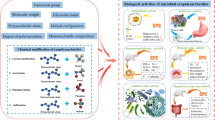
Abstract
Microbial exopolysaccharides (EPSs) are valuable extracellular macromolecules secreted as capsules or slime layers. Various microorganisms, including bacteria, yeasts, fungi, and algae have been studied for their ability to produce EPSs. Microbial EPSs exist as homopolysaccharides or heteropolysaccharides with various properties such as different monosaccharide compositions, structural conformation, molecular weight, and functional groups. They are cost-effective alternatives to plant and animal-derived polysaccharides because the microbial cells produced them in large quantities by biotechnological processes using low-cost substrates such as industrial wastes in a short time. Microbial EPSs are safe, biodegradable, and compatible polymers. They have extensive bioactivities, including antibacterial, antifungal, antiviral, antioxidant, antitumor, antidiabetic, antiulcer, anticoagulant, antiaging, immunomodulatory, wound healing, and cholesterol-lowering activities. Microbial EPSs owing to biological activities, special biochemical structures, and attractive physicochemical properties find plenty of potential applications in various industries. The enhancement of the production of EPSs and improving their properties can be provided by genetic engineering methods. The current review aims to provide a comprehensive examination of the therapeutic activities of microbial EPSs in infectious diseases and metabolic disorders, with a focus on the mechanisms involved. Also, the effect of the physicochemical characteristics of EPSs on these bioactivities was discussed to reveal the structure-activity relationship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microorganisms have considerable biosynthetic potentials to produce various bioactive substances with unique chemical scaffolds and functionalities. These compounds displayed pharmaceutical potential. For example, they suppress the growth of infectious bacteria and fungi, mitigate multiplication of cancerous cells, scavenge free radicals, reduce inflammation, and accelerate wound healing. These bioactive metabolites can be peptides, lipopeptides, polypeptides, lactones, fatty acids, polyketides, isocoumarins, terpenoids or exopolysaccharides (Ramezanzadeh et al. 2021; Salimi et al. 2022; Almasi et al. 2021; Salimi and Mohammadipanah 2021).
Polysaccharides can be produced by various natural sources like plants, animals and microorganisms (Li et al. 2022). Exopolysaccharides (EPS) are extracellular carbohydrate polymers which are synthesized by different microbial cells, including bacteria, yeasts, fungi, and microalgae during their growth and metabolism. EPSs can be secreted from microbial cells or attached to their cells (Arayes et al. 2023). EPSs are composed carbohydrates (main part) and some non-carbohydrate substituents like acetate, pyruvate, succinate, and phosphate. Various monosaccharides such as fructose, glucose, arabinose, mannose, rhamnose, and xylose can be present in EPS structure (Al-Nabulsi et al. 2022). EPSs provide microenvironments, which protect bacteria at extreme conditions, help bacterial colonization and pathogenicity, and facilitate genes and metabolites exchange within bacterial communities (Arayes et al. 2023). Microbial EPSs can be produced in a short time in high quantity under controlled conditions. Therefore, they can be cost-effective substituents to the plant and algal derived polysaccharides. Also, production cost can be reduced by using cheaper substrates, improving product yield through optimizing fermentation conditions and downstream processing, or developing higher yielding strains (Freitas et al. 2011).
According to the literature, microbial-derived EPSs have desirable biological functions and high-value applications as well as they have no toxic and side effects on body tissue cells. These biopolymers are biocompatible and biodegradable (Li et al. 2023). EPSs with favorable thermal stability and water retention ability have proper candidate for biomedical applications (Andrew and Jayaraman 2022). Due to the toxicity of chemical therapies or emerging resistance to current drugs(Al-Nabulsi et al. 2022), EPSs have the potential of replacing chemical drugs and are considered medically, pharmaceutically impotent carbohydrates (Ji et al. 2022; Zhou and Huang 2023). In addition to these, EPSs are being used for drug delivery, and scaffold synthesis in tissue engineering (Andrew and Jayaraman 2022). Also, the microbial EPSs have potential applications in food, feed, packaging, chemical, textile, agriculture, and cosmetic industries (Ates 2015; Tang and Huang 2022; Zhou and Huang 2023). Since great efforts have been made in recent years on characterization and studying the bioactivities of microbial EPSs, reviewing the recent articles helps better understanding between their properties and biomedical activities. In this review, we attempt to review research on bioactivities of microbial EPSs, including antibacterial, antifungal, antiviral, antioxidant, antiulcer, immunostimulant, or immunosuppressive activities. Furthermore, the impactful physicochemical properties of EPSs on their various biological activities will be reviewed.
