Abstract
This study aimed to isolate and characterize a native strain of Beauveria bassiana, coded as Bv065, showcasing its potential as a biological control agent targeting the palm weevil Dynamis borassi. Originating from a naturally infected D. borassi specimen collected in southwestern Colombia, the fungus underwent molecular identification and was identified as B. bassiana, exhibiting high sequence similarity with known reference strains. The physiological characterization revealed that Bv065 thrived within a temperature range of 25 to 30 °C and a pH range of 6 to 9. Moreover, the key carbon sources that allow optimal growth of the strain were identified through metabolic profiling, including sucrose, D-mannose, and γ-amino-butyric acid. These findings offer strategic insights for scalability and formulation methodologies. Additionally, enzymatic analyses unveiled robust protease activity within Bv065, crucial for catalysing insect cuticle degradation and facilitating host penetration, thus accentuating its entomopathogenic potential. Subsequent evaluations exposed Bv065’s pathogenicity against D. borassi, causing significant mortality within nine days of exposure, albeit exhibiting limited effectiveness against Rhynchophorus palmarum. This study underscores the importance of understanding optimal growth conditions and metabolic preferences of B. bassiana strains for developing effective biopesticides. The findings suggest Bv065 as a promising candidate for integrated pest management strategies in neotropical regions, particularly for controlling palm weevil infestations in coconut and peach palm cultivation. Future research avenues include refining mass production methodologies, formulating novel delivery systems, and conducting comprehensive field efficacy trials to unlock the full potential of Bv065 in fostering sustainable pest management practices. Overall, this study contributes to the growing body of knowledge on entomopathogenic fungi and their pivotal role in biological control, offering nuanced perspectives on eco-friendly alternatives to conventional insecticidal interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The palm weevils, D. borassi and R. palmarum, have emerged as significant phytosanitary concerns in the Colombian Pacific region, particularly affecting peach palm (Bactris gasipaes Kunth) and coconut (Cocos nucifera L.) cultivation (Gaviria et al. 2021; Gutiérrez et al. 2023). These insects’ larvae infest the palm’s stem and apical meristem, causing palm crown collapse and acting as vectors for diseases like red ring disease (Gerber and Giblin-Davis 1990; Oehlschlager et al. 2002). The identification of these species as pests began around 2010, initially attributing damages to the R. palmarum insect (Bautista-Giraldo et al. 2020). Further research confirmed D. borassi as the primary cause of inflorescence damage and palm decapitation in disturbed forests around Buenaventura, Colombia, resulting in damage of over 50% of inflorescences, which exhibited perforations at the apex, central, and basal regions (Bautista-Giraldo et al. 2020; Gaviria et al. 2021). These damages documented on palms of economic interest echoes symptoms first reported in 1998 on native palms (Couturier et al. 1998). Between 2015 and 2020, the onslaught of palm weevil attacks in Colombia had a substantial negative impact on peach palm and coconut yields (Agronet 2020). This impact is particularly noteworthy because, despite efforts to expand cultivation, the production of these crops in Colombia still falls significantly short of the growing demand. Since 2020, two major phytosanitary issues further exacerbated the situation, resulting in a noticeable decline in productivity and extensive damage, with palm weevils causing up to 70% damage, notably along the Pacific coast (Vásquez-Ordóñez et al. 2020; Gaviria et al. 2021).
Globally, traditional methods for managing palm pests encompass a range of practices, including maintaining overall plant and field sanitation, applying preventive chemical treatments to palm wounds, introducing insecticide-sand mixtures into young palm leaf axils, using curative chemical treatments on infested palms, incorporating biopesticides, and, as a last resort, removing heavily infested palms and incinerating them (Abbas 2010; Hussain et al. 2013; Faleiro et al. 2019). In Colombia, the recommended strategy for palm weevil management leans heavily on cultural practices, including mass trapping, which entices adult weevils using aggregation pheromones (Oehlschlager et al. 2002; Gutiérrez et al. 2023), eliminating breeding sites and removing affected palms (Alvarado et al. 2013; Oehlschlager 2016). Nevertheless, these approaches frequently fall short given the severity of the phytosanitary issue (Ahmed and Freed 2021; Gutiérrez et al. 2023). Moreover, the use of highly toxic synthetic insecticides by palm producers has been observed, an aspect not extensively investigated in Colombia, where concerns about the development of physiological and behavioral resistance within palm weevil populations are a distinct possibility (Gutiérrez 2020; Ahmed and Freed 2021). Additionally, the application of synthetic insecticides with little or no guidance may have significant implications for human and environmental health (Isman 2006; Devine and Furlong 2007).
An environmentally sustainable alternative for palm weevil control is the implementation of biological control via the use of entomopathogenic fungi. In the realm of integrated pest management, entomopathogenic fungi serve as pivotal biological control agents, particularly in tropical agricultural settings (McGuire and Northfield 2020). Notably, Beauveria and Metarhizium species have demonstrated their effectiveness as biological control agents against a range of agriculturally significant pests, including ticks (Sullivan et al. 2022a), whiteflies (Barra-Bucarei et al. 2020; Sani et al. 2020), citrus psyllids (Maluta et al. 2022) and red palm weevils (Yang et al. 2023). Their broad-spectrum efficacy encompasses all developmental stages of these pests, making them essential components of integrated pest management strategies. Their mechanisms of action involve outcompeting pathogens for nutrients and space while also altering growth conditions. Additionally, entomopathogenic fungi display cryptic behavior within plants, allowing them to establish themselves within plant tissues and maintain their pathogenicity characteristics (Akello et al. 2010). Remarkably, these fungi may even confer stress resistance proteins to the host plant (Gomez-Vidal et al. 2006). Furthermore, they provide protection against larval feeding, with their effectiveness extending for up to three months following inoculation (Ricaño et al. 2013).
B. bassiana, a naturally occurring soil-borne fungus belonging to the Hypocreales order and Clavicipitaceae family, is highly regarded for its entomopathogenic capabilities. It possesses the remarkable ability to infect and eliminate a diverse range of insect species, making it a top choice for pest control (Wahengbam et al. 2021). What sets B. bassiana apart is its inherent safety for humans, domestic animals, and the environment. This fungus poses no threat to non-target species and leaves no harmful residues in soil, water, or on crops (Meyling et al. 2009; Xiao et al. 2012). Numerous promising applications for sustainable and effective population control have emerged in the utilization of B. bassiana against palm weevils. Extensive research conducted in laboratory, semi-field, and field settings consistently demonstrates the pathogenicity of B. bassiana towards both larvae and adults of various Rhynchophorus species, resulting in significant mortality rates (Dembilio et al. 2010; Lo Verde et al. 2014; León Martínez et al. 2019; López-Luján et al. 2022). Furthermore, B. bassiana infections have been shown to reduce egg fertility by approximately 52%, with outcomes influenced by the sex of the treated parent (Dembilio et al. 2010). Exposure of R. ferrugineus larvae to B. bassiana can also impact larval development, leading to increased energy expenditure in defense against the microbial agent (Hussain et al. 2015). Notably, B. bassiana emits volatile organic compounds that function as repellents against the R. ferrugineus beetle while concurrently exerting a neurotoxic effect (Jalinas et al. 2022). Additionally, in the realm of population control, infected adult palm weevils, typically surviving for several days, partake in horizontal transmission by dispersing conidia from their cuticles to infect new insects (Dembilio et al. 2018; León-Martínez et al. 2019). Lastly, transgenerational effects have been observed, where the impact of B. bassiana extends to the survival rates of larvae descended from inoculated adults, signaling a broader ecological influence (Lo Verde et al. 2014).
