Abstract
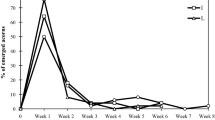
Tissue damage to seedlings can limit their later growth, and the further effects may be greater with increasing seedling age. Seedlings, however, can minimize the effect of damage through compensatory growth. Seedlings of Pharbitis purpurea grow in frequently disturbed habitats and generally tolerate damage to leaf tissues. We evaluated the compensatory responses of the cotyledon to different levels of defoliation and their effect on seedling growth and development. We also examined the relationship between seeding depth and compensatory growth. We tested seven defoliation treatments with one or both cotyledons and/or the apical meristem of seedlings removed from seeds buried at a seeding depth of either 2 or 5 cm. We then measured 12 growth traits of the seedlings to assess development and growth compensation. The area, thickness, biomass, and longevity of the remaining cotyledon were also measured to quantify increased growth as result of treatment effects at both seeding depths. The results showed that defoliation reduced seedling height, belowground length, and total biomass significantly in subsequent growth in all treatments. However, defoliation treatments had direct positive impacts on growth at 2 cm depth compared with 5 cm depth. In contrast, the compensation of cotyledon area (C area), biomass (C mass), and thickness (C thickness) was greater at 5 cm depth than at 2 cm depth. The results thus indicate that P. purpurea seedlings adopted a compensatory growth strategy to resist leaf loss and minimize any adverse effects using the remaining cotyledon. Increasing seeding depth can aggravate the compensatory growth of remain cotyledon after partial defoliation.




Similar content being viewed by others
References
Anten NPR, Martinez-Ramos M, Ackerly DD (2003) Defoliation and growth in an understory palm: quantifying the contributions of compensatory responses. Ecology 84:2905–2918
Armstrong DP, Westoby M (1993) Seedlings from large seeds tolerate defoliation better: a test using phylogenetically independent contrasts. Ecology 74:1092–1100
Barton KE (2008) Phenotypic plasticity in seedling defense strategies: compensatory growth and chemical induction. Oikos 117:917–925
Belsky AJ (1986) Does herbivory benefit plants? A review of the evidence. Am Nat 127:870–892
Boege K (2005) Influence of plant ontogeny on compensation to leaf damage. Am J Bot 92:1632–1640
Bonfil C (1998) The effects of seed size, cotyledon reserves, and herbivory on seedling survival and growth in Quercus rugosa and Q. laurina (Fagaceae). Am J Bot 85:79–87
Chazdon RL (1991) Effects of leaf and ramet removal on growth and reproduction of Geonoma congesta, a clonal understorey palm. J Ecol 79:1137–1146
Dech JP, Maun MA (2006) Adventitious root production and plastic resource allocation to biomass determine burial tolerance in woody plants from central Canadian coastal dunes. Ann Bot 98:1095–1105
Del-Val E, Crawley MJ (2005) Are grazing increaser species better tolerators than decreasers? An experimental assessment of defoliation tolerance in eight British grassland species. J Ecol 93:1005–1016
Fenner M, Thompson K (2005) The ecology of seeds, 2nd edn. Cambridge University Press, Cambridge
Ferraro DO, Oesterheld M (2002) Effect of defoliation on grass growth. A quantitative review. Oikos 98:125–133
Garwood NC (1996) Functional morphology of tropical tree seedlings. In: Swaine IMD (ed) The ecology of tropical forest tree seedlings. Parthenon, Carnforth, pp 59–129
Hanley ME, Fegan EL (2007) Timing of cotyledon damage affects growth and flowering in mature plants. Plant Cell Environ 30:812–819
Hanley ME, Lamont BB (2001) Herbivory, serotiny and seedling defence in Western Australian Proteaceae. Oecologia 126:409–417
Hanley ME, May OC (2006) Cotyledon damage at the seedling stage affects growth and flowering potential in mature plants. New Phytol 169:243–250
Hanley ME, Fenner M, Whibley H, Darvill B (2004) Early plant growth: identifying the end point of the seedling phase. New Phytol 163:61–66
Harper JL (1977) Population biology of plants. Academic, London
Hicks S, Turkington R (2000) Compensatory growth of three herbaceous perennial species: the effects of clipping and nutrient availability. Can J Bot 78:759–767
Hikosaka K, Takashima T, Kabeya D, Hirose T, Kamata N (2005) Biomass allocation and leaf chemical defence in defoliated seedlings of Quercus serrata with respect to carbon–nitrogen balance. Ann Bot 95:1025–1032
Ibarra-Manríquez G, Martínez Ramos M, Oyama K (2001) Seedling functional types in a lowland rain forest in Mexico. Am J Bot 88:1801–1812
Kitajima K (1996) Cotyledon functional morphology, patterns of seed reserve utilization and regeneration niches of tropical tree seedlings. In: Swaine IMD (ed) The ecology of tropical forest tree seedlings. Parthenon, Carnforth, pp 193–210
