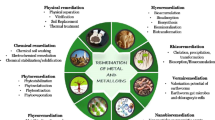
Abstract
Mercury is a highly toxic heavy metal whose emission sources can be both natural and the result of anthropic activity. Its polluting action on soils, and its ability to spread through the atmosphere and aquatic environments, constitutes a threat to human and environmental health; both for its bioaccumulation capacity and for biomagnification through the trophic chain. For this reason, there is a growing scientific and social interest in the reduction of this heavy metal in ecosystems. Bioremediation based on the use of microorganisms and/or plants is postulated as a sustainable alternative to traditional physicochemical methods. The main strategies used for this purpose (individually or in combination) are the volatilization of the contaminant, biosorption, phytoextraction and phytoremediation. All these tools are based on taking advantage of the natural and evolutionary capacity that different organisms have developed to adapt to the presence of various pollutants in the environment. Based on the consulted bibliography, these bioremediation methodologies focus on the use of microorganisms (freely or associated with plants) have been successfully applied in different ecosystems, postulating themselves as a respectful alternative for the future for the recovery of degraded environments. For these reasons there is a growing interest in the scientific community to design and use new techniques in a “One Health” context, which allow interpreting the positive impact of bioremediation. In this sense, the universalization of Omics techniques has allowed to abound in the knowledge of new bacterial taxa, and their biotechnological application. This study pretends to cover the present knowledge about mercury bioremediation techniques. In the same way, some new techniques and perspectives are presented in order to expand the frontiers of future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mercury as a pollutant
Mercury (Hg) is one of the most toxic heavy metals. Pollution by this element is a serious environmental problem, even at low concentrations, which affects all systems: soil, water and air (Munthe et al. 2019; Ballabio et al. 2021; Attwaters 2023).
Most of the environmental Hg is in the form of inorganic and organomercuric salts, except for atmospheric mercury. The most prevalent species in the environment are mercuric salts, such as HgCl2, Hg(OH), and HgS. In addition, CH3HgCl, and CH3HgOH are the main organomercurial compounds that together with other organic compounds are found in small fractions (Al-Sulaiti et al. 2022). The organomercurial compounds mentioned are compounds derived from methylmercury (MeHg or [CH3 Hg]+), one of the most dangerous Hg species, due to its high capacity to bioaccumulate in the tissues of organisms (Munthe et al. 2019; Gallorini and Loizeau 2021; Li et al. 2022b).
The most relevant Hg emitting sources are those of natural, anthropogenic and re-emission origin (Panagos et al. 2021; Sonke et al. 2023). It is estimated that since the beginning of the industrial revolution (AMAP/UNEP 2013), the amount of global atmospheric Hg has increased 10-fold and that throughout the post-industrial era to the present, the amount of Hg accumulated in soils and sediments has increased 3–10 times (Munthe et al. 2019).
The most important anthropogenic sources of Hg pollution are urban and industrial discharges, agricultural materials, mining, combustion, which emit from 2000 to 2200 tons annually, with the main source being the burning of fossil fuels and waste incineration (Munthe et al. 2019; Ballabio et al. 2021; Singh et al. 2023c; Zhang et al. 2023). These emissions come mainly from the metallurgical industry of non-ferrous materials, the main one being the Zn industry, followed by the large-scale production of Au, Cu and Al (Munthe et al. 2019; Yuwono et al. 2023). Finally, as a polluting source, there are reemissions, which are defined as Hg emissions derived from natural and anthropogenic past deposits. Under the right conditions, Hg deposits at the Earth’s surface can be suspended back into the atmosphere by various transport mechanisms. Annual Hg re-emission is estimated to be between 4,000t and 6,300t per year (Munthe et al. 2019; Ballabio et al. 2021). Most of this re-emitted Hg accumulates back in the soil.
Mercury-contaminated environments
Numerous ecosystems and environments contaminated with Hg are known, especially in regions with a high level of industrial activity and cities with large population volumes (Munthe et al. 2019) (Fig. 1).
The natural environments in which higher concentrations of Hg are detected are those indicated below:
Atmosphere
Elemental Hg (Hg0) and divalent Hg (Hg2+) are the majority species found in the atmosphere. The latter species is composed of gaseous divalent Hg (Hg2+) and divalent Hg particles (Hgρ) (Munthe et al. 2019; Dastoor et al. 2022; Yuan et al. 2023). Atmospheric Hg can be deposited in aquatic and terrestrial ecosystems through sedimentation and rainfall. The main specie of Hg that is deposited is the gaseous divalent Hg, coming mainly from anthropogenic and reemission sources; being the atmosphere the main recipient that collects and distributes them globally. In this way, atmospheric accumulation contributes significantly to the transport and discharge of Hg to multiple environments. Hg0 present in the atmosphere can be deposited in the medium after conversion to Hg2+ after its reduction by ozone (Saiz-Lopez et al. 2022).
Aquatic systems
Hg exhibits high bioavailability in aquatic systems, since under appropriate biochemical conditions, inorganic Hg is converted to MeHg by microorganisms (Gallorini and Loizeau 2021; Yue et al. 2023). It is known that the main source of biotransformation of Hg to MeHg in aquatic systems occurs in bed sediments as well as microorganisms along the water column (Gallorini and Loizeau 2021; Li et al. 2022b). This fact facilitates its accumulation in marine organisms, which can produce a high concentration of Hg in themselves (Liu et al. 2020; Scott and Black 2020; Li et al. 2022b). This problem is well described in some environments, such as river mouths, large cities, and the South China Sea (Xiang et al. 2018; Bernalte et al. 2020). The different aquatic systems, and mainly the oceans, act as Hg sinks, with the consequent ease of Hg biotransformation.
Soil
More than 90% of the emitted Hg ends up back in terrestrial ecosystems, with soil being the largest deposit of this metal (Ballabio et al. 2021; Yuwono et al. 2023). About 1–3% of the Hg present in soils is MeHg and the remaining percentage corresponds to different complexes, maintaining a small part as Hg0 (O’Connor et al. 2019). The capacity of the soil to accumulate Hg arouses scientific interest due to the easy transmissibility contaminants to organisms that develop in it. In this way it can be incorporated into the trophic chain, through its bioaccumulation in plants for human consumption or livestock. In turn, these compounds can move from soil deposits to the aquatic environment (Gębka et al. 2020; Yuwono et al. 2023), polluting the media, on a large scale. Likewise, the highest levels of Hg in soil have been found near urban centers with high industrial activity, as well as mining areas (Ballabio et al. 2021; Panagos et al. 2021). Hg forms, under natural conditions, primary complexes with Cl-, OH-, S2− and with organic compounds containing sulfur functional groups. Organic compounds have also been seen to be the dominant factor in Hg mobilization (Al-Sulaiti et al. 2022).