The aforementioned attributes collectively establish B. bassiana as an excellent candidate for controlling pest palm weevils. However, despite significant advances in the development of fungal biopesticides over the past two decades, the availability of commercial products for their use in controlling agriculturally significant pests remains limited. In Colombia, this constraint is underscored by the absence of registered biopesticides utilizing entomopathogenic fungi, where the only available products, exceeding ten in number, are hormone-based (Sullivan et al. 2022b). Additionally, there is a conspicuous absence of studies to date assessing the pathogenicity of entomopathogenic fungi against the palm weevil D. borassi.
The objective of this study was to comprehensively examine a native strain of the entomopathogenic fungus B. bassiana (Bv065), encompassing its molecular identification, growth characteristics, metabolic and enzymatic profiles, and its pathogenicity against two weevil species, D. borassi and R. palmarum. We postulated that the native B. bassiana strain (Bv065), isolated from an infected D. borassi specimen, would exhibit the attributes required for efficient pest control. Furthermore, our hypothesis included that this strain’s pathogenicity against D. borassi and R. palmarum weevils would rival, or potentially surpass, that of commercially available bioinsecticides. Additionally, through an in-depth analysis of the culturing conditions, and metabolic and enzymatic profile, we aimed to gather the foundational data necessary for the formulation of a bioinsecticide based on the studied B. bassiana strain.
Materials and methods
Fungal collection, isolation, and molecular identification
A native strain of the entomopathogenic fungus B. bassiana was isolated from an infected D. borassi individual found in an experimental peach-palm plantation at El Mira Research Centre in Tumaco, Colombia (coordinates: 78.70072 N, 1.55122 W). This location has an average temperature of 25.8 °C and an average annual precipitation of 3000 mm (IDEAM, 2022).
To promote sporulation on the insect’s surface, the infected insect was placed in a humid chamber at 25 °C for five days. Conidia samples were collected using a sterile entomological needle and inoculated onto potato dextrose agar (PDA Sigma-Aldrich, Darmstadt, Germany) supplemented with 0.1% w/v chloramphenicol antibiotic (Colmed International, Bogotá). The plates were then incubated for ten days at 25 °C until fungal growth became evident. Subsequently, several consecutive inoculations were made on PDA to isolate the fungi using the puncture method. Agar discs containing the fungi were preserved in a solution of 10% v/v glycerol (Sigma-Aldrich, Germany) and 0.1% Universal peptone (Merkc, Burlington Massachusetts, USA) v/w, and stored at − 80 °C. The isolate was registered in the Colombian Corporation of Agricultural Research’s (Agrosavia) Microorganisms Germplasm Bank (Mosquera, Colombia) RCN:129) and assigned the code Bv065. The collection of both, the insect and the fungi, was conducted under collecting permit 1466 of 2014 granted by the ANLA (Autoridad Nacional de Licencias Ambientales) to Agrosavia.
For molecular identification, total DNA was extracted using Zymo Research Fungal/bacterial DNA miniprep TM kit (Zymo Research, California, USA) following the manufacturer’s instructions. Partial amplification of the internal transcribed spacer region (ITS) and the elongation factor gene TEF1-α were amplified by polymerase chain reaction using the primers sets ITS1 (5ʹ-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5ʹ-TCCTCCGCTTATTGATATGC-3ʹ), and EF1-728F (5ʹ-CATCGAGAAGTTCGAGAAGG-3ʹ) and EF1-986R (5ʹ-TACTTGAAGGAACCCTTACC-3ʹ), respectively. The sequencing of amplified DNA fragments was conducted by Corpogen Corporation (https://www.corpogen.org/, Bogotá. Colombia). Ambiguous bases in the forward and reverse trace files were resolved by manually checking against the PHRED scores. The taxonomic identification of the isolate was carried out using the Mycobank and NCBI GenBank databases (https://www.mycobank.org; https://blast.ncbi.nlm.nih.gov) and the basic local alignment search analysis tool (BLASTn). The partial sequences of the ITS region and EF were deposited in GenBank with accession numbers OR135359 and OR136511, respectively.
Effect of temperature and pH on the growth of B. bassiana Bv065
We initiated pure cultures of B. bassiana Bv065 in PDA, incubating them at a consistent temperature of 25 °C for 10 days. Following this, we prepared a conidial suspension with a concentration of 1xx106 conidia mL−1 in a 0.1% v/v Tween 80® solution (Scharlau, Barcelona).
To evaluate the impact of different temperature conditions on the growth of B. bassiana Bv065, we initiated an experiment by inoculating PDA plates with a 2 µL conidia suspension. These plates were incubated for 15 days, spanning a temperature range of 8, 15, 20, 25, 30, and 35 °C. Importantly, we kept the pH of the PDA medium consistent without any adjustments. The pH value of the PDA medium was measured at 5.6 ± 0.2 at 25 °C.
Following the temperature assessment, we proceeded to examine growth under varying pH levels. To achieve this, we adjusted the pH of the PDA plates to values of 4, 5, 6, 7, 8, and 9 with hydrochloric acid (Sigma-Aldrich, Germany) HCl (1N) and potassium hydroxide solution (Sigma-Aldrich, Germany) KOH (1N). PDA plates were inoculated with the conidia suspension as previously described and incubated at 25 °C for 15 days.
In both experiments described above, each experimental unit comprised a Petri dish, and we included four replicates for each treatment, totaling 24 samples for each experiment. For each Petri dish, we determined the diametral growth rate (mm day−1) by averaging the measurements at two perpendicular points using a digital caliper.
Massive conidia production and germination of B. bassiana Bv065
To mass-produce B. bassiana Bv065, we followed the semisolid-state fermentation method detailed by Mejia et al. (2020) was used. The process consisted of the inoculation of aluminum trays filled with rice with a 2 mL suspension of 1 × 106 conidia mL−1, followed by incubation at 25 °C for 7 days. After this period, the plastic film covering the trays was replaced with paper towels to promote sporulation and drying. Subsequently, the dried conidia were collected and sieved through a 150 µm mesh.
To assess conidial germination, 10 mg of dried conidia were suspended in 990 µL of 0.1% v/v Tween 80, and 100 µL of serial dilutions were inoculated into Petri dishes containing water agar supplemented with 0.1% w/v malt extract and 5 ppm benomyl. These Petri dishes were then incubated at 25 °C for 24 h, and germinated conidia were counted under a light microscope (ZEISS Axio Lab.A1, Oberkochen, Germany) at 40X magnification. Conidia with germinating tubes longer than their width or diameter were considered germinated, as described by Grijalba et al. (2018). Furthermore, conidial yield was estimated by counting conidia in serial dilutions using a Neubauer chamber (Boeco, Hamburg, Germany).