Classification and properties of microbial EPSs
EPSs produced by microbial cells have diverse chemical composition, and they can be categorized into two groups of homopolysaccharides (HoPS) and heteropolysaccharides (HePS) (Fig. 1).
Homopolysaccharides
Homopolysaccharides (HoPSs) are contained of repeating one kind of monosaccharide with the molecular mass of ~ 107 Da (Panchal et al. 2022). They can be further classified into α-d-glucans, β-d-glucans, and fructans (Panchal et al. 2022; Bajpai et al. 2016; Nwodo et al. 2012). Examples of bacterial HoPSs with the main characteristics of them are summarized in Table 1.
Dextran and mutan belong to α-d-glucans. In contrary to dextran, mutan is a water-insoluble HoPS. Dextran (Fig. 1) is produced by various genera such as Leuconostoc, Lactobacillus, Limosilactobacillus, Weissella, Streptococcus, Lentilactobacillus, and Latilactobacillus. Streptococcus downei, Streptococcus mutans, Streptococcus salivarius, and Limosilactobacillus reuteri are examples of bacteria producing mutan. Alternan and reuteran are two other α-d-glucans, which are soluble in water. Alternan can be produced by Leuconostoc mesenteroides, L. citreum, and Streptococcus salivarius. The generation of reuteran was also reported by Limosilactobacillus reuteri (Jurášková et al. 2022). Curdlan, cellulose, and salecan are unbranched β-d-glucans, which among them, salecan is water-soluble (Guo et al. 2017; Sun and Zhang 2021). Curdlan (Fig. 1) is produced by Agrobacterium sp. ATCC 31,749 (Ruffing and Chen 2012), Cellulomonas flavigena KU (Kenyon and Buller 2002), Alcaligenes faecalis, Rhizobium meliloti, and Agrobacterium radiobacter (Prete et al. 2021). Acetobacter, Pseudomonas, Agrobacterium, and Rhizobium genera have ability to generate cellulose (Vu et al. 2009). Agrobacterium sp. ZX09 can produce salecan as a HoPS (Sun and Zhang 2021). Levan, inulin, and fructooligosaccharide are members of fructan that all of them have solubility in water (Jurášková et al. 2022). Limosilactobacillus reuteri, L. mesenteroides, Streptococcus mutans, Bacillus subtilis, and Streptococcus salivarius are levan producer strains (Jurášková et al. 2022; Prete et al. 2021). Streptococcus mutans, Limosilactobacillus reuteri, Leuconostoc citreum, and Lactobacillus johnsonii have ability to generate inulin (Jurášková et al. 2022). Fructooligosaccharide is also detected in the culture of Lactobacillus reuteri 121 (Sun and Zhang 2021).