Effects of mercury pollution on systems and organisms
Hg in soil microbial communities
Hg, as a heavy metal and pollutant, exerts a strong biological and environmental pressure that affects the structure of microbial communities and their diversity (Mariano et al. 2020; Hu et al. 2023). The mer operon is one of the best-known bacterial defense systems against Hg (Manoj et al. 2020; Yadav et al. 2023). merA is the central gene of this operon, which codes for a mercuroreductase enzyme whose function is to catalyze the reduction of volatile Hg2+ to Hg0 (Harsonowati et al. 2023). These resistance genes are usually included in plasmids and other mobile gene elements, very ubiquitous in ecosystems. Two types of mer operon are known, capable of providing bacteria with Hg resistance (Naguib et al. 2019): (i) reduced spectrum operon which confers resistance only against inorganic Hg; (ii) broad-spectrum operon that, in addition to inorganic Hg resistance genes, include additional mer genes that confer organormercuric species resistance.
The biochemical process of inorganic Hg resistance is very similar between different bacterial species. In the case of bacteria with the reduced spectrum mer operon, there is a conversion of Hg2+ to Hg 0 mediated by a reductase enzyme produced by the merA gene, induced by Hg2+. This enzyme uses NADPH as an electron source. Organomercuric compounds resistance biochemical process varies according to the bacterial species. After the transport process, the bond between Hg and carbon is digested by a lyase enzyme, encoded in the merB gene, releasing Hg2+. The Hg cation is subsequently transformed into Hg0 by a mercuroreductase encoded by merA (Kumari et al. 2020) (Fig. 2).
The appearance of the contaminant in the soil favors the selection of those strains that present a greater tolerance to acquire resistance genetic elements that may be present in the environment. (Hall et al. 2020; Li et al. 2022a). In turn, Hg affects the composition of bacterial communities, decreasing their diversity (Mariano et al. 2020; Zheng et al. 2022; Hu et al. 2023). Likewise, the natural selection of tolerance to heavy metals can be linked, by a phenomenon of co-selection, to resistance to other compounds such as antibiotics (Robas et al. 2021b; Karnachuk et al. 2023; Tran et al. 2023), as well as the horizontal transfer of these resistances (Robas et al. 2021b; Li et al. 2022a; Kothari et al. 2023).
Hg in crops
Inorganic Hg can be incorporated and sequestered from soils into plant tissues by both stomal and non-stomal absorption (Zhou et al. 2021; Hussain et al. 2023; Singh et al. 2023a). The association of this heavy metal with functional groups of organic matter and various root exudates capable of retaining Hg in the soil (Eagles-Smith et al. 2018; Du et al. 2019; Hussain et al. 2023) favor the retention of Hg in ecosystems. The relationship between soils and plant activity is one of the most important environmental markers of soil inorganic Hg variation (Munthe et al. 2019; Ballabio et al. 2021).
Hg in the food chain
The presence of Hg in ecosystems allows its incorporation into the food chain (Li et al. 2022b; Basu et al. 2023; Han et al. 2023; Moslemi-Aqdam et al. 2023), negatively affecting, in the One health context, the health of ecosystems, especially the species that are at the highest levels of the chain (Li et al. 2022b). Most of the forms that Hg can take in nature are highly toxic to all species, even at low concentrations (Gil-Hernández et al. 2020; Marumoto et al. 2020; Basu et al. 2023). The processes known as bioaccumulation (accumulation of toxic substances in tissues) and biomagnification (increased accumulation of toxicants due to predation or consumption of other contaminated organisms), ultimately affect human health (Basu et al. 2023; Han et al. 2023; Moslemi-Aqdam et al. 2023) (Fig. 3).
The levels of Hg can be found in terrestrial vertebrates intended for human consumption are very low (Emami et al. 2023; Nava et al. 2023). However, numerous authors collect evidence of a high concentration of Hg in fish (Alemayehu et al. 2023; Frías-Espericueta et al. 2023). Despite the high concentration of Hg that can be found in the oceans, studies such as those conducted by Li et al. (2022b), show how water from rivers and lakes have a higher concentration of Hg than open waters and coasts. Hg and MeHg biomagnify along the food chain from phytoplankton to zooplankton to higher organisms (Frías-Espericueta et al. 2023; Han et al. 2023; Yue et al. 2023).
To address this environmental and health problem, the United Nations Minamata Convention on the Reduction of Hg Emissions and Use (AMAP/UNEP 2013) has developed regulations for the control of emissions to air, water, or waste and products under Federal environmental statutes, with the acts of “clean air, clean water and the recovery and conservation of natural resources” (Aldy et al. 2020).
Chronic exposure to this pollutant, especially through the consumption of seafood, can cause various neurological alterations, reproductive and immunological conditions and premature death (Gil-Hernández et al. 2020; Marumoto et al. 2020; Basu et al. 2023; Mallongi et al. 2023), especially affecting embryos and people suffering sustained exposure over time (Lee et al. 2023). Likewise, the case of Hg disease in humans that occurred in Minamata (Japan) between 1932 and 1968 is also well analyzed. During this period, an acetic acid factory dumped waste liquids with a high concentration of MeHg into Minamata Bay, where a large population subsisted on fishing for self-consumption. Even today, it has been observed that in populations engaged in subsistence fishing, between 1.5 and 17 out of every thousand children have cognitive disorders (mild mental retardation) due to the consumption of contaminated fish (Malagon-Rojas and Sonia 2018; Lin et al. 2023).