Metabolic profiling of B. bassiana Bv065
The metabolic profile of B. bassiana Bv065 was assesed using the Biolog FF MicroPlates™ (Biolog, Hayward, USA), a microplated dish containing up to 95 carbon sources and a control well. To initiate this evaluation, we suspended fungal spores in 15 mL of FF-IF MicroPlate™ (filamentous fungi-inoculation fluid) broth (Biolog), adjusting them to a concentration of 1 × 106 conidia mL−1. Subsequently, 100 µL of the conidia suspension were poured into each well of the microplates. The microplates were then subjected to an incubation period of 120 h at 25 °C, with constant agitation. This assay was conducted in triplicate to ensure robustness. We measured fungal growth at 750 nm and assimilation was assessed at 490 nm using a microplate reader (Cytation, Agilent Biotek, Santa Clara. USA), following the method described by Mchunu et al. in 2013.
Enzymatic profile of B. bassiana Bv065
For the determination of lipase, chitinase, and protease activities originating from B. bassiana (Bv065), dried conidia were obtained from semisolid-state fermentation. The extraction of these enzymes involved suspending 50 mg of conidia in 1 mL of a 1% v/v Tween® 80 (Sigma-Aldrich, Burlington) solution, followed by vortexing at a constant speed for 1 h. Afterward, we subjected the sample to centrifugation at 10,000 rpm for 10 min, and the resulting supernatant was collected for analysis. To quantify lipase activity, we followed the methodology outlined by Beys da Silva et al. (2010), and Glogauer et al. (2011). Chitinase activity assessment followed the protocol described by Santos et al. in 2017. In both cases, the reactions were carried out at 37 °C for 30 min, with absorbance measurements conducted at 400 nm using a Spectrophotometer (Cytation™ 3, Agilent Biotek, Santa Clara. USA). One unit of the enzyme was defined as the amount of enzyme that release 1 µmol of p-nitrophenol per minute at the conditions of the assay. To measure protease activity, we adhered to the procedure proposed by Cupp-Enyard (2008) was followed. Absorbance measurements were made at 660 nm using a Spectrophotometer (same as described above), and protease activity was expressed as the amount of enzyme needed to release 1 µmol of tyrosine per minute.
Weevil collection and maintenance
Adult R. palmarum and D. borassi specimens were collected from oil palm (Elaeis oleífera x Elaeis guineensis) and peach palm (Bactris gasipaes) plantations using bucket traps as described by Lohr & Parra (2014). The traps contained a mixture of 300 g of sugar cane or pineapple, 6-methyl-2-hepten-4-ol (Rochat et al. 1991)and 4-Methyl-5-nonanol (Giblin-Davis et al. 1997) as aggregation pheromones, and 4.5 mL of ethyl acetate as attractants. The traps were checked weekly in peach palms and fortnightly in oil palms. The collected adults were subsequently examined in the laboratory to identify their species and sex based on morphological characteristics. Pairs of each species were housed in 500 mL plastic containers with sugar cane and allowed to acclimate for one week under laboratory conditions (12:12 LD photoperiod, 26 °C air temperature, and 60–70% relative humidity) before conducting experiments.
Pathogenicity assessment of B. bassiana Bv065 against palm weevils
To evaluate the pathogenicity of B. bassiana Bv065, mortality experiments were carried out with two weevil species: D. borassi and R. palmarum. The experiments encompassed several treatments: (1) A suspension of B. bassiana Bv065 conidia at a concentration of 1 × 109 conidia mL−1. (2) A commercial bioinsecticide containing the entomopathogenic fungus Metarhizium anisopliae at a concentration of 1 × 109 conidia mL−1 (Metaran SC, Bioquirama, Rionegro, Colombia). (3) Another commercial bioinsecticide, which incorporated the entomopathogenic bacteria Bacillus thuringiensis at a concentration of 1 × 108 UFC mL−1 (BTK, Bioquirama). (4) As a positive control, we employed the insecticide fipronil at a concentration of 0.6 mg active ingredient mL−1 (Trust 200 SC, PRECISAGRO SAS, Bogotá). This concentration was determined as the lethal dose required to induce mortality in 90% of R. palmarum in laboratory trials (Martínez et al. 2019). (5) For the negative control, mineral water with a 0.1% v/v Tween® 80 solution was used.
At the beginning of the experiments, individual weevils were rinsed with clean mineral water and subsequently immersed for 30 s in their respective treatment solutions, all prepared in mineral water with 0.1% v/v Tween® 80. Each treatment group comprised a pair of insects, consisting of one female and one male, housed in a clean 500 mL plastic container with two pieces of split sugarcane stalks for sustenance. The experiment was carried out under controlled laboratory conditions (26 ± 1°C and 60 ± 5% RH). The sugar cane was replaced every two weeks, and weevil mortality was monitored daily over a two-week period. The dead specimens were identified by their characteristic posture, lying on their side with retracted legs. These deceased weevils were then transferred to individual Petri dishes containing damp filter paper to confirm the presence of fungal sporulation.
Data analysis
All statistical analyses were performed in R 4.1.1 (Core Team 2019) using RStudio (RStudio Team 2020). To investigate the impact of temperature on colony growth, we utilized a linear mixed-effects model (LMM) from the ‘lme4’ package (Bates et al. 2015). In this analysis, temperature and time (measured in days) were included as fixed effects. To account for the presence of repeated measurements, the experimental unit code was included as a random effect, addressing any temporal autocorrelation. Next, we conducted a similar LMM analysis to examine the influence of pH on colony growth. For this purpose, pH and time (day) were included as fixed effects, and the experimental unit code was again introduced as a random effect. In both cases, for the temperature and pH models, multiple comparisons were performed among treatments using the False Discovery Rate (FDR) method (Verhoeven et al. 2005) from the ‘emmeans’ package (Lenth et al. 2019).
To analyse the metabolic profile data, we computed the average fungal growth and substrate assimilation across all 96 substrates found in the microplates. These substrates were clustered into four groups using k-means (k = 4, based on preliminary data exploration of the within-cluster sum of squares). Following this, significant differences among these clusters were confirmed by conducting a Wilks’ lambda test within a multivariate analysis of variance (MANOVA). The Wilks test was used to determine pairwise differences between the clusters and adjusted the results for FDR using the ‘biotools’ package (Da Silva 2015). Furthermore, for the analysis of the enzymatic profile of B. bassiana Bv065 we applied descriptive statistics to the acquired measurements. Triplicate evaluations of chitinases, proteases, and lipases were used for the computation of both the mean and standard error for each distinct measurement.
Finally, to evaluate the pathogenicity of B. bassiana Bv065, a two-step analysis of the mortality data was conducted. Initially, the significance of the experimental factors and their interactions using a Cox Proportional Hazards Model were assessed, employing the survival package (Therneau 2016). This model included the experimental treatment, species identity, and sex as fixed effects. Subsequently, Kaplan–Meier Survival Curves was constructed for each weevil species separately using the survival package. These curves allowed us to compare the survival distributions of weevils subjected to different experimental treatments.
