Abstract
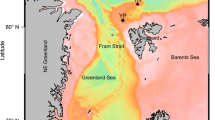
The thawing of snow and sea ice produces distinctive melt ponds on the surface of the Arctic sea ice, which covers a significant portion of the surface sea ice during summer. Melt-pond salinity impacts heat transfer to the ice below and the melting rate. It is widely known that melt ponds play a significant role in heat fluxes, ice-albedo feedback, and sea-ice energy balance. However, not much attention has been given to the fact that melt ponds also serve as a unique microbial ecosystem where microbial production begins as soon as they are formed. Here, we investigated the role of melt pond salinity in controlling the diversity and distribution of prokaryotic communities using culture-dependent and –independent approaches. The 16 S rRNA gene amplicon based next generation sequencing analysis retrieved a total of 14 bacterial phyla, consisting of 146 genera, in addition to two archaeal phyla. Further, the culture-dependent approaches of the study allowed for the isolation and identification of twenty-four bacterial genera in pure culture. Flavobacterium, Candidatus_Aquiluna, SAR11 clade, Polaribacter, Glaciecola, and Nonlabens were the dominant genera observed in the amplicon analysis. Whereas Actimicrobium, Rhodoglobus, Flavobacterium, and Pseudomonas were dominated in the culturable fraction. Our results also demonstrated that salinity, chlorophyll a, and dissolved organic carbon were the significant environmental variables controlling the prokaryotic community distribution in melt ponds. A significant community shift was observed in melt ponds when the salinity changed with the progression of melting and deepening of ponds. Different communities were found to be dominant in melt ponds with different salinity ranges. It was also observed that melt pond prokaryotic communities significantly differed from the surface ocean microbial community. Our observations suggest that complex prokaryotic communities develop in melt ponds immediately after its formation using dissolved organic carbon generated through primary production in the oligotrophic water.






Similar content being viewed by others
Data Availability
The datasets generated and/or analysed in the current study are available in the NCBI repository, BioProject number PRJNA770954. Submission information can be found at: http://www.ncbi.nlm.nih.gov/sra.
Code Availability
Codes used in the current study are available from the corresponding author upon reasonable request.
References
Abell GC, Bowman JP (2005) Ecological and biogeographic relationships of class Flavobacteria in the Southern Ocean. FEMS Microbiol Ecol 51(2):265–277
Akulava V, Miamin U, Akhremchuk K, Valentovich L, Dolgikh A, Shapaval V (2022) Isolation, physiological characterization, and antibiotic susceptibility testing of fast-growing Bacteria from the Sea-affected Temporary Meltwater ponds in the Thala Hills Oasis (Enderby Land, East Antarctica). Biology 11(8):1143
Ambrose WG, Quillfeldt Cv, Clough LM, Tilney PV, Tucker T (2005) The sub-ice algal community in the Chukchi sea: large-and small-scale patterns of abundance based on images from a remotely operated vehicle. Polar Biol 28:784–795
Archer SD, McDonald IR, Herbold CW, Lee CK, Cary CS (2015) Benthic microbial communities of coastal terrestrial and ice shelf Antarctic meltwater ponds. Front Microbiol 6:485
Archer SD, McDonald IR, Herbold CW, Lee CK, Niederberger TS, Cary C (2016) Temporal, regional and geochemical drivers of microbial community variation in the melt ponds of the Ross Sea region, Antarctica. Polar Biol 39(2):267–282