Heteropolysaccharides
Heteropolysaccharides (HePSs) are complex because of two or more kinds of monosaccharides in their structures. Pentose, hexose, N-acetylated monosaccharide, and non-carbohydrate units can be present in branched or unbranched HePSs (Jurášková et al. 2022; Abarquero et al. 2022). The molecular mass of HePSs is within 104 to 106 Da (Panchal et al. 2022). The structure of some HePSs is presented in Table 1. The backbone of gellan, wellan, and diutan are the same, and it constructs from l-rhamnopyranose, d-glucopyranose, and d-glucopyranuronic acid subunits. Gellan (Fig. 1) is linear, however, wellan, and diutan have different branched structures (Sun and Zhang 2021). All of them are water-soluble (Nadzir et al. 2021; González et al. 2019; Li et al. 2020), and their production reported by Sphingomonas and Pseudomonas strains (Sun and Zhang 2021). Kefiran is another branched HePS, which is made from d-glucopyranose and d-galactopyranose units. Kefiran is a water-soluble polysaccharide, and several species of Lactobacillus such as L. kefir, L. parakefir, L. kefiranofaciens, L. kefirgranum, and L. delbrueckii subsp. bulgaricus have the ability to generate it (Moradi and Kalanpour 2019). The composition of the xanthan backbone is similar to cellulose, while its branch structure makes from d-mannopyranose and d-glucopyranuronic acid. Xanthan (Fig. 1) is water-soluble EPS (Nadzir et al. 2021), which can be produced by Xanthomonas campestris and Sphingomonas paucimobilis (Jurášková et al. 2022). Hyaluronic acid (Fig. 1) with the composition of d-glucuronic acid and d-N-acetylglucosamine is linear water-soluble HePS (Sun and Zhang 2021; Nadzir et al. 2021). Streptococcus equi, S. zooepidemicus, S. pyogenes, Pasteurella multocida, and Cryptococcus neoformans are natural bacteria that produce hyaluronic acid (Sze et al. 2016). Alginate is linear HePS with solubility in water (S et al. 2020). Its subunits consist of d-manopyranuronic acid and l-guluronic acid (Sun and Zhang 2021). Pseudomonas aeruginosa and Azotobacter vinelandii are main producer of alginate (Nadzir et al. 2021).
Antibacterial activities
Microbial EPSs showed considerable inhibiting effects on adherence, colonization, or growth of various Gram-positive (e.g. Listeria monocytogenes, Micrococcus luteus, Bacillus subtilis, Bacillus cereus and Staphylococcus aureus, Staphylococcus petrasii, Enterococcus faecalis) and Gram-negative (e.g. Salmonella enteritidis, Escherichia coli, Heliobacter pylori, Acinetobacter baumannii, Proteus mirabilis, Enterobacter cloacae, Pseudomonas aeruginosa, Shigella flexneri) bacterial pathogens. These bacteriostatic or bactericidal EPSs are produced by bacteria belonging to Lactobacillus, Lactococcus, Streptococcus, Bifidobacterium, Bacillus, Weissella, Leuconostoc, Limosilactobacillus genera (Table 2). Some of these microbial EPSs showed broad-spectrum activities while others act specifically (Angelin and Kavitha 2020).
Antibacterial mechanisms of microbial EPSs may be related to disrupting the structure of bacterial cell membrane, cell wall, or respiratory chain, affecting cell division machinery (Hu et al. 2019; Hasheminya and Dehghannya 2020; Wu et al. 2010). Microbial EPSs cannot permeate to the other cells so probably impose their antibacterial activity by combining with oligopeptides or acyl-homoserine lactone in Gram-positive and Gram-negative bacteria, respectively. These compounds are biofilm-related signal molecules. EPSs via this mechanism disrupt cell communication and suppress formation of biofilm (Spanò et al. 2016). Therefore, microbial EPSs could be effective therapeutic molecules in ameliorating biofilm-related chronic and recurrent infections (Fig. 2).
Also, microbial EPSs via protecting their producing cells from a strong immunological response of the host (Paynich et al. 2017) or through acting as prebiotics enhance adherence and subsequent the colonization of microflora on host cells. So, they can competitively inhibit colonization of bacterial pathogens. Also, microbial EPSs can reduce the autoaggregation of bacterial pathogens and make bacterial pathogens more susceptible to immunological response inside the host (Dertli et al., 2015). EPS-producing probiotics can attach to microbial pathogens through their EPS. This coaggregation accelerates their antimicrobial functions through blocking the receptors or channels on the outer membrane of the Gram-negative pathogenic bacteria (Abdalla et al. 2021).
Microbial EPSs have various functional groups, including hydroxyl, phosphate, and carbonyl groups. It has been suggested that these functional groups are involved in the interaction of microbial EPSs with the cell membranes or cell walls of bacterial pathogens. So, they play a critical role in exerting antimicrobial activities (Fig. 3) (Riaz Rajoka et al. 2020).