Bioremediation
The potential capacity of microbial communities and their use for the improvement of plant production (Valle-Romero et al. 2023; Vlajkov et al. 2023; Nagrale et al. 2023), as well as the use of soil plant processing capacity to reduce the presence of a certain pollutant (Chandel et al. 2023; Sitarska et al. 2023; Nnaji et al. 2023) are issues that have been addressed from different perspectives. Bioremediation or biotechnological remediation of a contaminated environment is presented as an economical alternative with a lower negative impact on the environment (Daniel et al. 2022). The use of mercurotolerant microorganisms (Mathema et al. 2011) represents a great opportunity for the design and application of effective bioremediation processes (Yadav et al. 2023; Rojas-Solis et al. 2023; Gupta et al. 2023).
In the literature, a large number of works can be found regarding the biological methods used to remediate Hg with bacteria:
-
Volatilization of the contaminant: Bacteria possessing the mer operon are able to reduce Hg from Hg2+ to volatile Hg0 (Tanwer et al. 2022; Yadav et al. 2023; Yao et al. 2023). The mer operon codes for a mercuroreductase (merA), an organomercuric lyase (merB), a periplasmic protein for environmental Hg uptake (merP), inner membrane proteins related to Hg2+ transport (merT, merC, merE, merF and merG) and operon system regulatory proteins and expression (merR and merD) (Gionfriddo et al. 2020).
-
Biosorption of Hg: Defined as the ability of microorganisms to capture heavy metals by increasing their biomass (Vijayaraghavan and Yun 2008; Beveridge and Murray 1980). The heavy metal is retained in the bacterial cell wall, without the need for intracellular bioaccumulation (Jing et al. 2022; Baran et al. 2022; Yao et al. 2023; Zhao et al. 2023).
-
Phytoextraction (Yu et al. 2022; Sitarska et al. 2023; Nnaji et al. 2023):. A process by which it is intended to remove heavy metals through the use of plants: (i) Fitoextractors: when the metal accumulates in the aerial part (Wang et al. 2019), and (ii) Phytostabilizers: when accumulation occurs in the root (Dary et al. 2010; Ke et al. 2021).
-
Phytoremediation (or phytorhizoremediation): Technique that uses plant-microorganism interaction for the extraction and/or removal of soil contaminants (Raklami et al. 2022; Ruley et al. 2022; Sitarska et al. 2023; Rojas-Solis et al. 2023). This technique has a much more powerful and efficient effect by taking advantage of the synergistic action between the plant and microorganisms (Quiñones et al. 2021; Robas et al. 2021a; Senabio et al. 2023) (Fig. 4).
New opportunities for old Problems: plant-microorganism interaction
Plants and microorganisms show parallel patterns of heterogeneity because plants release a wide range of organic products (via exudate), which are consumed by soil microorganisms. In this way, it has been postulated that the taxonomic heterogeneity of plants improves plant productivity by making more efficient use of available resources. But this factor has to go hand in hand with microbial activity in the soil and the bioavailability of organic resources.
In the plant-microorganism interaction, two fundamental factors should be considered: (i) to know the way in which the plant selects the rhizospheric microbiota and the way of relating to the microorganism; (ii) understanding microbial functional diversity will help reveal underlying ecological processes. In plant-microorganism collaboration, the benefit of this synergy in soil recovery is diminished in the presence of contaminants (Irfan et al. 2023). In this way, it is shown how the impact of heavy metals alters and reduces the diversity of the soil microbial ecosystem (Mariano et al. 2020; Robas et al. 2021a, c; González et al. 2022).
Some microorganisms have the ability to protect plants against biotic or abiotic agents that compromise their normal development (Robas et al. 2022; Nagrale et al. 2023; El-Sayed et al. 2023), mainly by volatilization and bioadsorption mechanisms (Robas Mora et al. 2022; Yadav et al. 2023; Zhao et al. 2023). Phytoprotection is a very interesting quality with potential agronomic use in contaminated soils that would otherwise take long periods of time to recover.
Some pollutants, such as Hg, induce physiological and metabolic alterations in plants, such as the appearance of ROS (reactive oxygen species) and decreased plant growth (Çavuşoğlu et al. 2022). Likewise, it is known that the use of PGPB minimizes these effects (Pirzadah et al. 2018). One of the main effects that numerous environmental pollutants have on a plant is the increase in oxidative stress and the production of ROS (Çavuşoğlu et al. 2022; Carrasco-Gil et al. 2023; Flores-Cáceres et al. 2023; Magnuson and Sandheinrich 2023). The production of the enzymes superoxide dismutase (SOD), glutathione reductase (GR), catalase (CAT) and ascorbate peroxidase (APX) catalyze the degradation of ROS such as H202, HO-, 1O2 and the superoxide anion O− 2. This effect has also been observed in the cellular production of APX and GR enzymes when confronting different plant species with heavy metals (Liu et al. 2018; Azimychetabi et al. 2021). Therefore, enzyme activity is interpreted as a protective response against ROS, whose function is induced by the intracellular presence of Hg (González-Reguero et al. 2022).
Microorganisms and new tools for bioremediation
Tools for the characterization and description of new strains. Massive Sequencing (NGS) and Biomercuroremediator Suitability Index (BMRSI)
During the last four decades, the use of microbiological agents (fungi and bacteria) as an alternative to conventional chemicals has prevailed. The commonly used plant growth promoting bacteria (PGPB) species are those belonging to Bacillus (Etesami et al. 2023; Liu et al. 2023; Wróbel et al. 2023) and Pseudomonas genera (de Andrade et al. 2023; Singh et al. 2023b).
An indirect effect of phytoprotection is the ability of these bacteria to prevent or reduce damage to the plant by the action of certain pathogens (Nagrale et al. 2023; El-Sayed et al. 2023). To do this, the accompanying bacteria can induce resistance mechanisms of the plant itself. PGPBs can perform other beneficial functions against abiotic stress for plants, such as protecting against salinity, drought, or toxic environmental compounds, such as heavy metals (Rajendran and Sundaram 2020; Zerrouk et al. 2020; Danish et al. 2020; Wróbel et al. 2023).
