ appears to be in repulsion with the reduced susceptibility for fire blight, as reported by Kostick et al. (2021). While the current fruit quality standard for fresh market apples favors low levels of russet formation, this information could also be used by apple breeders interested in developing new cultivars with higher levels of russet.
Conclusions
In this study, two previously reported QTLs, on LG2 and LG6, were confirmed and characterized for the first time in “Honeycrisp”-derived germplasm. Five other putative QTLs were also detected. Non-additive interactions were observed at and across LGs 2 and 6. The QTLs characterized in this study can be targeted for DNA test development to be used in marker-assisted selection. The information presented in this study highlights the necessity for breeders to consider haplotypes for russet formation across loci. Future work should include validation of QTL and haplotype effects in other germplasm, development of trait-predictive DNA tests for use in apple breeding programs, and exploration of genome-wide prediction models for russet formation.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Barceló-Vidal C, Bonany J, Martín-Fernández JA, Carbó J (2013) Modelling of weather parameters to predict russet on ‘Golden Delicious’ apple. J Hortic Sci Biotechnol 88(5):624–630. https://doi.org/10.1080/14620316.2013.11513016
Baugher TA, Hogmire HW, Biggs AR, Lightner GW, Walter SI, Leach DW, Winfield T (1996) Packout audits of apples from five orchard management systems. HortTechnol 6(1):34–41. https://doi.org/10.21273/HORTTECH.6.1.34
Beach SA, Booth NO, Taylor OM (1905) The apples of New York, vol II. Albany, New York
Bedford DS, Luby JJ (2008) Apple Tree Named ‘Minneiska’. U.S. Plant Patent No. 18,812 P3. U.S. Trademark Office. https://ppubs.uspto.gov/dirsearch-public/print/downloadPdf/PP18812
Bianco L, Cestaro A, Sargent DJ, Banchi E, Derdak S, Di Guardo M, Salvi S, Jansen J, Viola R, Gut I, Laurens F, Chagné D, Velasco R, Van De Weg E, Troggio M (2014) Development and validation of a 20 K Single Nucleotide Polymorphism (SNP) whole genome genotyping array for apple (Malus × domestica Borkh). Plos Onehttps://doi.org/10.1371/journal.pone.0110377
Bink MCAM, Uimari P, Sillanpää MJ, Janss LLG, Jansen RC (2002) Multiple QTL mapping in related plant populations via a pedigree-analysis approach. Theor Appl Genet 104(5):751–762. https://doi.org/10.1007/s00122-001-0796-x
Bink MCAM, Boer MP, ter Braak CJF, Jansen J, Voorrips RE, van De Weg WE (2008) Bayesian analysis of complex traits in pedigreed plant populations. Euphytica 161(1–2):85–96. https://doi.org/10.1007/s10681-007-9516-1
Bink MCAM, Totir LR, ter Braak CJF, Winkler CR, Boer MP, Smith OS (2012) QTL linkage analysis of connected populations using ancestral marker and pedigree information. Theor Appl Genet 124(6):1097–1113. https://doi.org/10.1007/s00122-011-1772-8
Bink MCAM, Jansen J, Madduri M, Voorrips RE, Durel CE, Kouassi AB, Laurens F, Mathis F, Gessler C, Gobbin D, Rezzonico F, Patocchi A, Kellerhals M, Boudichevskaia A, Dunemann F, Peil A, Nowicka A, Lata B, Stankiewicz-Kosyl M, Jeziorek K, Pitera E, Soska A, Tomala K, Evans KM, Fernández-Fernández F, Guerra W, Korbin M, Keller S, Lewandowski M, Plocharski W, Rutkowski K, Zurawicz E, Costa F, Sansavini S, Tartarini S, Komjanc M, Mott D, Antofie A, Lateur M, Rondia A, Gianfranceschi L, van De Weg WE (2014) Bayesian QTL analyses using pedigreed families of an outcrossing species, with application to fruit firmness in apple. Theor Appl Genet 127(5):1073–1090. https://doi.org/10.1007/s00122-014-2281-3
Bus VGM, Rikkerink EHA, Caffier V, Durel CE, Plummer KM (2011) Revision of the nomenclature of the differential host-pathogen interactions of Venturia inaequalis and Malus. Annu Rev Phytopathol 49:391–413. https://doi.org/10.1146/annurev-phyto-072910-095339
Bussey DJ (2016) The illustrated history of apples in the United States and Canada. Wisconsin, Mount Horeb, p 226
Calenge F, Faure A, Goerre M, Gebhardt C, van De Weg WE, Parisi L, Durel CE (2004) Quantitative trait loci (QTL) analysis reveals both broad-spectrum and isolate-specific QTL for scab resistance in an apple progeny challenged with eight isolates of Venturia inaequalis. Phytopathol 94(4):370–379. https://doi.org/10.1094/PHYTO.2004.94.4.370