In all the models above, data distribution was identified using the library fitdistrplus (Delignette-Muller et al. 2019). The significance of terms in all the models was determined using Type-II analysis of variance (or Type-II ANOVA) with the ‘Anova’ function from the car library (Fox and Weisberg 2018). The library performance (Lüdecke et al. 2019) was used to inspect and plot the model diagnostics, and figures were made using the libraries sjPlot (Lüdecke 2016), survminer (Kassambara et al. 2017), and ggplot2 (Wickham 2016).
Results
Molecular identification of the isolated strain
The isolate Bv065 was identified as B. bassiana as evidenced by comparison with the GenBank sequences of ITS and TEF1-α fragments. For the former, 99.26% identity and an E-value of 0.0 were obtained when compared with the type material accession B. bassiana ARSEF (NR_111594.1). A 100% of identity with an E-value of 6 × 10−149 was observed when the Bv065 TEF1-α sequence was aligned with the sequence of accession B. bassiana isolate CVAD02 (P328925.1).
Effect of pH and temperature on the growth of B. bassiana Bv065
The interaction between temperature and time significantly influenced the mycelial growth of the fungus B. bassiana Bv065 (LMM: χ2 = 3685.1, P < 0.001). At the end of the experiment at day 18, it became evident that Petri dishes exposed to temperatures of 8 and 35 °C exhibited no fungal growth. Among the temperatures ranging from 15 to 30 °C, noticeable variations in growth rates were observed, albeit following a linear pattern. The optimal temperature range for B. bassiana Bv065 growth was found to be between 25 and 30 °C, where the growth rate measured 3.47 ± 0.11 mm day−1 (x̄ ± SE). Importantly, there was no statistically significant difference between growth at 25 °C and 30 °C. Conversely, mycelial growth at 15 and 20 °C was restricted and notably slower compared to the rates observed at 25 and 30 °C (Fig. 1A, Table 1).
Influence of different incubation temperatures (A) and pH levels (B) on the growth of B. bassiana Bv065 over 15 days. Diametral growth was quantified by averaging measurements taken at two orthogonal points employing a digital caliper. The significance of differences is indicated by letters in accordance with pair-wise comparisons
Additionally, the pH levels and time interacted significantly, affecting the growth of B. bassiana Bv065 (LMM: χ2 = 183.21, P < 0.001). The B. bassiana isolate Bv065 exhibited robust mycelial growth across a broad pH range from 5 to 9, with a growth rate of 4.17 ± 0.23 mm day−1 (x̄ ± SE). However, growth was notably diminished at a pH of 4 in comparison to the other pH levels evaluated (Fig. 1B, Table 1).
Massive conidia production and germination of B. bassiana Bv065
The B. bassiana Bv065 isolate yielded 6.8 × 109 ± 1.7 × 108 conidia g−1 of dry substrate (mean ± SE) following a 15-day semi-solid fermentation period. Notably, these conidia displayed a germination rate of 97 ± 1% within the first 24 h of incubation.
Metabolic profile of B. bassiana Bv065
Carbon source utilization profiles for B. bassiana Bv065 were analyzed using cluster analysis. The data generated for growth and substrate assimilation revealed four distinct substrate groups (MANOVA: F = 32.13, P < 0.001). The analysis showed that cluster I, composed of sucrose, D-Mannose, γ-amino-butyric acid, glycerol, and N-acetyl-D-glucosamine, exhibited the highest B. bassiana Bv065 growth and substrate assimilation. AT the cluster level, B. bassiana Bv065 achieved a growth of 1.50 ± 0.06 OD750nm (mean ± SE) and a substrate assimilation of 2.37 ± 0.16 OD490nm over a 120-h period (Fig. 2). Specifically, B. bassiana Bv065 achieved the greatest growth using sucrose, recording a value of 1.65 OD750nm (mean ± SE) and an assimilation of 2.09 OD490nm, followed by D-trehalose with a growth of 1.39 OD750nm (mean ± SE) and an assimilation of 1.83 OD490nm. On the other hand, glycerol exhibited the highest assimilation with a value of 2.89 OD490nm and a growth of 1.54 OD750nm.
Impact of different carbon sources on B. bassiana Bv065 growth and substrate assimilation over 120 h. Growth assessments were conducted at 750 nm absorbance, while assimilation measurements were performed at 490 nm. Substrates were categorized into four distinct groups through the application of k-means clustering. Statistical analysis via MANOVA affirmed the presence of significant differences among these groups (P < 0.001). Detailed information on the substrates and their corresponding growth and assimilation values can be found in Table S1 in the supplementary material
Furthermore, cluster II exhibited a 24% decrease in assimilation and a 39% reduction in B. bassiana Bv065 growth compared to substrates from cluster I. Meanwhile, carbon sources from Groups III and IV showed a significant decrease of 50 and 60%, respectively, in both assimilation and fungal growth. Within these latter two groups, organic acids constitute most substrates at 86%, followed by amino groups at 80%, disaccharides at 67%, monosaccharides at 87%, polyalcohols at 90%, and all compounds classified as carboxylic acids, ester, and glycosides. The complete list of substrates within each group, along with precise values for B. bassiana Bv065 growth and substrate assimilation, can be found in Table S1 in the supplementary material.
In the context of each cluster, the five substrates grouped within cluster I, displaying the highest assimilation and growth values, are classified as amino acids, amino compounds, disaccharides, monosaccharides, and polyalcohols. Within cluster II, nine amino acids, two organic acids, three disaccharides, two monosaccharides, one polyalcohol, and two polysaccharides are found. Meanwhile, clusters III and IV encompass the remainder of the 72 evaluated substrates previously mentioned. In this study, it was observed that B. bassiana Bv065 achieved the highest assimilation and growth in 14 out of the 94 substrates comprising the Biolog FF™ plate.
In the comparative analysis of assimilation and growth results among different substrate groups, it was observed that the 15 amino acids exhibited the highest assimilation average, reaching a value of 1.73 ± 0.59 OD490nm. Meanwhile, the highest growth average was attained by the various disaccharides 0.79 ± 0.35 OD750nm followed by the polysaccharides 0.70 ± 0.20 OD750nm. The amino acids with assimilation values exceeding 2.00 OD490nm and a growth between 0.69 OD750nm and 1.28 OD750nm correspond to γ-amino-butyric acid, L-proline, L-glutamic acid, L-serine, N-acetyl-L-glutamic acid, glycyl-L-glutamic acid and L-alanyl-glycine. Disaccharides with assimilation exceeding 1.5 OD490nm and greater growth ranging from 1.24 OD750nm to 1.65 OD750nm correspond to sucrose, D-Trehalose, and Maltose. Meanwhile, monosaccharides with assimilations surpassing 1.5 OD490nm correspond to D-mannose, β-methyl-D-glucoside and α-D-glucose, with growth ranging between 1.12 OD750nm and 1.56 OD750nm. Finally, B. bassiana Bv065 exhibited the lowest assimilation and growth values among compounds classified within the groups of dicarboxylic acids and esters, ranging between 0.48 OD490nm to 0.51 OD490nm for assimilation and 0.30 OD750nm to 0.25 OD750nm for growth, respectively.