The main activities that allow characterizing a strain as PGPB have been the production of different phytohormones (like 3-indolacetic acid; IAA) and growth promoting activities (Basu et al. 2021; Hsu et al. 2021; Valle-Romero et al. 2023; Nagrale et al. 2023; Gupta et al. 2023). Likewise, it is necessary to know the Hg minimal bactericidal concentration (MBC) as a measure of its mercurotolerant capacity (Mathema et al. 2011). To assess these factors in an integrated wayRobas et al. (2021a); González et al. (2021) propose the use of the BMRSI (Biomercuroremedial Suitability Index):
BMRSI= [IAA (mg.mL− 1) + ACCd (1/0) + SID (cm) + PO43− (1/0)] + [MBC Hg (mg.mL− 1)]
Where 1 = Presence; 0 = Absence; IAA: Indole-3-acetic acid production; ACCd: ACC deaminase activity; SID: Siderophores production; PO43−: Phosphates solubilization capacity; MBC: Hg minimal bactericidal concentration.
The development of genetics and whole genome sequencing (through Next Generation Sequencing (NGS) and Whole Genome Sequencing (WGS) are fundamental tools for the description of new bacterial strains, their taxonomic reassignment (Mora et al. 2022; Shu and Huang 2022) or the molecular study of uncultivable species (Cycil et al. 2020) introducing a new paradigm in the study of microorganisms. Likewise,the use of new bioinformatics tools has allowed the discovery of new genes and molecular mechanisms of residence to metals such as Hg (Douglas et al. 2020; Moon et al. 2020).
Community study tools. Cenoantibiogram and Omics-
The presence of antibiotics in soils is an inherent fact because soil systems are the habitat of numerous microbial species that naturally produce them. Antibiotics may, at sub-inhibitory concentrations, have different functions such as the activation/deactivation of virulence factors or the regulation of microbial communication systems (Li et al. 2021; Chow et al. 2021; Moori Bakhtiari et al. 2023). The effect of antibiotic resistance in different populations may be due to various factors, including the effect of microbial communication processes and/or ecological competition, such as quorum-sensing/ quorum-quenching (Li et al. 2021; Wang et al. 2023), as well as the response to abiotic factors and genetic co-selection with resistance mechanisms to the presence of heavy metals. One of these tools that allows the study of antibiotic resistance of a complex microbial community is the so-called cenoantibiogram (Mora et al. 2017). The interpretation of the cenoantibiogram does not seek to characterize all the different mechanisms that explain each of the antibiotic resistances detected but the overall behavior of the soil microbial community compared to the most commonly used antibiotics. This technique is suggested as a possible bioindicator of both the evolution of the edaphic community and the comparison between different communities.
Likewise, there is no doubt that there is a growing interest in the use of techniques such as metagenomics, transcriptomics, proteomics and metabolomics for the study of soil samples and the use of microorganisms in bioremediation processes (Chakdar et al. 2022; Jhariya and Pal 2022; Sharma et al. 2022; López and dos Santos Silva 2023; Sevak et al. 2023). Methods based on the functional analysis of DNA libraries can be a great source for the discovery of new genes for resistance to different pollutants, such as heavy metals (Sharma et al. 2021; Chen et al. 2022). Similarly, transcriptomic and proteomic analysis offers an opportunity for understanding the expression of these genes and the different mechanisms of resistance, their expression and their regulation (Jhariya and Pal 2022; Lata et al. 2023; López and dos Santos Silva 2023; Shyam et al. 2023).
Future prospects
Bioremediation and the use of biotechnological methods for the removal of environmental pollutants, such as Hg, is proposed as an economical and environmentally friendly alternative. Therefore, the development of new technologies that allow effective environmental remediation is, at present, a field of research of great scientific interest. To this end, it is necessary to develop procedures and techniques that allow the correct taxonomic ascription or classification of new PGPB with potential biotechnological use. In order to successfully employ new microbial species in bioremediation processes, a deeper understanding of the mechanisms that promote microbial activity (such as communication mechanisms mediated by quorum sensing) and the metabolism of pollutants under various ecological conditions (through the aforementioned processes of mobilization/immobilization, translocation, transformation, biosorption or bioaccumulation) is needed. “Omics” tools will continue to be key to the discovery of new microbial species, genetic mechanisms of transformation (metagenomics); gene expression in different biological contexts (metatranscriptomics); the synthesis and participation of proteins in new metabolic pathways (metaproteomics) as well as the identification of metabolites not yet described with therapeutic, industrial, or remedial potential (metabolomics).
It is necessary to expand the knowledge of the mechanisms that allow the exosimbiont association or mutualistic interaction between organisms, especially those established between plants and microorganism, which allow an optimization of the use of organisms for the recovery of degraded environments. Finally, obtaining genetically modified microorganisms will allow the development of more effective methods and techniques for the restoration of the balanced conditions of deteriorated ecosystems.
Data Availability
There are no data associated with this publication.