Antifungal activities
Some microbial EPSs, especially negatively charged ones, show antifungal activity. The negative charge provides better electrostatic interactions with fungi. L. rhamnosus GG produces EPS, which inhibited the hyphal formation of Candida in in vitro cell culture. Furthermore, this EPS in a gut model, decreases the hyphal elongation of C. albicans. The dextran of Weissella confuse has the ability to significantly inhibit the biofilm formation of C. albicans SC5314. Moreover, EPS produced by Lactobacillus strains shows antifungal activity (Abdalla et al. 2021). The EPS of Gloeocapsa sp. and Nostoc entophytum prevents the growth of C. albicans (Najdenski et al. 2013). According to the report of Abinaya et al. (2018), Bacillus licheniformis Dahb1 EPS showed antibiofilm activity toward C. albicans.
Antiviral effects
Microbial EPSs can exert their antiviral effects locally or systemically. In local mode action, the EPSs directly interact with either the viruses or the receptors on the host cell. So, block viral adsorption while in systemic mode actions microbial EPSs stimulate the innate and adaptive immunity or suppress viral replication enzymes (Saadat et al. 2019).
Some microbial EPSs, mainly sulfated polysaccharides like dextran exhibited both inhibitory mode actions (Bell and Lu, 2010; Lin and Huang 2022). L. sakei MN1-derived dextran inhibited infectious pancreatic necrosis virus and infectious hematopoietic necrosis virus. The in vivo treatment of trout with the EPS, decreased their mortality rate of these viruses and considerably enhanced the expression of interferon (IFN)-1 (Vázquez et al., 2017). In the following, the antiviral activities of some microbial exopolysaccharides are represented.
Treated cells with EPS 26a-derived Lactobacillus spp. completely suppressed viral adsorption and the formation of infectious human adenovirus C serotype 5 particles as well as their release (Biliavska et al. 2019).
Kim et al. (2018), demonstrated that L. plantarum LRCC5310 EPS hindered the attachment of the rotavirus and subsequently reduced diarrhea duration, epithelial lesions, rotavirus replication in the intestine, and the recovery time of young mice. Also, L. delbrueckii TUA4408L-derived HePS reduced viral replication and regulated inflammatory response consequently enhanced the resistance of porcine intestinal epitheliocytes to rotavirus infection. This EPS considerably increased the expression of the antiviral (IFN)-β, MxA, and RNase L (Kanmani et al. 2018a; Mizuno et al. 2020), also reported that the EPS of S. thermophilus ST538 activated TLR3 in porcine intestinal epitheliocytes subsequently modulated the innate antiviral immune response.
Microbial EPS also can suppress respiratory viruses. Kanmani et al. (2018b), demonstrated that oral administration of L. delbrueckii OLL1073R-1 HePS considerably reduced influenza virus titer and increased IgA and IgG1. Furthermore, it activated natural killer cells. Also, Lactobacillus plantarum SN35N-derived EPS suppressed the influenza A virus and Vesivirus Feline calicivirus (Noda et al. 2021). Also, EPSs from Haloarcula hispanica ATCC33960 suppressed binding spike protein of SARS-CoV-2 to Vero E6 and bronchial epithelial BEAS-2B cells (Xu et al. 2022b).
Microbial EPSs also can be considered promising anti-herpes virus polymers. Since, Bacillus licheniformis-derived EPS-1 impaired Herpes Simplex Virus type 2 (HSV-2) replication in human peripheral blood mononuclear cells (PBMC) through induction of IL-12, IFN-g, IFN-a, TNF-a, and IL-18 (Arena et al. 2006). Also, EPS-2 produced by Geobacillus thermodenitrificans hindered HSV-2 replication in PBMC through the induction of cytokine production (Arena et al. 2009; El Awady et al. 2019), reported antiviral activities of Streptomyces hirsutus NRC2018-derived EPS on HSV1, Hepatitis A virus, and Coxsackie B-4. Also, Reichert et al. (2017), demonstrated that EPS of A. platensis hindered koi herpesvirus replication in common carp brain cells. Finally, Arthrospira platensis-derived EPS exhibited inhibitory activities on vaccinia and ectromelia viruses (Radonić et al. 2011), and EPS from Weissella paramesenteroides MN2C2 exhibited antiviral activity against Coxsackie virus (Amer et al. 2021).