Enzimatic profile of B. bassiana Bv065
Proteases exhibited the highest activity among the enzymes measured, with levels reaching 3.69 ± 0.16 U mL−1 (mean ± SE). Conversely, chitinases showed a lower activity level, measuring 0.06 ± 0.01 U mL−1 (mean ± SE). Lipases, on the other hand, displayed no detectable activity, registering levels of 0 U mL−1 (mean ± SE).
Pathogenicity assessment of B. bassiana Bv065 against palm weevils
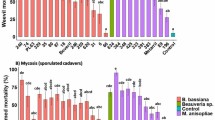
The Cox Proportional Hazards Model revealed a significant interaction between the experimental treatment applied to the weevils and their species identity (CoxPH: χ2 = 22.71, P < 0.001). However, neither the weevils’ sex nor the interaction of sex with treatment or species identity showed statistical significance. Therefore, sex was not considered as a fixed effect in subsequent analyses.
Furthermore, when examining survival distribution for each species separately, we observed that D. borassi weevils experienced 100% mortality when exposed to the positive control treatment with fipronil (P < 0.001). These insects perished within 48 h. Additionally, when D. borassi weevils were treated with B. bassiana Bv065, 50% of the test subjects died within nine days, and by day 15, approximately 94% of the insects had perished as well (P < 0.001). None of the other experimental treatments resulted in significant mortality for D. borassi (Fig. 3A). On the other hand, R. palmarum had its survival impacted solely by exposure to the control treatment, the insecticide fipronil, similar to D. borassi. In this case, all R. palmarum insects died within 48 h (P < 0.001). However, R. palmarum weevils did not exhibit significant susceptibility to treatment with B. bassiana Bv065 or any of the other experimental treatments (Fig. 3B).
Evaluation of B. bassiana Bv065 pathogenicity. Survival analysis comparing the mortality of adult palm weevils of the species D. borassi (A) and R. palmarum (B) following treatment with B. bassiana Bv065, a negative control (water), a positive control (fipronil), and two commercial biological insecticides (Bacillus and Metharizium). *** indicates significant difference when compared with the positive control treatment
Discussion
This study represents an initial exploration into the isolation and characterisation of a strain of B. bassiana adept at managing a prevalent pest that impacts coconut and peach palm cultivation in neotropical regions, particularly targeting the weevil D. borassi (Gaviria et al. 2021; Hussain et al. 2022). Understanding the optimal growth conditions for a biological control agent is pivotal for crafting a highly effective and enduring biopesticide for field application. Factors such as temperature, humidity, radiation, and pH play pivotal roles in influencing the viability, persistence, and efficacy of entomopathogenic fungi throughout their production, storage, and application phases.
In our investigation, the B. bassiana Bv065 strain exhibited the highest growth rate within the temperature range of 25 to 30 °C, a range widely acknowledged for optimal growth among entomopathogenic fungi (Hallsworth and Magan 1999). Conversely, diminished growth at alternative temperatures could be attributed to phenomena such as protein denaturation and membrane disorganisation, thereby impeding the fungus’s growth and germination (Fernandes et al. 2009). Additionally, the B. bassiana Bv065 strain demonstrated optimal growth within a pH range of 6 to 8, indicating the strain’s proficiency in effectively regulating cytosolic pH (Hallsworth and Magan 1996; Gharibzahedi et al. 2014). Moreover, considering the typical pH range of insect cuticles, which range between 6 to 8, it is reasonable to assume that optimal growth at this pH leads to the expression of enzymes involved in the degradation and penetration of the insect cuticle by the fungus (Leger 1995).
Furthermore, we found that metabolites such as sucrose, D-mannose, γ-amino-butyric acid, glycerol, and N-acetyl-D-glucosamine play a major role in B. bassiana Bv065 strain metabolism. These metabolites were highly assimilated and induced the greatest growth of B. bassiana Bv065. These results align with those obtained by Canfora et al. (2017), who demonstrated that substrates such as N-acetyl-D-glucosamine, glycerol, D-trehalose, and γ-Amino-n-Butyric Acid, among others, are highly metabolised by Beauveria species. The affinity with N-acetyl-D-glucosamine reflects the relevance of B. bassiana Bv065 as an entomopathogen, as this metabolite is a component of the polysaccharide chitin found in the exoskeletons of arthropods (Le Guen et al. 2017). Additionally, the substrate for mass-producing fungus may influence important traits; for instance, it has been shown that sucrose and glycerol can influence the production of secondary metabolites such as polyketides and terpenes by entomopathogenic fungi, including the Beauveria genus (Zhang et al. 2020). Such secondary metabolites are known for their bioactive properties and their essential role in the pathogenicity of entomopathogenic fungi (Pedrini 2022; Toopaang et al. 2023).
The metabolic profile of B. bassiana has been identified as a major factor in the role of this fungal species as an entomopathogen. It has been established that B. bassiana is capable of growing in different environmental conditions due to its metabolic plasticity (Luo et al. 2015). Thus, the metabolic shift of B. bassiana is influenced by the availability and composition of carbon sources, impacting its growth, conidiation, stress tolerance, and pathogenicity (Mohamed et al. 2021; Gutiérrez Román et al. 2022). Additionally, the metabolic preferences of this genus for specific carbon sources are linked to its ability to repair DNA damage, improve growth, and modulate its lifecycle (Wang et al. 2019; Ren et al. 2021).
Moreover, the assimilation of substrates as a carbon source is highly dependent on the production of enzymes that catalyse their degradation. These enzymes are also considered crucial pathogenicity factors of entomopathogenic fungi (Golzan et al. 2023). In semi-solid fermentation, the Bv065 strain mainly produced proteases and chitinases, while lipases were not detected. Proteases play a crucial role in the pathogenicity of entomopathogenic fungi, aiding in the degradation of the insect cuticle and haemolymph, and facilitating invasion and growth within the host (Leger 1995; Khan et al. 2016). Similarly, chitinases may play a crucial role in this entomopathogenic fungus, particularly in cell wall morphogenesis and host-parasite interactions (Sahai and Manocha 1993). These enzymes are involved in the degradation of chitin, a major component of insect exoskeletons, allowing the fungus to penetrate and infect its host (Gooday et al. 1986). In the degradation process of the insect cuticle, N-acetyl-D-glucosamine is released (Liu et al. 2023), which in this study proved to be one of the substrates that significantly influenced fungal growth.