References
Al-Sulaiti MM, Soubra L, Al-Ghouti MA (2022) The Causes and Effects of Mercury and Methylmercury Contamination in the Marine Environment: a review. Curr Pollution Rep 8:249–272. https://doi.org/10.1007/s40726-022-00226-7
Aldy J, Kotchen M, Evans M et al (2020) Deep flaws in a mercury regulatory analysis. Science 368:247–248
Alemayehu D, Rudra P, Mathews S et al (2023) Assessment of Mercury concentrations in Water and Fish tissue analysis in Kaw Lake, Oklahoma, 2022. J Environ Prot 14:50–65
AMAP/UNEP (2013) AMAP/UNEP technical background report for the global mercury assessment 2013: final technical report; output
Attwaters M (2023) A solution for mercury pollution? Nat Rev Microbiol 21:67–67
Azimychetabi Z, Sabokdast Nodehi M, Karami Moghadam T, Motesharezadeh B (2021) Cadmium stress alters the essential oil composition and the expression of genes involved in their synthesis in peppermint (Mentha piperita L). Ind Crops Prod 168:113602. https://doi.org/10.1016/j.indcrop.2021.113602
Ballabio C, Jiskra M, Osterwalder S et al (2021) A spatial assessment of mercury content in the European Union topsoil. Sci Total Environ 769:144755
Baran MF, Yildirim A, Acay H et al (2022) Adsorption performance of Bacillus licheniformis sp. bacteria isolated from the soil of the Tigris River on mercury in aqueous solutions. null 102:2013–2028. https://doi.org/10.1080/03067319.2020.1746779
Basu A, Prasad P, Das SN et al (2021) Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability 13:1140
Basu N, Bastiansz A, Dórea JG et al (2023) Our evolved understanding of the human health risks of mercury. Ambio 1–20
Bernalte E, Arévalo S, Pérez-Taborda J et al (2020) Rapid and on-site simultaneous electrochemical detection of copper, lead and mercury in the Amazon river. Sens Actuators B 307:127620
Beveridge T, Murray R (1980) Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol 141:876–887
Carrasco-Gil S, Ortega-Villasante C, Sobrino-Plata J et al (2023) Attenuation of mercury phytotoxicity with a high nutritional level of nitrate in alfalfa plants grown hydroponically. Plant Stress 7:100131. https://doi.org/10.1016/j.stress.2023.100131
Çavuşoğlu D, Macar O, Kalefetoğlu Macar T et al (2022) Mitigative effect of green tea extract against mercury (II) chloride toxicity in Allium cepa L. model. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-17781-z
Chakdar H, Thapa S, Srivastava A, Shukla P (2022) Genomic and proteomic insights into the heavy metal bioremediation by cyanobacteria. J Hazard Mater 424:127609
Chandel S, Dar RA, Singh D et al (2023) Plant assisted bioremediation of Heavy Metal Polluted Soils. In: Pandey VC (ed) Bio-Inspired Land Remediation. Springer International Publishing, Cham, pp 85–114
Chen G, Bai R, Zhang Y et al (2022) Application of metagenomics to biological wastewater treatment. Sci Total Environ 807:150737
Chow LKM, Ghaly TM, Gillings MR (2021) A survey of sub-inhibitory concentrations of antibiotics in the environment. J Environ Sci 99:21–27. https://doi.org/10.1016/j.jes.2020.05.030
Cycil LM, DasSarma S, Pecher W et al (2020) Metagenomic insights into the diversity of halophilic microorganisms indigenous to the Karak Salt Mine, Pakistan. Front Microbiol 11:1567
Daniel AJ, Enzo ER, Juliana MS et al (2022) The current approach to soil remediation: a review of physicochemical and biological technologies, and the potential of their strategic combination. J Environ Chem Eng 107141
Danish S, Zafar-ul-Hye DrM, Hussain S et al (2020) Mitigation of drought stress in maize through inoculation with drought tolerant ACC deaminase containing PGPR under axenic conditions. Pak J Bot 52. https://doi.org/10.30848/PJB2020-1(7)
Dary M, Chamber-Pérez M, Palomares A, Pajuelo E (2010) In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J Hazard Mater 177:323–330
Dastoor A, Wilson SJ, Travnikov O et al (2022) Arctic atmospheric mercury: sources and changes. Sci Total Environ 839:156213
de Andrade LA, Santos CHB, Frezarin ET et al (2023) Plant growth-promoting Rhizobacteria for Sustainable Agricultural production. Microorganisms 11. https://doi.org/10.3390/microorganisms11041088
Douglas GM, Maffei VJ, Zaneveld JR et al (2020) PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 38:685–688
Du B, Zhou J, Zhou L et al (2019) Mercury distribution in the foliage and soil profiles of a subtropical forest: process for mercury retention in soils. J Geochem Explor 205:106337
Eagles-Smith CA, Silbergeld EK, Basu N et al (2018) Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 47:170–197
El-Sayed MH, Kobisi AE-NA, Elsehemy IA, El-Sakhawy and MA (2023) Rhizospheric-derived Nocardiopsis alba BH35 as an effective Biocontrol Agent Actinobacterium with Antifungal and Plant Growth-Promoting Effects: in Vitro Studies. J Microbiol Biotechnol 33:1–14. https://doi.org/10.4014/jmb.2301.01001
Emami MH, Saberi F, Mohammadzadeh S et al (2023) A review of heavy metals accumulation in red meat and meat products in the Middle East. J Food Prot 100048
Etesami H, Jeong BR, Glick BR (2023) Potential use of Bacillus spp. as an effective biostimulant against abiotic stresses in crops—A review. Curr Res Biotechnol 5:100128. https://doi.org/10.1016/j.crbiot.2023.100128
Flores-Cáceres ML, Ortega-Villasante C, Carril P et al (2023) The early oxidative stress Induced by Mercury and Cadmium is modulated by Ethylene in Medicago sativa seedlings. https://doi.org/10.3390/antiox12030551. Antioxidants 12:
Frías-Espericueta MG, Sánchez-Betancourt A, Ruelas-Inzunza J et al (2023) Total Mercury and Selenium in wild shrimp from Coastal Lagoons of Northwest Mexico: Human Health risk Assessment. Bull Environ Contam Toxicol 110:1–5
Gallorini A, Loizeau J-L (2021) Mercury methylation in oxic aquatic macro-environments: a review. J Limnol 80
Gębka K, Saniewska D, Bełdowska M (2020) Mobility of mercury in soil and its transport into the sea. Environ Sci Pollut Res 27:8492–8506
Gil-Hernández F, Gómez-Fernández AR, la Torre-Aguilar MJ et al (2020) Neurotoxicity by mercury is not associated with autism spectrum disorders in spanish children. Ital J Pediatr 46:1–7