Anticancer activities
Microbial EPSs have displayed antiproliferative properties against various cancers, including colon, breast, pancreatic, leukemia, and cervical cancers (Jurášková et al. 2022). The chemical characteristics of microbial EPSs like molecular composition, molecular weight, the presence of uronic acid and sulfate groups as well as β-type glycosidic bonds are influential factors in their anticancer activities (Ismail and Nampoothiri 2013; (Wang et al. 2014a; Hou et al. 2021). Microbial EPSs probably through the following mechanisms exert their anticancer activities: act as antioxidants, bind to genotoxic carcinogens, induce apoptosis, and improve immunity (Fig. 4) (Koller et al. 2008).
L. plantarum and L. rhamnosus-derived EPSs can bind to various mutagens, like 2-nitrofluorene, heterocyclic amines, and 4-nitroquinoline-N-oxide reduce their mutagenic potential (Tsuda et al. 2008; Thapa and Zhang 2009).
Studies have shown that microbial EPSs can be effective against various colon cancer cell lines, including HT-29, Caco-2, and CT26. Antiproliferative effects of EPSs produced by L. casei 01 (Liu et al. 2011a) and L. plantarum 70,810 (Wang et al. 2014a), L. rhamnosus ATCC 9595 (Kim et al. 2006), L. brevis and L. delbrueckii subsp. bulgaricus on the HT-29 malignant cell line was reported.
EPSs with antioxidant activity may suppress cancers. It has been shown that the anti-HT-29 activity of L. plantarum 70,810 EPS can be related to its antioxidant activity and it was increased after acetylation modification (Wang et al. 2014a). Also, L. rhamnosus SHA111 EPS with ability to scavenge hydroxyl and superoxide radicals displayed antitumor activity against the Caco-2 cell line (Rajoka et al. 2018).
Also, microbial EPSs through apoptosis induction can exert their anticancer activities. Apoptosis can occur through caspase-dependent intrinsic and extrinsic pathways. In the intrinsic pathway caspase-3, caspase-9, BCl-2, and BAX are expressed and expression of caspase-8 and caspase-10 are done in the extrinsic pathway. Caspase-3 activation is indicating that cell shrinkage, nuclear fragmentation, and chromatin condensation have been occurred in cancerous cells without affecting surrounding healthy cells or tissues (Angelin and Kavitha 2020).
For example, Lactobacillus kefiri EPS the upregulated the expression of Cytochrome-c, Bax, Bad, Caspase-3, -8, and -9 in HT-29 cancerous cells (Rajoka et al. 2019). Also, Lactobacillus strain SB27-derived EPS increased activation of caspase-3 and subsequently induced apoptosis and arrested cell cycle. Moreover, Lactobacillus casei SB27 EPSs (LW1 and LW2) significantly inhibited the proliferation of HT-29 colorectal cancer cells through upregulation of Bad, Bax, Caspase-3, and -8 gene expressions (Di et al. 2017).
Kim et al. (2010), reported the Lactobacillus acidophilus 606 EPS exert its antitumourigenic activity against HT-29 colon cancer cells by activating autophagic cell death which was promoted through inducing of Beclin-1, Grp78, Bcl-2, and Bak.
Tukenmez et al. (2019), showed that EPSs of four Lactobacillus spp. were capable to induce apoptosis in HT-29 via increasing the expression of Bax, Caspase-3 and -9 while decreasing Bcl-2 and Survivin. Among these EPSs, EPS of L. delbrueckii ssp. bulgaricus B3 which contained the highest amount of mannose and the lowest amount of glucose showed the highest apoptosis induction.
Anticancer activity of microbial EPSs on other colon cancer cell lines like Caco-2 and CT26 have also been reported. For example, Lactobacillus fermentum YL-11 EPS suppressed the proliferation of HT-29 and Caco-2 colon cancer cells (Wei et al. 2019). El-Debb et al. (2018), reported that the HePS produced by L. acidophilus 20,079 displayed anti-Caco-2 activity via apoptotic and NF-κB inflammatory pathways. Also, The Lactobacillus acidophilus 20,079-derived EPS suppressed cell proliferation of the CaCo-2 cell line (El-Deeb et al. 2018).