Regarding the pathogenicity of B. bassiana Bv065 strain against palm weevils, our results indicate that this strain is capable of infecting and killing adults of the species D. borassi, with greater effectiveness than other commercially available entomopathogens. The high mortality caused by this strain to individuals of D. borassi can be explained by the specificity of this fungus strain with this weevil species, as the original isolation was made after a D. borassi weevil naturally infected in field conditions. Conversely, the Bv065 strain was not significantly effective in causing lethal effects to the weevil species R. palmarum, which has shown susceptibility to other B. bassiana strains as reported in other studies (León-Martínez et al. 2019; Dalbon et al. 2024). Also, B. bassiana has been effective for controlling other palm weevil species like R. ferrugineus (Dembilio et al. 2010; Yang et al. 2023). Although the Bv065 strain may not cause significant lethal effects on palmarum, there may be sublethal effects of B. bassiana infection as suggested by previous studies. B. bassiana may cause a transgenerational effect on female weevils inoculated and that managed to survive infection. The offspring of such females (both in the egg and larvae stages) have lower survival rates and are more susceptible to B. bassiana. Additionally, surviving insects of the infection may act as potential vectors for the propagation of the entomopathogen and facilitate horizontal transmission of the entomopathogen (Lo Verde et al. 2015; León Martínez et al. 2019).
Since D. borassi and R. palmarum have been recognized as pests of economic importance for the crops of Bactris gasipaes and Cocos nucifera (Bautista-Giraldo et al. 2020; Gaviria et al. 2021; Gutiérrez et al. 2023), the results obtained with the Bv065 strain show great potential for its use in the field, where different dissemination strategies can be employed such as trunk applications (Güerri-Agulló et al. 2010), passage traps for the inoculation of adults (Dembilio et al. 2018), release of contaminated males (León Martínez et al. 2019); where the control objective is the adults. Additionally, exploratory studies have shown the potential use of B. bassiana in endotherapy (i.e., trunk injection) for the control of weevil larvae inside the palms (Sutanto et al. 2023; Husain et al. 2024).
Overall, the B. bassiana Bv065 strain exhibited distinctive traits across diverse conditions, reflecting the natural habitat of palm weevils in Colombia. The optimal growth range for B. bassiana Bv065 in terms of temperature and pH provides the basis for optimizing mass production and identifying bottlenecks for use in field conditions. Furthermore, the metabolic profiling assessment of B. bassiana Bv065 enabled the identification of different carbon sources that would increase the growth of this entomopathogenic fungus. This serves the development of a culture medium conducive to high fungal growth, sporulation, and biological activity. Additionally, it facilitated the identification of substances viable for use in the large-scale production process and subsequent formulation of a bio-pesticide, utilizing this strain as the primary active ingredient. Finally, the promising preliminary pathogenicity assessments show that this strain is effective for the control of the pervasive palm weevil D. borassi.
Data Availability
The data that support the findings of this study are available on request from the corresponding author, YG
References
Abbas MST (2010) IPM of the red palm weevil, Rhynchophorus ferrugineus. In: Integrated management of arthropod pests and insect borne diseases. Springer, pp 209–233
Agronet (2020) Reporte: Área, Producción y Rendimiento Nacional por Cultivo: Chontaduro. In: Ministerio de Agricultura y Desarrollo Rural
Ahmed R, Freed S (2021) Biochemical resistance mechanisms against chlorpyrifos, imidacloprid and lambda-cyhalothrin in Rhynchophorus ferrugineus (Olivier)(Coleoptera: Curculionidae). Crop Prot 143:105568
Akello J, Dubois T, Coyne D, Kyamanywa S (2010) The pathogenicity of Beauveria bassiana: what happens after an endophytic phase in plants? Commun Agric Appl Biol Sci 75:273–278
Alvarado HL, Montes LG, Gomes H et al (2013) Patogenicidad de cepas de Metarhizium anisopliae (L.) y Beauveria bassiana sobre Rhynchophorus palmarum. Revista Palmas 34:15–24
Barra-Bucarei L, González MG, Iglesias AF et al (2020) Beauveria bassiana multifunction as an endophyte: Growth promotion and biologic control of Trialeurodes vaporariorum, (Westwood) (Hemiptera: Aleyrodidae) in tomato. InSects 11:591
Bates D, Maechler M, Bolker B (2014) Walker S (2015) lme4: Linear mixed-effects models using Eigen and S4. R Package Version 1:1–7
Bautista-Giraldo MA, Armbrecht I, Vásquez-Ordoñez AA (2020) The weevil Dynamis borassi (Coleoptera: Curculionidae: Dryophthorinae) associated with native palms in forests and disturbed areas in Buenaventura, Colombia. Rev Colomb Entomol 46:
Canfora L, Abu-Samra N, Tartanus M, et al (2017) Co-inoculum of Beauveria brongniartii and B. bassiana shows in vitro different metabolic behaviour in comparison to single inoculums. Sci Rep 7:13102
Couturier G, O’Brien CW, Kahn F (1998) Astrocaryum carnosum and A. chonta (Palmac), New Host for the Weevil Dynamis borassi (Curculionidae: Rhynchophorinae). Principes 42:227–228
Cupp-Enyard C (2008) Sigma’s non-specific protease activity assay-casein as a substrate. JoVE (Journal of Visualized Experiments) e899
Da Silva AR (2015) Biotools: tools for biometry and applied statistics in agricultural science. R package version 2:
Da Silva WOB, Santi L, Schrank A, Vainstein MH (2010) Metarhizium anisopliae lipolytic activity plays a pivotal role in Rhipicephalus (Boophilus) microplus infection. Fungal Biol 114:10–15
Dalbon VA, Acevedo JPM, Ribeiro Junior KAL et al (2024) Native Entomopathogenic Fungi Isolated from Rhynchophorus palmarum (Linnaeus, 1758) in Northeast Brazil. InSects 15:159
Delignette-Muller ML, Dutang C, Pouillot R, et al (2019) Package ‘fitdistrplus’
Dembilio Ó, Moya P, Vacas S et al (2018) Development of an attract-and-infect system to control Rhynchophorus ferrugineus with the entomopathogenic fungus Beauveria bassiana. Pest Manag Sci 74:1861–1869
Dembilio Ó, Quesada-Moraga E, Santiago-Álvarez C, Jacas JA (2010) Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the Red Palm Weevil, Rhynchophorus ferrugineus. J Invertebr Pathol 104:214–221