Celton JM, Chagné D, Tustin SD, Terakami S, Nishitani C, Yamamoto T, Gardiner SE (2009) Update on comparative genome mapping between Malus and Pyrus. BMC Res Notes 2:1–7. https://doi.org/10.1186/1756-0500-2-182
Chagné D, Crowhurst RN, Troggio M, Davey MW, Gilmore B, Lawley C, Vanderzande S, Hellens RP, Kumar S, Cestaro A, Velasco R, Main D, Rees JD, Iezzoni A, Mockler T, Wilhelm L, van de Weg E, Gardiner SE, Bassil N, Peace C (2012) Genome-wide SNP detection, validation, and development of an 8 K SNP array for apple. PLoS One 7(2). https://doi.org/10.1371/journal.pone.0031745
Clark MD, Schmitz CA, Rosyara UR, Luby JJ, Bradeen JM (2014) A consensus ‘Honeycrisp’ apple (Malus x domestica) genetic linkage map from three full-sib progeny populations. Tree Genet Genomes 10:627–639. https://doi.org/10.1007/s11295-014-0709-1
Daccord N, Celton JM, Linsmith G, Becker C, Choisne N, Schijlen E, van De Geest H, Bianco L, Micheletti D, Velasco R, Di Pierro EA, Gouzy J, Rees DJG, Guérif P, Muranty H, Durel CE, Laurens F, Lespinasse Y, Gaillard S, Aubourg S, Quesneville H, Weigel D, van De Weg E, Troggio M, Bucher E (2017) High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat Genet 49(7):1099–1106. https://doi.org/10.1038/ng.3886
Eccher T, Hajnajari J (2006) Fluctuations of endogenous Gibberellin A4 and A7 content in apple fruits with different sensitivity to Russet. Acta Hortic 727:537–544. https://doi.org/10.17660/ActaHortic.2006.727.66
Evans KM, Barritt BH, Konishi BS, Dilley MA, Brutcher LJ, Peace CP (2010) WA 2. Apple. HortScience 45(4):668–669. https://doi.org/10.21273/HORTSCI.45.4.668
Falginella L, Cipriani G, Monte C, Gregori R, Testolin R, Velasco R, Troggio M, Tartarini S (2015) A major QTL controlling apple skin russeting maps on the linkage group 12 of ‘Renetta Grigia di Torriana.’ BMC Plant Biol 15(1):150. https://doi.org/10.1186/s12870-015-0507-4
Faust M, Shear CB (1972) Russeting of Apples, an Interpretive Review. HortSci 7(3):233–235
Galli P, Broggini GAL, Kellerhals M, Gessler C, Patocchi A (2010) High-resolution genetic map of the Rvi15 (Vr2) apple scab resistance locus. Mol Breed 26(4):561–572. https://doi.org/10.1007/s11032-010-9391-7
Howard NP, Troggio M, Durel CE, Muranty H, Denancé C, Bianco L, Tillman J, van de Weg E (2021) Integration of Infinium and Axiom SNP array data in the outcrossing species Malus × domestica and causes for seemingly incompatible calls. BMC Genomics 22(1):1–18. https://doi.org/10.1186/s12864-021-07565-7
Howard NP, van de Weg E, Tillman J, Tong CBS, Silverstein KAT, Luby JJ (2018) Two QTL characterized for soft scald and soggy breakdown in apple (Malus × domestica) through pedigree-based analysis of a large population of interconnected families. Tree Genet Genomes 14(1). https://doi.org/10.1007/s11295-017-1216-y
Kass RE, Raftery AE (1995) Bayes factors. J Am Stat Assoc 90(430):773–795. https://doi.org/10.2307/2291091
Kostick SA, Teh SL, Norelli JL, Vanderzande S, Peace C, Evans KM (2021) Fire blight QTL analysis in a multi-family apple population identifies a reduced-susceptibility allele in ‘Honeycrisp.’ Hortic Res 8(1). https://doi.org/10.1038/s41438-021-00466-6
Kostick SA, Luby JJ (2022) Apple fruit size QTLs on chromosomes 8 and 16 characterized. Agronomy 12(6):1279. https://doi.org/10.3390/agronomy12061279
Kunihisa M, Moriya S, Abe K, Okada K, Haji T, Hayashi T, Kim H, Nishitani C, Terakami S, Yamamoto T (2014) Identification of QTLs for fruit quality traits in Japanese apples: QTLs for early ripening are tightly related to preharvest fruit drop. Breed Sci 64(3):240–251. https://doi.org/10.1270/jsbbs.64.240
Kunihisa M, Moriya S, Abe K, Okada K, Haji T, Hayashi T, Kawahara Y, Itoh R, Itoh T, Katayose R, Kanamori H, Matsumoto T, Si M, Sasaki H, Matsumoto T, Nishitani C, Terakami S, Yamamoto T (2016) Genomic dissection of a ‘Fuji’ apple cultivar: re-sequencing, SNP marker development, definition of haplotypes, and QTL detection. Breed Sci 515:499–515. https://doi.org/10.1270/jsbbs.16018