Gionfriddo CM, Stott MB, Power JF et al (2020) Genome-resolved metagenomics and detailed geochemical speciation analyses yield new insights into microbial mercury cycling in geothermal springs. Appl Environ Microbiol 86:e00176–e00120
González D, Robas M, Probanza A, Jiménez PA (2021) Selection of mercury-resistant PGPR strains using the BMRSI for bioremediation purposes. Int J Environ Res Public Health 18:9867
González D, Robas M, Fernández V et al (2022) Comparative metagenomic study of Rhizospheric and Bulk Mercury-Contaminated soils in the Mining District of Almadén. Front Microbiol 13
González-Reguero D, Robas-Mora M, Probanza A, Jiménez PA (2022) Evaluation of the oxidative stress alleviation in Lupinus albus var. Orden Dorado by the inoculation of four plant growth-promoting bacteria and their mixtures in mercury-polluted soils. Front Microbiol 13
Gupta R, Khan F, Alqahtani FM et al (2023) Plant growth–promoting Rhizobacteria (PGPR) assisted bioremediation of Heavy Metal Toxicity. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-023-04545-3
Hall JP, Harrison E, Pärnänen K et al (2020) The impact of mercury selection and conjugative genetic elements on community structure and resistance gene transfer. Front Microbiol 11:1846
Han Y, Jiang Y, Xiong X et al (2023) Mercury biomagnification at higher rates than the global average in aquatic ecosystems of the Qinghai-Tibet Plateau. J Hazard Mater 453:131408
Harsonowati W, Rahayuningsih S, Yuniarti E et al (2023) Bacterial metal-scavengers newly isolated from indonesian Gold Mine-Impacted area: Bacillus altitudinis MIM12 as Novel Tools for Bio-Transformation of Mercury. Microb Ecol 1–15
Hsu S-H, Shen M-W, Chen J-C et al (2021) The photosynthetic bacterium Rhodopseudomonas palustris strain PS3 exerts plant growth-promoting Effects by stimulating Nitrogen Uptake and elevating auxin levels in expanding Leaves. Front Plant Sci 12:93
Hu H, Gao Y, Yu H et al (2023) Mechanisms and biological effects of organic amendments on mercury speciation in soil–rice systems: a review. Ecotoxicol Environ Saf 251:114516
Hussain S, Jianjun Y, Hussain J et al (2023) The rhizospheric transformation and bioavailability of mercury in pepper plants are influenced by selected chinese soil types. Environ Geochem Health 45:41–52
Irfan M, Aslam H, Maqsood A et al (2023) Changes in Plant Microbiome in response to abiotic stress. Plant Microbiome for Plant Productivity and sustainable agriculture. Springer, pp 99–119
Jhariya U, Pal S (2022) Proteomic, genomic, and Metabolomic understanding and Designing for Bioremediation of Environmental Contaminants. Omics Insights in Environmental Bioremediation. Springer, pp 415–435
Jing X, Lu T, Sun F et al (2022) Microbial transformation to remediate mercury pollution: strains isolation and laboratory study. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-022-04158-z
Karnachuk OV, Beletsky AV, Rakitin AL et al (2023) Antibiotic-resistant Desulfovibrio produces H2S from supplements for animal farming. Microorganisms 11:838
Ke T, Guo G, Liu J et al (2021) Improvement of the Cu and Cd phytostabilization efficiency of perennial ryegrass through the inoculation of three metal-resistant PGPR strains. Environ Pollut 271:116314. https://doi.org/10.1016/j.envpol.2020.116314
Kothari A, Kumar P, Gaurav A et al (2023) Association of antibiotics and heavy metal arsenic to horizontal gene transfer from multidrug-resistant clinical strains to antibiotic-sensitive environmental strains. J Hazard Mater 443:130260. https://doi.org/10.1016/j.jhazmat.2022.130260
Kumari S, Jamwal R, Mishra N, Singh DK (2020) Recent developments in environmental mercury bioremediation and its toxicity: A review. Environmental Nanotechnology, Monitoring & Management 13:100283
Lata S, Sharma S, Kaur S (2023) OMICS approaches in Mitigating Metal Toxicity in comparison to conventional techniques used in Cadmium Bioremediation. Water Air Soil Pollut 234:1–22
Lee S, Kim JH, Moon H-B et al (2023) Effects of mercury exposure on fetal body burden and its association with infant growth. Environ Res 217:114780
Li Y, Xia L, Chen J et al (2021) Resistance elicited by sub-lethal concentrations of ampicillin is partially mediated by quorum sensing in Pseudomonas aeruginosa. Environ Int 156:106619
Li W, Zhang W-G, Zhang M-S et al (2022a) Environmentally relevant concentrations of mercury facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Sci Total Environ 852:158272. https://doi.org/10.1016/j.scitotenv.2022.158272
Li Z, Chi J, Wu Z et al (2022b) Characteristics of plankton hg bioaccumulations based on a global data set and the implications for aquatic systems with aggravating nutrient imbalance. Front Environ Sci Eng 16:1–13
Lin Y-C, Chang W-H, Li T-C et al (2023) Health risk of infants exposed to lead and mercury through breastfeeding. Exposure and Health 15:255–267
Liu Y-R, Delgado-Baquerizo M, Bi L et al (2018) Consistent responses of soil microbial taxonomic and functional attributes to mercury pollution across China. Microbiome 6:1–12
Liu M, Xiao W, Zhang Q et al (2020) Methylmercury bioaccumulation in deepest ocean fauna: implications for ocean mercury biotransport through food webs. Environ Sci Technol Lett 7:469–476
Liu Y, Yue Z, Sun Z, Li C (2023) Harnessing native Bacillus spp. for sustainable wheat production. Appl Environ Microbiol 89:e01247–e01222. https://doi.org/10.1128/aem.01247-22
López AMQ, dos Santos Silva AL (2023) Proteomics and Bioremediation Using Prokaryotes. Genomics Approach to Bioremediation: Principles, Tools, and Emerging Technologies 485–502
Magnuson JT, Sandheinrich MB (2023) Relation among Mercury, Selenium, and biomarkers of oxidative stress in Northern Pike (Esox lucius). https://doi.org/10.3390/toxics11030244. Toxics 11:
Malagon-Rojas J, Sonia D (2018) Evaluation of the degree of contamination by mercury and other toxic substances, and its impact on human health in the populations of the Atrato river basin. as a consequence of mining activities