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman J, Smith JA, Struhl K (1992) Short protocols in molecular biology. New York 275:28764–28773
Avcı B, Krüger K, Fuchs BM, Teeling H, Amann RI (2020) Polysaccharide niche partitioning of distinct Polaribacter clades during North Sea spring algal blooms. ISME J 14(6):1369–1383
Bowman JP, McCAMMON SA, BROWN JL, McMEEKIN TA (1998) Glaciecola punicea gen. nov., sp. nov. and Glaciecola pallidula gen. nov., sp. nov.: psychrophilic bacteria from Antarctic sea-ice habitats. Int J Syst Evol Microbiol 48(4):1213–1222
Brinkmeyer R, Glöckner F-O, Helmke E, Amann R (2004) Predominance of β-proteobacteria in summer melt pools on Arctic pack ice. Limnol Oceanogr 49(4):1013–1021
Cardozo-Mino MG, Fadeev E, Salman-Carvalho V, Boetius A (2022) Spatial distribution of Arctic Bacterioplankton abundance is linked to distinct Water masses and Summertime Dynamics (Fram Phytoplankton Strait, 79◦ N) Bloom. Front Microbiol 12:1–14
Chen M, Jung J, Lee YK, Hur J (2018) Surface accumulation of low molecular weight dissolved organic matter in surface waters and horizontal off-shelf spreading of nutrients and humic-like fluorescence in the Chukchi Sea of the Arctic Ocean. Sci Total Environ 639:624–632
Choi T-H, Lee HK, Lee K, Cho J-C (2007) Ulvibacter antarcticus sp. nov., isolated from Antarctic coastal seawater. Int J Syst Evol Microbiol 57(12):2922–2925
Chong J, Liu P, Zhou G, Xia J (2020) Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat Protoc 15(3):799–821
Comiso JC (2012) Large decadal decline of the Arctic multiyear ice cover. J Clim 25(4):1176–1193
Diamond R, Sime LC, Schroeder D, Guarino M-V (2021) The contribution of melt ponds to enhanced Arctic sea-ice melt during the last interglacial. The Cryosphere 15(11):5099–5114
Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MG (2020) PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 38(6):685–688
Eicken H, Grenfell T, Perovich D, Richter-Menge J, Frey K (2004) Hydraulic controls of summer Arctic pack ice albedo. J Geophys Res Oceans 109:C8
Eronen-Rasimus E, Piiparinen J, Karkman A, Lyra C, Gerland S, Kaartokallio H (2016) Bacterial communities in Arctic first‐year drift ice during the winter/spring transition. Environ Microbiol Rep 8(4):527–535
Fernández-Méndez M, Katlein C, Rabe B, Nicolaus M, Peeken I, Bakker K, Flores H, Boetius A (2015) Photosynthetic production in the central Arctic Ocean during the record sea-ice minimum in 2012. Biogeosciences 12(11):3525–3549
Fernández-Méndez M, Turk-Kubo KA, Buttigieg PL, Rapp JZ, Krumpen T, Zehr JP, Boetius A (2016) Diazotroph diversity in the sea ice, melt ponds, and surface waters of the Eurasian Basin of the Central Arctic Ocean. Front Microbiol 7:1884
Fetterer F, Untersteiner N (1998) Observations of melt ponds on Arctic Sea Ice. J Geophys Res Oceans 103(C11):24821–24835
Fuhrman JA, Cram JA, Needham DM (2015) Marine microbial community dynamics and their ecological interpretation. Nat Rev Microbiol 13(3):133–146
Galgani L, Piontek J, Engel A (2016) Biopolymers form a gelatinous microlayer at the air-sea interface when Arctic sea ice melts. Sci Rep 6(1):29465
Gawor J, Grzesiak J, Sasin-Kurowska J, Borsuk P, Gromadka R, Górniak D, Świątecki A, Aleksandrzak-Piekarczyk T, Zdanowski MK (2016) Evidence of adaptation, niche separation and microevolution within the genus Polaromonas on Arctic and Antarctic glacial surfaces. Extremophiles 20:403–413
Geilfus N-X, Galley R, Crabeck O, Papakyriakou T, Landy J, Tison J-L, Rysgaard S (2015) Inorganic carbon dynamics of melt-pond-covered first-year sea ice in the Canadian Arctic. Biogeosciences 12(6):2047–2061