Zhou et al. (2017), demonstrated the inhibitory activity of Lactobacillus plantarum NCU116-derived EPS on the proliferation and survival of CT26 cell line (a murine colorectal carcinoma cell line) through induction of apoptosis.
Inhibitory activity of microbial EPSs on other cancerous cells is also reported. For example, L. plantarum and L. helveticus EPSs suppressed breast cancer and gastric cancer cell lines, respectively (Ismail and Nampoothiri 2013; Li et al. 2014). Pediococcus pentosaceus M41 EPS displayed inhibitory activity against Caco-2 and MCF-7 cells (Ayyash et al. 2020c). Lactococcus lactis subsp. lactis EPS was found to affect the production of inflammatory cytokines and considerably increased TNF-α and inducible nitric oxide (NO) synthase release in MCF-7 cells in comparison with control cells (Wu et al. 2016).
Microbial EPS also can exert their anticancer activity through stimulating cell-mediated immune responses, like tumoricidal activity of natural killer cells, the proliferation of t-lymphocyte, and phagocytic capacity of mononuclear cells. In this regard, it has been reported that L. lactis subsp. lactis EPS induced the apoptosis of MCF17 cells along with nuclear condensation and cell shrinkage, enhancing intracellular calcium levels and production of inflammatory cytokine (Wu et al. 2016).
Lactobacillus plantarum RJF4 EPS showed inhibitory activity against the MiaPaCa2-pancreatic cancer cell line. Its antiproliferative activity can be due to its antioxidant activities (Dilna et al. 2015; Chen et al. 2015), demonstrated that Pseudoaltermonas sp. S-5 EPS suppressed the proliferation of human leukemia K562 cells.
Sungur et al., reported the inhibitory effect of L. gasseri strains-derived EPSs on proliferation of cervical cancerous cells. These EPSs induced apoptosis, upregulated expression of Bax and Caspase-3 in Hela cells (Sungur et al. 2017).
EPS of Bacillus mycoides BS4 displayed antitumor activity on human hepatocellular carcinoma and colorectal adenocarcinoma cells. This microbial EPS demonstrated low cytotoxicity against the normal cell baby hamster kidneys (Farag et al. 2020). Therefore, microbial EPSs can be considered promising natural polymers to develop antitumor drugs with lower side effects than current chemical drugs.
Antioxidant activities
Microbial exopolysaccharides have displayed significant antioxidative activities (Table 3). Their subunits, monosaccharides, are considered reducing sugars because they possess aldoses and ketoses or they can interconvert into either form. The antioxidant potential of microbial EPS can be related to their various functional groups, including hydroxyl, carboxyl, sulfate, sulfhydryl, acetyl, carbonyl, sulfhydryl, thioether and amide groups. These functional groups donate electron pairs, lose a proton, or facilitate the metal binding process (Fig. 3). Subsequently, convert free radicals to stable substances. For instance, the phenomenal scavenging ability of chitosan is due to its hydroxyl and amino groups. Also, it has been stated that negatively charged functional groups by generating an acidic environment could facilitate EPS hydrolysis. Therefore, more exposed hemiacetal hydroxyl groups enhance antioxidant activity (Andrew and Jayaraman 2020; Lin and Huang 2022; Li and Huang 2022; Zhou et al. 2022).
In this regard, chemical modification of the naturally occurring EPSs can be a promising and easy approach to make them more potent antioxidants. Phosphorylation, selenylation, carboxymethylation, sulfation, and acetylation are some of the possible and influential chemical modifications on microbial EPSs. In addition to functional groups, the monosaccharide constituent also affects antioxidant activities of EPSs. It has been observed that EPSs containing neutral monosaccharides like d-galactose, fucose, arabinose, mannose, glucose, and glucuronic acid showed more the antioxidant activities (Andrew and Jayaraman 2020).
Immunomodulatory activities
Microbial EPSs can regulate the actions of innate and adaptive immunity, though acting as immunomodulatory agents (Tables 4 and 5). They interact with dendritic cells and macrophages, stimulate the proliferation of T/B lymphocytes and natural killer cells, improve antibody production, enhance cell tumoricidal activity, and mononuclear cell phagocytic capacity, increase the function of chemokines as well as affect the production of pro-inflammatory (IL-6, IL-12, TNF-α, and NO) and anti-inflammatory cytokines (IL-4 and IL-10) (Fig. 5) (Li and Shah 2016; Rajoka et al. 2020).
