Abstract
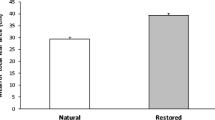
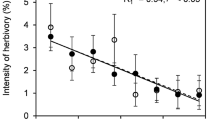
We examined the effects of leaf herbivory by the dorcas gazelle, Gazella dorcas, on the compensatory growth of the geophyte Pancratium sickenbergeri (Amaryllidaceae) in the Negev desert, Israel. In three populations exposed to different levels of herbivory, we removed different amounts of photosynthetic leaf area from plants in five clipping treatments: 0, 25, 50%-dispersed over all leaves, 50%-entire area of half the leaves, 100%. The population with the lowest level of herbivory showed the lowest relative regrowth rate after clipping. In the population with a constantly high level of herbivory, plants in intermediate-clipping treatments overcompensated in leaf area after clipping. For all the populations, clipped plants produce more new leaves than unclipped plants. In the population with the highest level of herbivory, clipping treatments did not have a significant effect on the number of fruits per plant. In addition, we did not find a trade-off between investments in growth and reproduction in this population. Our results indicated that, in the desert lily, herbivores may select for plant mechanisms that compensate after damage as a tolerant strategy to maintain fitness.



Similar content being viewed by others
References
Avila-Sakar G, Leist LL, Stephenson AG (2003) Effects of the spatial pattern of leaf damage on growth and reproduction: nodes and branches. J Ecol 91:867–879
Avila-Sakar G, Stephenson AG (2006) Effects of the spatial pattern of leaf damage on growth and reproduction: whole plants. Int J Plant Sci 167:1021–1028
Belsky AJ (1986) Does herbivory benefit plants? A review of the evidence. Am Nat 127:870–892
Belsky AJ, Carson WP, Jensen CL, Fox GA (1993) Overcompensation by plants: herbivore optimization or red herring? Evol Ecol 7:109–121
Bold H, Alexopoulos CJ, Delevoryas T (1987) Morphology of plants and fungi. Harper & Row, New York
Crawley MJ (1985) Reduction of oak fecundity by low density herbivore populations. Nature 314:163–164
Crawley MJ (1987) Benevolent herbivores? Trends Ecol Evol 2:167–168
Dafni A, Shmida A, Avishai M (1981) Leafless autumnal-flowering geophytes in the Mediterranean region—Phytogeographical, ecological and evolutionary aspects. Plant Syst Evol 137:181–193
Garrish RS, Lee TD (1989) Physiological integration in Cassia fasciculata Michx. inflorescence removal and defoliation experiments. Oecologia 81:279–284
Hendrix SD (1988) Herbivory and its impact on plant reproduction. In: Lovett J, Lovett L (eds) Plant reproductive ecology: patterns and strategies. Oxford Univ. Press, Oxford, pp 246–263
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Q Rev Biol 67:283–335
Inouye DW (1982) The consequences of herbivory: a mixed blessing for Jurines mollis (Asteraceae). Oikos 39:269–272
Jaremo J, Nilsson P, Tuomi J (1996) Plant compensatory growth: herbivory or competition? Oikos 77:238–247
Kulman HM (1971) Effects of insect defoliation on growth and mortality of trees. Annu Rev Entomol 16:289–324
Larcher W (1975) Physiological plant ecology. Springer-Verlag, Berlin
Lawes MJ, Nanni RF (1993) The density, habitat use and social organization of the dorcas gazelles (Gazella dorcas) in the Makhtesh Ramon, Negev desert, Israel. J Arid Environ 24:177–196
Lee TD, Bazzaz FA (1980) Effects of defoliation and competition on growth and reproduction in the annual plant Abutilon theophrasti. J Ecol 68:813–821
Lowman MD (1982) Effects of different rates and methods of leaf area removal on rain forest seedlings of coachwood. Austr J Bot 30:477–483
Marquis RJ (1984) Leaf herbivores decrease fitness of a tropical plant. Science 226:537–539
Marquis RJ (1992) A bite is a bite is a bite? Constraints on response to folivory in Piper arieianum (Piperaceae). Ecology 73:143–152
Marshall DL (1989) Integration of response to defoliation within plants of two species of Sesbania. Funct Ecol 3:207–214
Mauricio R, Bowers MD, Bazzaz FA (1993) Pattern of leaf damage affects fitness of the annual plant Raphanus sativus (Brassicaceae). Ecology 74:2066–2071
McNaughton SJ (1979a) Grazing as optimization process: grass-ungulate relationships in the Serengeti. Am Nat 113:691–703
McNaughton SJ (1979b) Grassland herbivore dynamics. In: Sinclair ARE, Norton-Griffiths M (eds) Serengeti: dynamics of an ecosystems. Chicago Univ. Press, Chicago, pp 46–81
McNaughton SJ (1983) Compensatory plant growth as a response to herbivory. Oikos 40:329–336
McNaughton SJ (1986) On plants and herbivores. Am Nat 128:765–770
Noy-Meir I (1993) Compensating growth of grazed plants and its relevance to the use of rangelands. Ecol Appl 3:32–34