Giebel H-A, Wolterink M, Brinkhoff T, Simon M (2019) Complementary energy acquisition via aerobic anoxygenic photosynthesis and Carbon Monoxide oxidation by Planktomarina temperata of the Roseobacter group. FEMS Microbiol Ecol 95(5):fiz050
Gifford SM, Zhao L, Stemple B, DeLong K, Medeiros PM, Seim H, Marchetti A (2020) Microbial niche diversification in the Galápagos Archipelago and its response to El Niño. Front Microbiol 11:575194
Giovannoni SJ, Bibbs L, Cho J-C, Stapels MD, Desiderio R, Vergin KL, Rappé MS, Laney S, Wilhelm LJ, Tripp HJ (2005) Proteorhodopsin in the ubiquitous marine bacterium SAR11. Nature 438(7064):82–85
Gordon LI, Jennings Jr JC, Ross AA, Krest JM (1993) A suggested protocol for continuous flow automated analysis of seawater nutrients (phosphate, nitrate, nitrite and silicic acid) in the WOCE Hydrographic Program and the Joint Global Ocean fluxes Study. WOCE Hydrographic Program Office Methods Manual WHPO 68/91:1–52
Gosink JJ, Woese CR, Staley JT (1998) Polaribacter gen. nov., with three new species, P. irgensii sp. nov., P. franzmannii sp. nov. and P. filamentus sp. nov., gas vacuolate polar marine bacteria of the Cytophaga-Flavobacterium-Bacteroides group and reclassification of ‘Flectobacillus glomeratus’ as Polaribacter glomeratus comb. Nov. Int J Syst Evol Microbiol 48(1):223–235
Gradinger R (2003) Sea ice microorganisms. Encyclopedia of environmental microbiology
Hahn MW (2009) Description of seven candidate species affiliated with the phylum Actinobacteria, representing planktonic freshwater bacteria. Int J Syst Evol Microbiol 59(0 1):112
Hahnke RL, Bennke CM, Fuchs BM, Mann AJ, Rhiel E, Teeling H, Amann R, Harder J (2015) Dilution cultivation of marine heterotrophic bacteria abundant after a spring phytoplankton bloom in the N orth S ea. Environ Microbiol 17(10):3515–3526
Han D, Kang I, Ha HK, Kim HC, Kim O-S, Lee BY, Cho J-C, Hur H-G, Lee YK (2014) Bacterial communities of surface mixed layer in the Pacific sector of the western Arctic Ocean during sea-ice melting. PLoS ONE 9(1):e86887
Hatam I, Charchuk R, Lange B, Beckers J, Haas C, Lanoil B (2014) Distinct bacterial assemblages reside at different depths in Arctic Multiyear Sea Ice. FEMS Microbiol Ecol 90(1):115–125
Hatam I, Lange B, Beckers J, Haas C, Lanoil B (2016) Bacterial communities from Arctic Seasonal Sea ice are more compositionally variable than those from multi-year sea ice. ISME J 10(10):2543–2552
Heberle H, Meirelles GV, da Silva FR, Telles GP, Minghim R (2015) InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform 16(1):1–7
Inoue J, Kikuchi T, Perovich DK (2008) Effect of heat transmission through melt ponds and ice on melting during summer in the Arctic Ocean. J Geophys Res Oceans 113:C5
Joint I, Mühling M, Querellou J (2010) Culturing marine bacteria–an essential prerequisite for biodiscovery. Microb Biotechnol 3(5):564–575
Jung SW, Park JS, Kown OY, Kang J-H, Shim WJ, Kim Y-O (2010) Effects of crude oil on marine microbial communities in short term outdoor microcosms. J Microbiol 48:594–600
Jung J, Hong S-B, Chen M, Hur J, Jiao L, Lee Y, Park K, Hahm D, Choi J-O, Yang EJ (2020) Characteristics of methanesulfonic acid, non-sea-salt sulfate and organic carbon aerosols over the Amundsen Sea, Antarctica. Atmos Chem Phys 20(9):5405–5424
Jung J, Lee Y, Cho KH, Yang EJ, Kang SH (2022) Spatial distributions of riverine and marine dissolved organic carbon in the western Arctic Ocean: results from the 2018 Korean expedition. J Geophys Res Oceans 127(7):e2021JC017718
Kang I, Lee K, Yang S-J, Choi A, Kang D, Lee YK, Cho J-C (2012) Genome sequence of Candidatus Aquiluna sp. strain IMCC13023, a marine member of the Actinobacteria isolated from an arctic fjord. Am Soc Microbiol