Kitajima K (2003) Impact of cotyledon and leaf removal on seedling survival in three tree species with contrasting cotyledon functions. Biotropica 35:429–434
Lee TD, Bazzaz FA (1980) Effects of defoliation and competition on growth and reproduction in the annual plant Abutilon theophrasti. J Ecol 813–821
Lehtila K, Syrjanen K (1995) Compensatory responses of two Melampyrum species after damage. Funct Ecol 9:511–517
Lennartsson T, Nilsson P, Tuomi J (1998) Induction of overcompensation in the field gentian, Gentianella campestris. Ecology 79:1061–1072
Li QY, Zhao WZ, Fang HY (2006) Effects of sand burial depth and seed biomass on seedling emergence and growth of Nitraria sphaerocarpa. Plant Ecol 185:191–198
Mabry CM, Wayne PW (1997) Defoliation of the annual herb Abutilon theophrasti: mechanisms underlying reproductive compensation. Oecologia 111:225–232
Martens B, Trumble JT (1987) Structural and photosynthetic compensation for leafminer (Diptera: Agromyzidae) injury in lima beans. Environ Entomol 16:374–378
Maschinski J, Whitham TG (1989) The continuum of plant responses to herbivory: the influence of plant association, nutrient availability, and timing. Am Nat 134:1–19
Maun MA (1998) Adaptations of plants to burial in coastal sand dunes. Can J Bot 76:713–738
Maun MA, Lapierre J (1986) Effects of burial by sand on seed germination and seedling emergence of four dune species. Am J Bot 73:450–455
McNaughton SJ (1983) Compensatory plant growth as a response to herbivory. Oikos 40:329–336
Meyer GA (1998) Mechanisms promoting recovery from defoliation in goldenrod (Solidago altissima). Can J Bot 76:450–459
Moriondo M, Orlandini S, Villalobos FJ (2003) Modelling compensatory effects of defoliation on leaf area growth and biomass of sunflower (Helianthus annuus L.). Eur J Agron 19:161–171
Oesterheld M, McNaughton SJ (1991) Effect of stress and time for recovery on the amount of compensatory growth after grazing. Oecologia 85:305–313
Rosenthal JP, Kotanen PM (1994) Terrestrial plant tolerance to herbivory. Trends Ecol Evol 9:145–148
Ruiz-R N, Ward D, Saltz D (2008) Leaf compensatory growth as a tolerance strategy to resist herbivory in Pancratium sickenbergeri. Plant Ecol 198:19–26
Seiwa K, Watanabe A, Saitoh T, Kannu H, Akasaka S (2002) Effects of burying depth and seed size on seedling establishment of Japanese chestnuts, Castanea crenata. Forest Ecol Manag 164:149–156
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Thomson VP, Cunningham SA, Ball MC, Nicotra AB (2003) Compensation for herbivory by Cucumis sativus through increased photosynthetic capacity and efficiency. Oecologia 134:167–175
Tiffin P (2000) Mechanisms of tolerance to herbivore damage: what do we know? Evol Ecol 14:523–536
Van Staalduinen MA, Anten NPR (2005) Differences in the compensatory growth of two co-occurring grass species in relation to water availability. Oecologia 146:190–199
Wallace SK, Eigenbrode SD (2002) Changes in the glucosinolate–myrosinase defense system in Brassica juncea cotyledons during seedling development. J Chem Ecol 28:243–256
Welter SC (1989) Arthropod impact on plant gas exchange. In: Bernays IEA (ed) Insect–plant interactions. CRC, Boca Raton, pp 135–150
Wise MJ, Abrahamson WG (2007) Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. Am Nat 169:443–454
Yanful M, Maun MA (1996) Effects of burial of seeds and seedlings from different seed sizes on the emergence and growth of Strophostyles helvola. Can J Bot 74:1322–1330
Zhang H, Zhou D, Matthew C, Wang P, Zheng W (2008) Photosynthetic contribution of cotyledons to early seedling development in Cynoglossum divaricatum and Amaranthus retroflexus. N Z J Bot 46:39–48
Zhao W, Chen SP, Lin GH (2008) Compensatory growth responses to clipping defoliation in Leymus chinensis (Poaceae) under nutrient addition and water deficiency conditions. Plant Ecol 196:85–99
Zheng Y, Xie Z, Yu Y, Jiang L, Shimizu H, Rimmington GM (2005) Effects of burial in sand and water supply regime on seedling emergence of six species. Ann Bot 95:1237–1245
Zheng W, Yang JY, Luan ZH, Wang P, Zhang HX, Zhou DW (2011) Compensatory growth and photosynthetic responses of Pharbitis purpurea seedlings to clipped cotyledon and second leaf. Photosynthetica 49(1):21–28
Acknowledgments
We greatly appreciate Dr. Beth Hazen for his assistance in the revision of this paper. This study was funded by the National Key Basic Research Program (2007CB106801).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zheng, W., Li, G., Huang, Y. et al. Compensatory growth responses of seedlings of Pharbitis purpurea (Convulvulaceae) to tissue removal at different seeding depths of seeds. Ecol Res 27, 569–576 (2012). https://doi.org/10.1007/s11284-011-0922-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-011-0922-4