Mallongi A, Rauf A, Astuti R et al (2023) Ecological and human health implications of mercury contamination in the coastal water. Global J Environ Sci Manage 9:261–274
Manoj SR, Karthik C, Kadirvelu K et al (2020) Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: a review. J Environ Manage 254:109779
Mariano C, Mello IS, Barros BM et al (2020) Mercury alters the rhizobacterial community in brazilian wetlands and it can be bioremediated by the plant-bacteria association. Environ Sci Pollut Res 27:13550–13564. https://doi.org/10.1007/s11356-020-07913-2
Marumoto M, Sakamoto M, Marumoto K et al (2020) Mercury and Selenium localization in the Cerebrum, Cerebellum, Liver, and kidney of a Minamata Disease Case. Acta Histochem Cytochem 20–00009
Mathema VB, Thakuri BKC, Sillanpää M, Shrestha RA (2011) Study of mercury (II) chloride tolerant bacterial isolates from Baghmati River with estimation of plasmid size and growth variation for the high mercury (II) resistant Enterobacter spp. J Biotech Res 3:72
Moon K, Jeon JH, Kang I et al (2020) Freshwater viral metagenome reveals novel and functional phage-borne antibiotic resistance genes. Microbiome 8:1–15
Moori Bakhtiari N, Ezzati Givi M, Goudarzi S (2023) Effect of sub-inhibitory concentrations of antibiotic on the production and N-acetylglucosamine scale of methicillin- resistant Staphylococcus aureus biofilm. J Hellenic Vet Med Soc 74:5363–5370. https://doi.org/10.12681/jhvms.29576
Mora MR, Gómez PAJ, Valbuena C, Probanza A (2017) Effect of the type of Vitis vinifera cultivation in the cenophenoresistome and metabolic profiling (CLPP) of edaphic bacterial communities. J Agricultural Sci Technol A 7:522–536
Mora MR, Pastrana VMF, Reguero DG et al (2022) Oxidative stress protection and growth promotion activity of Pseudomonas mercuritolerans sp. nov., in forage plants under mercury abiotic stress conditions. Front Microbiol 13
Moslemi-Aqdam M, Low G, Low M et al (2023) Estimates, spatial variability, and environmental drivers of mercury biomagnification rates through lake food webs in the canadian subarctic. Environ Res 217:114835
Munthe J, Kindbom K, Parsmo R, Yaramenka K (2019) Technical Background Report to the Global Mercury Assessment 2018
Nagrale DT, Chaurasia A, Kumar S et al (2023) PGPR: the treasure of multifarious beneficial microorganisms for nutrient mobilization, pest biocontrol and plant growth promotion in field crops. World J Microbiol Biotechnol 39:100. https://doi.org/10.1007/s11274-023-03536-0
Naguib MM, Khairalla AS, El-Gendy AO, Elkhatib WF (2019) Isolation and characterization of mercury-resistant bacteria from wastewater sources in Egypt. Can J Microbiol 65:308–321. https://doi.org/10.1139/cjm-2018-0379
Nava V, Di Bella G, Fazio F et al (eds) (2023) Hg Content in EU and Non-EU Processed Meat and Fish Foods. Applied Sciences 13:793
Nnaji ND, Onyeaka H, Miri T, Ugwa C (2023) Bioaccumulation for heavy metal removal: a review. SN Appl Sci 5:125. https://doi.org/10.1007/s42452-023-05351-6
O’Connor D, Hou D, Ok YS et al (2019) Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: a critical review. Environ Int 126:747–761
Panagos P, Jiskra M, Borrelli P et al (2021) Mercury in European topsoils: anthropogenic sources, stocks and fluxes. Environ Res 201:111556
Pirzadah TB, Malik B, Tahir I et al (2018) Characterization of mercury-induced stress biomarkers in Fagopyrum tataricum plants. Int J Phytoremediation 20:225–236
Quiñones MA, Fajardo S, Fernández-Pascual M et al (2021) Nodulated white lupin plants growing in contaminated soils accumulate unusually high mercury concentrations in their nodules, roots and especially cluster roots. Horticulturae 7:302
Rajendran S, Sundaram L (2020) Degradation of heavy metal contaminated soil using plant growth promoting rhizobacteria (PGPR): assess their remediation potential and growth influence of Vigna radiata. L. Int J Agricultural Technol 16:365–376
Raklami A-M, Abdelilah AU, Oufdou Khalid AU, Baslam Marouane TI (2022) Plants—Microorganisms-Based bioremediation for heavy metal cleanup: recent developments, phytoremediation techniques, regulation mechanisms, and molecular responses. Int J Mol Sci 23. https://doi.org/10.3390/ijms23095031
Robas M, Jiménez PA, González D, Probanza A (2021a) Bio-Mercury Remediation Suitability Index: a novel proposal that compiles the PGPR features of bacterial strains and its potential use in phytoremediation. Int J Environ Res Public Health 18:4213
Robas M, Probanza A, González D, Jiménez PA (2021b) Mercury and Antibiotic Resistance Co-Selection in Bacillus sp. Isolates from the Almadén Mining District. Int J Environ Res Public Health 18:8304
Robas Mora M, Jiménez Gómez PA, González Reguero D, Probanza Lobo A (2022) Effect of Plant Growth-Promoting Bacteria on Biometrical Parameters and Antioxidant Enzymatic Activities of Lupinus albus var. Orden Dorado Under Mercury Stress. Frontiers in Microbiology 13
Rojas-Solis D, Larsen J, Lindig-Cisneros R (2023) Arsenic and mercury tolerant rhizobacteria that can improve phytoremediation of heavy metal contaminated soils. PeerJ 11:e14697. https://doi.org/10.7717/peerj.14697
Ruley JA, Amoding A, Tumuhairwe JB, Basamba TA (2022) Chap. 14 - Rhizoremediation of petroleum hydrocarbon–contaminated soils: A systematic review of mutualism between phytoremediation species and soil living microorganisms. In: Bhat RA, Tonelli FMP, Dar GH, Hakeem K (eds) Phytoremediation. Academic Press, pp 263–296
Saiz-Lopez A, Acuña AU, Mahajan AS et al (2022) The chemistry of mercury in the stratosphere. Geophys Res Lett 49:e2022GL097953
Scott AF, Black FJ (2020) Mercury bioaccumulation and biomagnification in Great Salt Lake ecosystems. Great Salt Lake Biology. Springer, pp 435–461
Senabio JA, de Campos Pereira F, Pietro-Souza W et al (2023) Enhanced mercury phytoremediation by Pseudomonodictys pantanalensis sp. nov A73 and Westerdykella aquatica P71. Brazilian J Microbiol. https://doi.org/10.1007/s42770-023-00924-4
Sevak P, Pushkar B, Mazumdar S (2023) Mechanistic evaluation of chromium bioremediation in Acinetobacter junii strain b2w: a proteomic approach. J Environ Manage 328:116978