Owen DF (1980) How plants may benefit from the animals that eat them. Oikos 35:230–235
Owen DF, Wiegert RG (1976) Do consumers maximizes plant fitness? Oikos 27:488–492
Paige KN (1992) Overcompensation in response to mammalian herbivory: from mutualistic to antagonistic interaction. Ecology 73:2076–2085
Paige KN (1999) Regrowth following ungulate herbivory in Ipomopsis aggregata: geographic evidence for overcompensation. Oecologia 118:316–323
Paige KN, Whitham TG (1987) Overcompensation in response to mammalian herbivory: the advantage of being eaten. Am Nat 129:407–416
Painter EL, Belsky AJ (1993) Application of herbivore optimization theory to rangelands of the western United States. Ecol Appl 3:2–9
Price EAC, Hutchings MJ (1992) Studies of growth in the clonal herb Glechoma hederacea. II. The effects of selective defoliation. J Ecol 80:39–47
Ruiz N, Ward D, Saltz D (2002a) Crystals of calcium oxalate in leaves: constitutive or induced defense? Funct Ecol 16:99–105
Ruiz N, Ward D, Saltz D (2002b) Responses of Pancratium sickenbergeri to simulated bulb herbivory: combining defense and tolerance strategies. J Ecol 90:472–479
Ruiz N, Saltz D, Ward D (2006a) The effects of herbivory and resource variability on the production of a second inflorescence by the desert lily, Pancratium sickenbergeri. Plant Ecol 186:47–55
Ruiz N, Ward D, Saltz D (2006b) Population differentiation and the effects of herbivory and sand compaction on the subterranean growth of a desert lily. J Hered 97:409–416
Ruiz N, Saltz D, Ward D (2006c) Signal selection in a desert lily Pancratium sickenbergeri. Evol Ecol Res 8:1461–1474
Saltz D, Ward D (2000) Responding to a three-pronged attack: desert lilies subject to herbivory by dorcas gazelles. Plant Ecol 148:127–138
Saltz D, Schmidt H, Rowen M, Karnieli A, Ward D, Schmidt I (1999) Assessing grazing impacts by remote sensing in hyper-arid environments. J Range Manage 52:500–507
Shea MM, Watson MA (1989) Patterns of leaf and flower removal: their effect on fruit growth in Chamaenerion angustifolium (fireweed). Amer J Bot 76:884–890
Tiffin P, Rausher MD (1999) Genetic constraints and selection acting on tolerance to herbivory in the common morning glory Ipomoea purpurea. Am Nat 154:700–716
Tuomi J, Nilsson P, Astrom M (1994) Plant compensatory responses-bud dormancy as an adaptation to herbivory. Ecology 75:1429–1436
Vail SG (1992) Selection for overcompensatory plant responses to herbivory: a mechanism for the evolution of plant-herbivore mutualism. Am Nat 139:1–8
Van Der Heyden F, Stock WD (1996) Regrowth of a semiarid shrub following simulated browsing: the role of reserve carbon. Funct Ecol 10:647–653
Van Der Meijden E, Wijn M, Verkaar HJ (1988) Defence and regrowth, alternative plant strategies in the struggle against herbivores. Oikos 51:355–363
Verkaar HJ (1988) Are defoliators beneficial for their host plant in terrestrial ecosystems—a review? Acta Bot Neerl 37:137–152
Wandera JL, Richards JH, Muller RJ (1992) The relationship between relative growth rate, meristematic potential and compensatory growth of semiarid-land shrubs. Oecologia 90:391–398
Ward D, Olsvig-Whittaker L (1993) Plant species diversity at the junction of two desert biogeographic zones. Biodivers Lett 1:172–185
Ward D, Saltz D (1994) Foraging at different spatial scales: dorcas gazelles foraging for lilies in the Negev desert. Ecology 75:48–58
Ward D, Olsvig-Whittaker L, Lawes MJ (1993) Vegetation-environment relationships in a Negev desert erosion cirque. J Veg Sci 4:83–94
Ward D, Spiegel M, Saltz D (1997) Gazelle herbivory and interpopulation differences in calcium oxalate content of leaves of a desert lily. J Chem Ecol 23:333–346
Ward D, Saltz D, Olsvig-Whittaker L (2000) Distinguishing signal from noise: long-term studies of vegetation in Makhtesh Ramon erosion cirque, Negev desert, Israel. Plant Ecol 150:27–36
Watson MA, Casper BB (1984) Morphogenetic constraints on patterns of carbon distribution in plants. Ann Rev Ecol Syst 15:233–258
Wilkinson L (1997) Systat: the system for statistics. SYSTAT, Inc., Evanson, IL
Acknowledgments
We thank Iris Musli, Ofrit Gilan, and Betina Berendock for assistance in the field. We thank the anonymous reviewers for their insights. This study was supported by the Israel Science Foundation. This is publication number 587 of the Mitrani Department for Desert Ecology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz-R, N., Ward, D. & Saltz, D. Leaf compensatory growth as a tolerance strategy to resist herbivory in Pancratium sickenbergeri . Plant Ecol 198, 19–26 (2008). https://doi.org/10.1007/s11258-007-9381-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-007-9381-y