Devine GJ, Furlong MJ (2007) Insecticide use: contexts and ecological consequences. Agric Human Values. https://doi.org/10.1007/s10460-007-9067-z
Faleiro JR, Ferry M, Yaseen T, Al-Dobai S (2019) Overview of the gaps, challenges and prospects of red palm weevil management. Arab Journal of Plant Protection 37:
Fernandes ÉKK, Moraes AML, Pacheco RS et al (2009) Genetic diversity among Brazilian isolates of Beauveria bassiana: comparisons with non-Brazilian isolates and other Beauveria species. J Appl Microbiol 107:760–774
Fox J, Weisberg S (2018) An R Companion to Applied Regression. Sage Publications, Thousand Oaks, CA
Gaviria J, Montoya-Lerma J, Armbrecht I et al (2021) Dynamis borassi (Coleoptera: Curculionidae), a new potential pest to the palms (Arecaceae): an early warning for the palm producers. Florida Entomologist 104:107–116
Gerber K, Giblin-Davis RM (1990) Association of the red ring nematode and other nematode species with the palm weevil, Rhynchophorus palmarum. J Nematol 22:143
Gharibzahedi SMT, Razavi SH, Mousavi SM (2014) Characterization of bacteria of the genus Dietzia: an updated review. Ann Microbiol 64:1–11
Giblin-Davis RM, Gries R, Gries G et al (1997) Aggregation pheromone of palm weevil, Dynamis borassi. J Chem Ecol 23:2287–2297
Glogauer A, Martini VP, Faoro H et al (2011) Identification and characterization of a new true lipase isolated through metagenomic approach. Microb Cell Fact 10:1–15
Golzan SR, Talaei-Hassanloui R, Homayoonzadeh M, Safavi SA (2023) Role of cuticle-degrading enzymes of Beauveria bassiana and Metarhizium anisopliae in virulence on Plodia interpunctella (Lepidoptera, Pyralidae) larvae. J Asia Pac Entomol 26:102038
Gomez-Vidal S, Lopez-Llorca LV, Jansson H-B, Salinas J (2006) Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron 37:624–632
Gooday GW, Humphreys AM, McIntosh WH (1986) Roles of chitinases in fungal growth. Chitin in nature and technology 83–91
Grijalba EP, Espinel C, Cuartas PE et al (2018) Metarhizium rileyi biopesticide to control Spodoptera frugiperda: Stability and insecticidal activity under glasshouse conditions. Fungal Biol 122:1069–1076
Güerri-Agulló B, Gómez-Vidal S, Asensio L et al (2010) Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: a SEM study. Microsc Res Tech 73:714–725. https://doi.org/10.1002/jemt.20812
Gutiérrez Román AIF, Laynes Zela PF, Acuña Payano RK et al (2022) Production of sustainable proteins through the conversion of insects to proteins using beauveria bassiana cultures. Front Sustain Food Syst 5:760274
Gutiérrez Y (2020) Multiple mechanisms in which agricultural insects respond to environmental stressors: canalization, plasticity and evolution. Revista De Ciencias Agrícolas 37:90–99
Gutiérrez Y, Santos‐Holguín JM, Moncayo V, Guzmán FA (2023) Spatiotemporal dynamics of a palm weevils’ outbreak and susceptibility of peach palm (Bactris gasipaes Kunth) landraces in a germplasm bank in southwestern Colombia. Annals of Applied Biology
Hallsworth JE, Magan N (1999) Water and temperature relations of growth of the entomogenous fungi Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces farinosus. J Invertebr Pathol 74:261–266
Hallsworth JE, Magan N (1996) Culture age, temperature, and pH affect the polyol and trehalose contents of fungal propagules. Appl Environ Microbiol 62:2435–2442
Husain M, Sutanto KD, Rasool KG et al (2024) Translocation and survival of trunk injected Beauveria bassiana (Hypocreales: Cordycipitaceae) in healthy date palm trees. Journal of King Saud University-Science 36:103077
Hussain A, Rizwan-ul-Haq M, Al-Ayedh H et al (2015) Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. Biocontrol 60:849–859
Hussain A, Rizwan-ul-Haq M, Al-Jabr AM, Al-Ayied HY (2013) Managing invasive populations of red palm weevil: A worldwide perspective. J Food Agric Environ 11:456–463
Hussain Z, Sarwar ZM, Akbar A et al (2022) Spatiotemporal Distribution Patterns of Pest Species (Lepidoptera: Noctuidae) Affected by Meteorological Factors in an Agroecosystem. Agriculture 12:2003
Isman MB (2006) Botanical Insecticides, Deterrents, and Repellents in Modern Agriculture and an Increasingly Regulated World. Annu Rev Entomol. https://doi.org/10.1146/annurev.ento.51.110104.151146
Jalinas J, Lopez-Moya F, Marhuenda-Egea FC, Lopez-Llorca LV (2022) Beauveria bassiana (Hypocreales: Clavicipitaceae) volatile organic compounds (VOCs) repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of Fungi 8:843
Kassambara A, Kosinski M, Biecek P (2017) survminer: Drawing Survival Curves using’ggplot2’. R Package Version 03:1
Khan S, Nadir S, Wang X et al (2016) Using in silico techniques: Isolation and characterization of an insect cuticle-degrading-protease gene from Beauveria bassiana. Microb Pathog 97:189–197
Le Guen Y, Chassagne P, Le Heiget G, et al (2017) Allyl 4, 6-O-benzylidene-2-deoxy-2-trichloroacetamido-β-d-glucopyranoside. In: Carbohydrate Chemistry. CRC Press, pp 333–342
Leger RJ (1995) The role of cuticle-degrading proteases in fungal pathogenesis of insects. Can J Bot 73:1119–1125
Lenth R, Singmann H, Love J, et al (2019) Package ‘emmeans’
León Martínez GA, Campos-Pinzón JC, Arguelles-Cárdenas JH (2019) Patogenicidad y autodiseminación de cepas promisorias de hongos entomopatógenos sobre Rhynchophorus palmarum L. (Coleoptera: Dryophthoridae). Agronomía Mesoamericana 631–646. https://doi.org/10.15517/am.v30i3.36184
León-Martínez GA, Campos-Pinzón JC, Arguelles-Cárdenas JH (2019) Patogenicidad y autodiseminación de cepas promisorias de hongos entomopatógenos sobre Rhynchophorus palmarum L.(Coleoptera: Dryophthoridae). Agronomy Mesoamerican 631–646
Liu J-H, Dong J-C, Gao J-J et al (2023) Three chitin deacetylase family members of Beauveria bassiana modulate Asexual Reproduction and Virulence of Fungi by Mediating Chitin Metabolism and affect fungal parasitism and Saprophytic Life. Microbiol Spectr 11:e04748-e4822
Lo Verde G, Torta L, Mondello V et al (2014) Pathogenicity Bioassays of Isolates of Beauveria Bassiana on Rhynchophorus Ferrugineus. Pest Manag Sci. https://doi.org/10.1002/ps.3852
Lo Verde G, Torta L, Mondello V et al (2015) Pathogenicity bioassays of isolates of Beauveria bassiana on Rhynchophorus ferrugineus. Pest Manag Sci 71:323–328
Löhr B, Parra PP (2014) Manual de trampeo del picudo negro de las palmas, Rhynchophorus palmarum, en trampas de feromona adaptadas a la situación particular de pequeños productores de la costa del Pacífico Colombiano.