Sharma P, Kumar S, Pandey A (2021) Bioremediated techniques for remediation of metal pollutants using metagenomics approaches: a review. J Environ Chem Eng 9:105684
Sharma P, Singh SP, Iqbal HM, Tong YW (2022) Omics approaches in bioremediation of environmental contaminants: an integrated approach for environmental safety and sustainability. Environ Res 211:113102
Shu W-S, Huang L-N (2022) Microbial diversity in extreme environments. Nat Rev Microbiol 20:219–235
Shyam K, Kumar N, Chandel H et al (2023) Omics Technologies in Environmental Microbiology and Microbial Ecology: Insightful Applications in Bioremediation Research. Genomics Approach to Bioremediation: Principles, Tools, and Emerging Technologies 433–454
Singh AD, Khanna K, Kour J et al (2023a) Critical review on biogeochemical dynamics of mercury (hg) and its abatement strategies. Chemosphere 137917
Singh P, Singh RK, Li H-B et al (2023b) Nitrogen fixation and phytohormone stimulation of sugarcane plant through plant growth promoting diazotrophic Pseudomonas. Biotechnol Genet Eng Rev 0:1–21. https://doi.org/10.1080/02648725.2023.2177814
Singh S, Dhyani S, Pujari PR (2023c) Coal-fired thermal power plants and Mercury Risks: Status and Impacts to realize Minamata Convention promises. Anthropocene Sci 1–9
Sitarska M, Traczewska T, Filarowska W et al (2023) Phytoremediation of mercury from water by monocultures and mixed cultures pleustophytes. J Water Process Eng 52:103529. https://doi.org/10.1016/j.jwpe.2023.103529
Sonke JE, Angot H, Zhang Y et al (2023) Global change effects on biogeochemical mercury cycling. Ambio 1–24
Tanwer N, Bumbra P, Khosla B, Laura JS (2022) Mercury pollution and its bioremediation by microbes. Microbes and Microbial Biotechnology for Green Remediation. Elsevier, pp 651–664
Tran TQ, Park M, Lee JE et al (2023) Analysis of antibiotic resistance gene cassettes in a newly identified Salmonella enterica sv. Gallinarum strain in Korea. Mob DNA 14:4. https://doi.org/10.1186/s13100-023-00292-8
Valle-Romero P, García-López JV, Redondo-Gómez S et al (2023) Biofertilization with PGP Bacteria improve Strawberry Plant Performance under Sub-Optimum Phosphorus fertilization. Agronomy 13. https://doi.org/10.3390/agronomy13020335
Vijayaraghavan K, Yun Y-S (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Vlajkov V, Pajčin I, Vučetić S et al (2023) Bacillus-loaded Biochar as Soil Amendment for Improved Germination of Maize Seeds. https://doi.org/10.3390/plants12051024. Plants 12:
Wang Y, Meng D, Fei L et al (2019) A novel phytoextraction strategy based on harvesting the dead leaves: Cadmium distribution and chelator regulations among leaves of tall fescue. Sci Total Environ 650:3041–3047. https://doi.org/10.1016/j.scitotenv.2018.10.072
Wang M, Lian Y, Wang Y, Zhu L (2023) The role and mechanism of quorum sensing on environmental antimicrobial resistance. Environ Pollut 322:121238. https://doi.org/10.1016/j.envpol.2023.121238
Wróbel M, Śliwakowski W, Kowalczyk P et al (2023) Bioremediation of Heavy Metals by the Genus Bacillus. Int J Environ Res Public Health 20. https://doi.org/10.3390/ijerph20064964
Xiang Y, Wang Y, Zhang C et al (2018) Water level fluctuations influence microbial communities and mercury methylation in soils in the three Gorges Reservoir, China. J Environ Sci 68:206–217
Yadav V, Manjhi A, Vadakedath N (2023) Mercury remediation potential of mercury-resistant strain Rheinheimera metallidurans sp. nov. isolated from a municipal waste dumping site. Ecotoxicol Environ Saf 257:114888
Yao H, Wang H, Ji J et al (2023) Isolation and identification of Mercury-Tolerant Bacteria LBA119 from molybdenum-lead mining soils and their removal of Hg2+. https://doi.org/10.3390/toxics11030261. Toxics 11:
Yu F, Tang S, Shi X et al (2022) Phytoextraction of metal(loid)s from contaminated soils by six plant species: a field study. Sci Total Environ 804:150282. https://doi.org/10.1016/j.scitotenv.2021.150282
Yuan C-S, Chiang K-C, Yen P-H et al (2023) Long-range transport of atmospheric speciated mercury from the eastern waters of Taiwan Island to northern South China Sea. Environ Pollut 318:120899
Yue F, Li Y, Zhang Y et al (2023) Elevated methylmercury in Antarctic surface seawater: the role of phytoplankton mass and sea ice. Science of The Total Environment 163646
Yuwono SB, Banuwa IS, Suryono Suryono S, Somura H (2023) Mercury pollution in the soil and river water of the Ratai watershed by artisanal and small-scale gold mining activities in Pesawaran District, Lampung, Indonesia. Journal of Degraded and Mining Lands Management
Zerrouk IZ, Rahmoune B, Auer S et al (2020) Growth and aluminum tolerance of maize roots mediated by auxin-and cytokinin-producing Bacillus toyonensis requires polar auxin transport. Environ Exp Bot 176:104064
Zhang Y, Zhang P, Song Z et al (2023) An updated global mercury budget from a coupled atmosphere-land-ocean model: 40% more re-emissions buffer the effect of primary emission reductions. One Earth 6:316–325
Zhao M, Zheng G, Kang X et al (2023) Aquatic Bacteria Rheinheimera tangshanensis New ability for Mercury Pollution removal. Int J Mol Sci 24. https://doi.org/10.3390/ijms24055009
Zheng X, Cao H, Liu B et al (2022) Effects of Mercury Contamination on Microbial Diversity of different kinds of Soil. Microorganisms 10:977
Zhou J, Obrist D, Dastoor A et al (2021) Vegetation uptake of mercury and impacts on global cycling. Nat Reviews Earth Environ 2:269–284
Funding
This research was funded by FUNDACIÓN UNIVERSITARIA SAN PABLO CEU AND BANCO SANTANDER, grant number FUSP-BS-PPC01/2014.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Daniel González-Reguero, Marina Robas-Mora, Agustín Probanza Lobo and Pedro A. Jiménez Gómez. The first draft of the manuscript was written by Daniel González-Reguero and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
González-Reguero, D., Robas-Mora, M., Probanza Lobo, A. et al. Bioremediation of environments contaminated with mercury. Present and perspectives. World J Microbiol Biotechnol 39, 249 (2023). https://doi.org/10.1007/s11274-023-03686-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03686-1