Lashbrooke J, Aharoni A, Costa F (2015) Genome investigation suggests MdSHN3, an APETALA2-domain transcription factor gene, to be a positive regulator of apple fruit cuticle formation and an inhibitor of russet development. J Exp Bot 66(21):6579–6589. https://doi.org/10.1093/jxb/erv366
Luby JJ, Howard NP, Tillman JR, Bedford DS (2022) Extended pedigrees of apple cultivars from the University of Minnesota Breeding Program elucidated using SNP array markers. HortSci 57(3):472–477. https://doi.org/10.21273/HORTSCI16354-21
Muranty H, Denancé C, Feugey L, Crépin JL, Barbier Y, Tartarini S, Ordidge M, Troggio M, Lateur M, Nybom H, Paprstein F, Laurens F, Durel CE (2020) Using whole-genome SNP data to reconstruct a large multi-generation pedigree in apple germplasm. BMC Plant Biol 20(1):1–18. https://doi.org/10.1186/s12870-019-2171-6
Di Pierro EA, Gianfranchceschi L, Di Guardo M, Koehorst-van Putten HJ, Kruisselbrink JW, Longhi , Troggio M, Bianco L, Muranty H, Pagliarani G, Tartarini S, Letschka T, Lozano Luis L, Garkava-Gustavsson L, Micheletti D, Bink MC, Voorrips RE, Aziz E, Velasco R, Laurens F, van de Weg WE (2016) A high-density, multi-parental SNP genetic map on apple validates a new mapping approach for outcrossing species. Hortic Res. https://doi.org/10.1038/hortres.2016.57
Scharwies JD, Grimm E, Knoche M (2014) Russeting and relative growth rate are positively related in “Conference” and “Condo” pear. HortScience 49(6):746–749. https://doi.org/10.21273/HORTSCI.49.6.746
Sugar D, Hilton RJ, VanBuskirk PD (2005) Effects of Kaolin particle film and rootstock on tree performance and fruit quality in ‘Doyenne du Comice’ pear. HortScience 40(6):1726–1728. https://doi.org/10.21273/HORTSCI.40.6.1726
Takeuchi Y, Nishio S, Terakami S, Takada N, Kato H, Saito T (2021) Haplotype structure analysis of a locus associated with fruit skin type on chromosome 8 in Japanese pear. Tree Genet Genomes 17(1). https://doi.org/10.1007/s11295-020-01483-7
USDA National Agricultural Statistics Service (2021) Noncitrus Fruits and Nuts 2020 Summary. United States Department of Agriculture
Vanderzande S, Howard NP, Cai L, da Silva Linge C, Antanaviciute L, Bink MCAM, Kruisselbrink JW, Bassil N, Gasic K, Iezzoni A, van de Weg E, Peace C (2018) High-quality, genome-wide SNP genotypic data for pedigreed germplasm of the diploid outbreeding species apple, peach, and sweet cherry through a common workflow. Plos One https://doi.org/10.1371/journal.pone.0210928
Verma S, Evans K, Guan Y, Luby JJ, Rosyara UR, Howard NP, Bassil N, Bink MCAM, van de Weg WE, Peace CP (2019) Two large-effect QTLs, Ma and Ma3, determine genetic potential for acidity in apple fruit: breeding insights from a multi-family study. Tree Genet Genomes 15(2). https://doi.org/10.1007/s11295-019-1324-y
Wang YZ, Dai MS, Cai DY, Zhang S, Shi ZB (2016) A review for the molecular research of russet/semi-russet of sand pear exocarp and their genetic characters. Sci Hortic 210:138–142. https://doi.org/10.1016/j.scienta.2016.07.019
Funding
This research was funded by the Minnesota State Agricultural Experiment Station, University of Minnesota Projects MIN-21–040 and MIN-21–097, and by the United States Department of Agriculture, National Institute of Food and Agriculture Specialty Crop Initiative Projects 2009–51181-05808 and 2014–51181-22378.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The University of Minnesota receives royalty payments related to the “Honeycrisp,” “Minnewashta,” “Wildung,” “Minneiska,” and “MN55” apple cultivars. J.J.L., and the University of Minnesota have a royalty interest in these cultivars. These relationships have been reviewed and managed by the University of Minnesota in accordance with its conflicts of interest policies. Author N.P.H. is employed by Fresh Forward Breeding and Marketing.
Additional information
Communicated by D. Chagné
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Powell, A.A., Kostick, S.A., Howard, N.P. et al. Elucidation and characterization of QTLs for Russet formation on apple fruit in ‘Honeycrisp’-derived breeding germplasm. Tree Genetics & Genomes 19, 5 (2023). https://doi.org/10.1007/s11295-022-01582-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-022-01582-7