López-Luján LM, Ramírez-Restrepo S, Bedoya-Pérez JC et al (2022) Bioactivity of Fungi Isolated From Coconut Growing Areas Against Rhynchophorus palmarum. Pesqui Agropecu Bras. https://doi.org/10.1590/s1678-3921.pab2022.v57.02882
Lüdecke D (2016) sjPlot: data visualization for statistics in social science. R package version 2:
Lüdecke D, Makowski D, Waggoner P (2019) Performance: assessment of regression models performance. R Package Version 04:2
Luo F, Wang Q, Yin C et al (2015) Differential metabolic responses of Beauveria bassiana cultured in pupae extracts, root exudates and its interactions with insect and plant. J Invertebr Pathol 130:154–164
Maluta N, Castro T, Lopes JRS (2022) Entomopathogenic fungus disrupts the phloem-probing behavior of Diaphorina citri and may be an important biological control tool in citrus. Sci Rep 12:7959
Martínez LC, Plata-Rueda A, Rodríguez-Dimaté FA et al (2019) Exposure to insecticides reduces populations of Rhynchophorus palmarum in oil palm plantations with Bud Rot disease. InSects 10:111
McGuire AV, Northfield TD (2020) Tropical occurrence and agricultural importance of Beauveria bassiana and Metarhizium anisopliae. Front Sustain Food Syst 4:6
Mejía C, Espinel C, Forero M et al (2020) Improving ecological fitness of Beauveria bassiana conidia to control the sugar cane borer Diatraea saccharalis. Biocontrol Sci Technol 30:513–530
Meyling NV, Lübeck M, Buckley E, et al (2009) Community Composition, Host Range and Genetic Structure of the Fungal Entomopathogen Beauveria in Adjoining Agricultural and Seminatural Habitats. Mol Ecol. https://doi.org/10.1111/j.1365-294x.2009.04095.x
Mohamed RA, Ren K, Mou Y-N et al (2021) Genome-wide insight into profound effect of carbon catabolite repressor (Cre1) on the insect-pathogenic lifecycle of Beauveria bassiana. Journal of Fungi 7:895
Oehlschlager AC (2016) Palm Weevil Pheromones–Discovery and Use. J Chem Ecol. https://doi.org/10.1007/s10886-016-0720-0
Oehlschlager AC, Chinchilla C, Castillo G, Gonzalez L (2002) Control of red ring disease by mass trapping of Rhynchophorus palmarum (Coleoptera: Curculionidae). Florida Entomologist 507–513
Pedrini N (2022) The entomopathogenic fungus Beauveria bassiana shows its toxic side within insects: Expression of genes encoding secondary metabolites during pathogenesis. Journal of Fungi 8:488
Ren K, Mou Y, Tong S et al (2021) SET1/KMT2-governed histone H3K4 methylation coordinates the lifecycle in vivo and in vitro of the fungal insect pathogen Beauveria bassiana. Environ Microbiol 23:5541–5554
Ricaño J, Güerri-Agulló B, Serna-Sarriás MJ et al (2013) Evaluation of the pathogenicity of multiple isolates of Beauveria bassiana (Hypocreales: Clavicipitaceae) on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) for the assessment of a solid formulation under simulated field conditions. Florida Entomologist 96:1311–1324
Rochat D, Malosse C, Lettere M et al (1991) Male-produced aggregation pheromone of the American palm weevil, Rhynchophorus palmarum (L.)(Coleoptera, Curculionidae): Collection, identification, electrophysiogical activity, and laboratory bioassay. J Chem Ecol 17:2127–2141
Sahai AS, Manocha MS (1993) Chitinases of fungi and plants: their involvement in morphogenesis and host—parasite interaction. FEMS Microbiol Rev 11:317–338
Sani I, Ismail SI, Abdullah S et al (2020) A review of the biology and control of whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), with special reference to biological control using entomopathogenic fungi. InSects 11:619
Sullivan CF, Parker BL, Skinner M (2022) A review of commercial Metarhizium-and Beauveria-based biopesticides for the biological control of ticks in the USA. InSects 13:260
Sutanto KD, Al-Shahwan IM, Husain M et al (2023) Field evaluation of promising indigenous entomopathogenic fungal isolates against red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of Fungi 9:68
Team RC (2019) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Team Rs (2020) RStudio: integrated development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/
Therneau T (2016) A Package for Survival Analysis in S. version 2.38. 2015. Reference Source
Toopaang W, Panyawicha K, Srisuksam C et al (2023) Metabolomic analysis demonstrates the impacts of polyketide synthases PKS14 and PKS15 on the production of beauvericins, bassianolide, enniatin A, and ferricrocin in entomopathogen Beauveria bassiana. Metabolites 13:425
Vásquez-Ordóñez AA, Löhr B, Marvaldi AE (2020) Comparative morphology of the larvae of the palm weevils dynamis borassi (fabricius) and rhynchophorus palmarum (linnaeus) (curculionidae: dryophthorinae): two major pests of peach palms in the neotropics. Pap Avulsos Zool. https://doi.org/10.11606/1807-0205/2020.60.special-issue.27
Verhoeven KJF, Simonsen KL, McIntyre LM (2005) Implementing false discovery rate control: increasing your power. Oikos 108:643–647
Wahengbam J, Bhushan LS, Patil JB, Pathma J (2021) Insecticides Derived from Natural Products: Diversity and Potential Applications. Current Trends in Microbial Biotechnology for Sustainable Agriculture 403–437
Wang D-Y, Fu B, Tong S-M et al (2019) Two photolyases repair distinct DNA lesions and reactivate UVB-inactivated conidia of an insect mycopathogen under visible light. Appl Environ Microbiol 85:e02459-e2518
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer
Xiao G, Ying S, Zheng P et al (2012) Genomic perspectives on the evolution of fungal entomopathogenicity in beauveria bassiana. Sci Rep. https://doi.org/10.1038/srep00483
Yang T-H, Wu L-H, Liao C-T et al (2023) Entomopathogenic fungi-mediated biological control of the red palm weevil Rhynchophorus ferrugineus. J Asia Pac Entomol 26:102037
Zhang L, Fasoyin OE, Molnár I, Xu Y (2020) Secondary metabolites from hypocrealean entomopathogenic fungi: novel bioactive compounds. Nat Prod Rep 37:1181–1206
Acknowledgements
The initial isolation of fungi from the infected weevil was made possible with the kind assistance of Cenipalma (La Providencia research station). We extend our gratitude to Jose L. Preciado, Elvira H. Estrilla, and Juan C. Gonzáles for their valuable contributions in maintaining the weevil traps and collecting the insects. This research received funding from the Ministry of Agriculture and Rural Development (Colombia) and the Colombian Corporation for Agricultural Research AGROSAVIA, specifically through the “Colección de Microorganismos con Interés en Control Biológico conservado y caracterizado” (Tv21) project.
Funding
Open Access funding provided by Colombia Consortium. Ministry of Agriculture and Rural Development (Colombia), Agrosavia
Author information
Authors and Affiliations
Contributions
YG, LU, CVA, JLR and CM designed the study. KAA, CO, JMS, JLG and CM collected the data. YG, CVA and LU analysed the data. YG, KAA, CO, JMS, JLG, CM, CVA and LU wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Ethical approval
All applicable national guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gutiérrez, Y., Alarcón, K.A., Ortiz, C. et al. Isolation and characterization of a native strain of the entomopathogenic fungus Beauveria bassiana for the control of the palm weevil Dynamis borassi (Coleoptera: Curculionidae) in the neotropics. World J Microbiol Biotechnol 40, 260 (2024). https://doi.org/10.1007/s11274-024-04044-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-024-04044-5