Kenzaka T, Yamaguchi N, Tani K, Nasu M (1998) rRNA-targeted fluorescent in situ hybridization analysis of bacterial community structure in river water. Microbiol 144(8):2085–2093
Kim J, Kim K, Cho J, Kang YQ, Yoon H-J, Lee Y-W (2018) Satellite-based prediction of Arctic sea ice concentration using a deep neural network with multi-model ensemble. Remote Sens 11(1):19
Kolmakova OV, Gladyshev MI, Rozanov AS, Peltek SE, Trusova MY (2014) Spatial biodiversity of bacteria along the largest Arctic river determined by next-generation sequencing. FEMS Microbiol Ecol 89(2):442–450
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Kwok R (2018) Arctic sea ice thickness, volume, and multiyear ice coverage: losses and coupled variability (1958–2018). Environ Res Lett 13(10):105005
Lambo AJ, Patel TR (2006) Cometabolic degradation of polychlorinated biphenyls at low temperature by psychrotolerant bacterium Hydrogenophaga sp. IA3-A. Curr Microbiol 53:48–52
Lane D (1991) 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Thurber RLV, Knight R (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31(9):814–821
Lee SH, McRoy CP, Joo HM, Gradinger R, Cui X, Yun MS, Chung KH, Kang S-H, Kang C-K, Choy EJ (2011) Holes in progressively thinning Arctic Sea ice lead to new ice algae habitat. Oceanography 24(3):302–308
Lee SH, Stockwell DA, Joo HM, Son YB, Kang CK, Whitledge TE (2012) Phytoplankton production from melting ponds on Arctic Sea Ice. J Geophys Res Oceans 117:C4
Lee J, Kang S-H, Yang EJ, Macdonald AM, Joo HM, Park J, Kim K, Lee GS, Kim J-H, Yoon J-E (2019) Latitudinal distributions and controls of bacterial community composition during the summer of 2017 in western Arctic surface waters (from the Bering Strait to the Chukchi Borderland). Sci Rep 9(1):16822
Liu S-B, Chen X-L, He H-L, Zhang X-Y, Xie B-B, Yu Y, Chen B, Zhou B-C, Zhang Y-Z (2013) Structure and ecological roles of a novel exopolysaccharide from the Arctic Sea ice bacterium Pseudoalteromonas sp. strain SM20310. Appl Environ Microbiol 79(1):224–230
Liu J, Song M, Horton RM, Hu Y (2015) Revisiting the potential of melt pond fraction as a predictor for the seasonal Arctic sea ice extent minimum. Environ Res Lett 10(5):054017
Manz W, Wendt-Potthoff K, Neu T, Szewzyk U, Lawrence J (1999) Phylogenetic composition, spatial structure, and dynamics of lotic bacterial biofilms investigated by fluorescent in situ hybridization and confocal laser scanning microscopy. Microb Ecol 37(4):225–237
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet j 17(1):10–12
Maslanik J, Fowler C, Stroeve J, Drobot S, Zwally J, Yi D, Emery W (2007) A younger, thinner Arctic ice cover: increased potential for rapid, extensive sea-ice loss. Geophys Res Lett 34:24
Mundy C, Gosselin M, Ehn JK, Belzile C, Poulin M, Alou E, Roy S, Hop H, Lessard S, Papakyriakou TN (2011) Characteristics of two distinct high-light acclimated algal communities during advanced stages of sea ice melt. Polar Biol 34:1869–1886
Netzer R, Henry IA, Ribicic D, Wibberg D, Brönner U, Brakstad OG (2018) Petroleum hydrocarbon and microbial community structure successions in marine oil-related aggregates associated with diatoms relevant for Arctic conditions. Mar Pollut Bull 135:759–768
Nicolaus M, Katlein C, Maslanik J, Hendricks S (2012) Changes in Arctic Sea ice result in increasing light transmittance and absorption. Geophys Res Lett 39:24
Papale M, Giannarelli S, Francesconi S, Di Marco G, Mikkonen A, Conte A, Rizzo C, De Domenico E, Michaud L, Giudice AL (2017) Enrichment, isolation and biodegradation potential of psychrotolerant polychlorinated-biphenyl degrading bacteria from the Kongsfjorden (Svalbard Islands, high Arctic Norway). Mar Pollut Bull 114(2):849–859