It has been reported that acidic HePSs containing phosphate in their composition exert a pro-inflammatory effect and induce the immune response. According to the studies, the presence of the phosphate group and its subsequent chemical de-phosphorylation actives immune system through eliciting different immune cells like macrophages and lymphocytes (Saadat et al. 2019). The phosphate-containing dextran from Lactobacillus mesenteroides improve host immunity more compared to native dextran (Sato et al. 2004).
Immunomodulator activities of microbial EPSs may be interconnected to gut microbiota. Most EPSs can enhance the diversity and balance of microorganisms in the gut by promoting the growth of the intestinal microbiota. Several EPSs-derived from lactic acid bacteria (LAB), such as Lactobacillus plantarum, Pediococcus pentosaceus, Weissella cibaria, and Weissella confusa showed prebiotic characteristics and could encourage the growth of a probiotic strain, Bifidobacterium bifidum DSM 20,456, in vitro. Moreover, LAB derived EPSs can attach to intestinal epithelial cells, thereby hinder pathogen adhesion or stimulate immune cells (Chaisuwan et al. 2020).
The structures and the physicochemical characteristics of microbial EPSs play a pivotal role in their immunomodulatory potential. These properties include monosaccharide composition, molecular weight, electric charges, functional groups, linkage patterns, water solubility, and microstructures It has been reported that negatively charged EPS and/or small-size molecules have stimulating activities, while neutral and large EPS act as a suppressor (Fig. 3) (Werning et al. 2022; Ji et al. 2021).
Antiulcer activities
Helicobacter pylori infection and the usage of non-steroidal anti-inflammatory drugs are the major causes of peptic ulcers. The beneficial effect of some bacterial EPSs has been described in this context (Saadat et al. 2019; Nagaoka et al. 1994), reported that the oral feeding of isolated EPSs from Bifidobacterium breve YIT4014 and 4043, and B. bifidum YIT4007 exhibited antiulcer activity in rat models. The intragastric administration of purified EPS obtained from Streptococcus thermophiles CRL 1190 dissolved in reconstituted skim milk had an antiulcer effect in gastritis-induced mice. Whereas the suspension of the EPS in water did not show a protective effect, it assumes that the interaction of EPS and milk protein provides this gastroprotective effect (Rodríguez et al. 2009).
Other biomedical activities
In addition to the mentioned bioactivities of microbial EPSs, some other applications have been described for them. Antidiabetic property is one of the microbial EPS activities, which is measured by the inhibition of α-amylase and α-glucosidase. This inhibitory activity by the prevention of carbohydrate hydrolysis is helpful to diabetics. The extracted EPS from Enterococcus faecium MS79 showed 91 and 92% inhibitory activities against α-amylase and α-glucosidase, respectively (Ayyash et al. 2020d). EPS produced by several marine cyanobacteria with the potential to inhibit α-glucosidase showed antidiabetic activity. The isolated EPS from Pseudanabaena sp. and Chroococcus sp. inhibited α-glucosidase activity by 14.02 and 13.00%, respectively (Priatni et al. 2016). The α-amylase/α-glucosidase inhibitory mechanism of EPS is not clear. It seems that EPS by attaching to the active site of enzymes or substrates blocks hydrolysis (Ayyash et al. 2020d). The oral administration of purified EPS from Sorangium cellulosum NUST06 significantly reduced blood glucose levels in healthy and diabetic mice. Although the mechanism of action of EPS is not obvious, it is assumed that EPS by the activation of insulin receptors and enhancement of glucose utilization takes part in lowering glucose levels (Ding et al. 2004). Similarly, the administration of levan isolated from Bacillus licheniformis decreased plasma glucose levels by 52% in diabetic rats. The hypoglycaemic role of levan can be related to the stimulation of Langerhans islets, the increase of peripheral sensitivity to remnant insulin, and its antioxidant activity (Dahech et al. 2011; Ghoneim et al. 2016), in an in vivo study found that Bacillus subtilis sp. suppress produced EPS, and