Peeb A, Dang NP, Truu M, Nõlvak H, Petrich C, Truu J (2022) Assessment of hydrocarbon degradation potential in microbial communities in Arctic Sea Ice. Microorganisms 10(2):328
Perovich DK, Richter-Menge JA (2009) Loss of sea ice in the Arctic. Annual Rev Mar Sci 1:417–441
Piontek J, Galgani L, Nöthig EM, Peeken I, Engel A (2021) Organic matter composition and heterotrophic bacterial activity at declining summer sea ice in the central Arctic Ocean. Limnol Oceanogr 66:S343–S362
Pitt A, Schmidt J, Koll U, Hahn MW (2021) Aquiluna Borgnonia gen. nov., sp. nov., a member of a Microbacteriaceae lineage of freshwater bacteria with small genome sizes. Int J Syst Evol Microbiol 71(5):004825
Qin QL, Wang ZB, Cha QQ, Liu SS, Ren XB, Fu HH, Sun ML, Zhao DL, McMinn A, Chen Y (2022) Biogeography of culturable marine bacteria from both poles reveals that ‘everything is not everywhere’at the genomic level. Environ Microbiol 24(1):98–109
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41(D1):D590–D596
Rapp J (2014) Bacterial diversity in sea ice, melt ponds, water column, ice algal aggregates and deep-sea sediments of the Central Arctic Ocean. AWI, Bremen, Germany, p 99
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584
Rösel A, Kaleschke L (2013) Detection of melt ponds on Arctic Sea Ice with optical satellite data. Springer
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Serreze M, Walsh J, Chapin F, Osterkamp T, Dyurgerov M, Romanovsky V, Oechel W, Morison J, Zhang T, Barry R (2000) Observational evidence of recent change in the northern high-latitude environment. Clim Change 46:159–207
Sizova M, Panikov N (2007) Polaromonas hydrogenivorans sp. nov., a psychrotolerant hydrogen-oxidizing bacterium from alaskan soil. Int J Syst Evol Microbiol 57(3):616–619
Sørensen HL, Thamdrup B, Jeppesen E, Rysgaard S, Glud RN (2017) Nutrient availability limits biological production in Arctic Sea ice melt ponds. Polar Biol 40:1593–1606
Steindler L, Schwalbach MS, Smith DP, Chan F, Giovannoni SJ (2011) Energy starved Candidatus Pelagibacter ubique substitutes light-mediated ATP production for endogenous carbon respiration. PLoS ONE 6(5):e19725
Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M (2014) Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 9(8):e105592
Teeling H, Fuchs BM, Becher D, Klockow C, Gardebrecht A, Bennke CM, Kassabgy M, Huang S, Mann AJ, Waldmann J (2012) Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 336(6081):608–611
Tripp HJ, Kitner JB, Schwalbach MS, Dacey JW, Wilhelm LJ, Giovannoni SJ (2008) SAR11 marine bacteria require exogenous reduced sulphur for growth. Nature 452(7188):741–744
Underwood GJ, Michel C, Meisterhans G, Niemi A, Belzile C, Witt M, Dumbrell AJ, Koch BP (2019) Organic matter from Arctic sea-ice loss alters bacterial community structure and function. Nat Clim Change 9(2):170–176
Vipindas PV, Jabir T, Venkatachalam S, Yang EJ, Anand Jain, Krishnan KP (2023) Vertical segregation and phylogenetic characterization of archaea and archaeal ammonia monooxygenase gene in the water column of the western Arctic Ocea. Extremophiles 27:241–216
Vipindas PV, Venkatachalam S, Jabir T, Yang EJ, Cho K-H, Jung J, Lee Y, Krishnan KP (2022) Water mass controlled vertical stratification of bacterial and archaeal communities in the western arctic ocean during summer sea-ice melting. Microb Ecol 1–14
Von Scheibner M, Sommer U, Jürgens K (2017) Tight coupling of Glaciecola spp. and diatoms during cold-water phytoplankton spring blooms. Front Microbiol 8:27
Weisse T, Müller H, Pinto-Coelho RM, Schweizer A, Springmann D, Baldringer G (1990) Response of the microbial loop to the phytoplankton spring bloom in a large prealpine lake. Limnol Oceanogr 35(4):781–794
West NJ, Lepère C, Manes C-LO, Catala P, Scanlan DJ, Lebaron P (2016) Distinct spatial patterns of SAR11, SAR86, and Actinobacteria diversity along a transect in the ultra-oligotrophic South Pacific Ocean. Front Microbiol 7:234
Xing P, Hahnke RL, Unfried F, Markert S, Huang S, Barbeyron T, Harder J, Becher D, Schweder T, Glöckner FO (2015) Niches of two polysaccharide-degrading Polaribacter isolates from the North Sea during a spring diatom bloom. ISME J 9(6):1410–1422
Xu D, Kong H, Yang E-J, Li X, Jiao N, Warren A, Wang Y, Lee Y, Jung J, Kang S-H (2020) Contrasting community composition of active microbial eukaryotes in melt ponds and sea water of the Arctic Ocean revealed by high throughput sequencing. Front Microbiol 11:1170
Yackel J, Barber D, Hanesiak J (2000) Melt ponds on sea ice in the Canadian Archipelago: 1. Variability in morphological and radiative properties. J Geophys Res Oceans 105(C9):22049–22060
Yang A, Yen C (2012) PCR optimization of BOX-A1R PCR for microbial source tracking of Escherichia coli in waterways. J Exp Microbiol Immunol 16:85–89
Yoon S-H, Ha S-M, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67(5):1613
Zeng Y, Yu Y, Li H, He J, Lee SH, Sun K (2013) Phylogenetic diversity of planktonic bacteria in the Chukchi Borderland region in summer. Acta Oceanol Sin 32:66–74
Zhang T, Zhuang Y, Jin H, Li K, Ji Z, Li Y, Bai Y (2019) Comparison of Phytoplankton communities between Melt ponds and Open Water in the Central Arctic Ocean. J Ocean Univ China 18(3):573–579
Acknowledgements
The authors wish to express their sincere gratitude to the Director, NCPOR, Ministry of earth sciences, for providing the necessary facilities for carrying out this research. This research was a part of the project titled ‘Korea-Arctic Ocean Warming and Response of Ecosystem (K-AWARE, KOPRI, 1525011760)’, funded by the Ministry of Oceans and Fisheries, Korea. The authors also thank Asian Forum for Polar Sciences (AFOPS) for facilitating the Arctic expedition in 2019. The authors thank the captain and crew of the IBRV ARAON who were most helpful in all shipboard operations. This is NCPOR contribution number J-50/2023-24.
Funding
This work was supported by the Ministry of earth sciences, Govt. of India and the Ministry of Oceans and Fisheries, Republic of Korea. Grant numbers [K-AWARE, KOPRI, 1525011760]. Authors Puthiya Veettil Vipindas, Siddarthan Venkatachalam, Thajudeen Jabir, Anand Jain, and Kottekkatu Padinchati Krishnan have received research support from NCPOR, India. Authors Jinyoung Jung and Eun Jin Yang have received research support from KOPRI, Korea.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Puthiya Veettil Vipindas, Thajudeen Jabir, Siddarthan Venkatachalam, Jinyoung Jung and Eun Jin Yang, The first draft of the manuscript was written by Puthiya Veettil Vipindas and edited by Anand Jain and Kottekkatu Padinchati Krishnan. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
This manuscript does not contain any studies with human participants.
Consent to publish
This manuscript does not contain any studies with human participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vipindas, P., Venkatachalam, S., Jabir, T. et al. Salinity-controlled distribution of prokaryotic communities in the Arctic sea-ice melt ponds. World J Microbiol Biotechnol 40, 25 (2024). https://doi.org/10.1007/s11274-023-03850-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03850-7