had the ability to decrease total cholesterol, low-density lipoprotein, very low-density lipoprotein, and triglycerides. Therefore, this EPS can be reduced the risk of hyperglycemia, dyslipidemia, and cardiovascular disease in diabetic rats. Jin et al. (2012), described that the oral feeding of diabetic mice with selenium-enriched EPS isolated from Enterobacter cloacae Z0206 caused a significant decrease in blood glucose levels, total cholesterol, and triglycerides. EPSs produced by Lactobacillus plantarum GA06 and GA11 had also 36.7% and 28.6% in vitro cholesterol removal efficiency, respectively. It seems that these EPSs had a binding ability to cholesterol (Avci et al. 2020). The EPS of Limosilactobacillus fermentum NCDC400 (EPS400) also showed high cholesterol-lowering activity in in vitro study (90.32%) (Gawande et al. 2021). One of the important properties of EPS produced by Leuconostoc mesenteroides LM187 was its cholesterol-lowering capability with the rate of 53% (Zhang et al. 2021a). The Feeding of mice with EPS-producing Lactobacillus paracasei NFBC 338 and L. mucosae DPC 6426 reduced cholesterol levels in serum and liver (London et al. 2014). Several Lactobacillus species (Uyen et al. 2021)d delbrueckii subsp. bulgaricus B3 (Tok and Aslim 2010) also had cholesterol removal activity.
Sulfated EPS released from Synechocystis aquatilis Sauvageau B90.79 showed anticoagulant and complement-modulating activities (Volk et al. 2006). The EPS produced by Alteromonas infernus after chemical modification of sulfation and depolymerization found anticoagulant activity (Jouault et al. 2001). Lactobacillus plantarum HY7714 produces an EPS with skin anti-aging activity. This EPS by the improvement of cytotoxicity induced by UVB and cellular hydration capacity can repair skin damage (Lee et al. 2021; Shirzad et al. 2018), reported the anti-elastase, anti-collagenase, antioxidant, and wound healing activities of EPSs generated by some Lactobacilli, which are converted into appropriate agents for skin anti-aging.
According to the conducted research, EPSs produced by some marine bacteria through the induction of proliferation and migration in fibroblasts and keratinocytes have wound-healing activity. EPS produced by Alteromonas sp. PRIM-28 (Sahana and Rekha 2019), Polaribacter sp. SM1127 (Sun et al. 2020), Pantoea sp. YU16-S3 (Sahana and Rekha 2020), and Lactiplantibacillus plantarum EI6 (Zaghloul and Ibrahim 2022) are examples of bioactive molecules, which can be used in wound-care products.
Improving the production and properties of microbial EPS by genetic engineering
Bacterial species generally produce EPS through four well-known mechanisms: the Wzx/Wzy- dependent pathway, the ATP-binding cassette (ABC) transporter-dependent pathway, the synthase-dependent pathway, and the extracellular synthesis by use of a single sucrase protein (Rana and Upadhyay 2020; Schmid et al. 2015). In each of these pathways, several enzyme-encoding genes take part in EPS biosynthesis. These genes usually cluster within bacterial genomes or plasmids (Schmid et al. 2015; Sun and Zhang 2021). Moreover, some housekeeping genes, which have a role in the formation of sugar nucleotides are important for EPS biosynthesis (Bajpai et al. 2016). By improving our knowledge about these genes and their regulations, the yield and properties of EPS can be altered through genetic engineering methods. Transposon engineering, degenerate PCR, gene knockout, gene overexpression, and gene editing by the CRISPR system can be used for generating modified EPS with new biological activities (Sun and Zhang 2021). Some successful research in which EPS production or its properties improved is briefly describes as follows. The yield of EPS in Streptococcus thermophiles enhanced from 0.17 to 0.31 g/mol when galU (UDP-glucose pyrophosphorylase) and pgmA (phosphoglucomutase) overexpressed simultaneously (Levander et al. 2002). By the overexpression of the nox gene in recombinant Lactobacillus casei LC-nox, the yield of EPS by 75% rising reached 263.7 mg/L in aerobic culture condition. nox encodes NADH oxidase which is related to energy metabolism and redox status (Li et al. 2015; Song et al. 2018), found that by the overexpression of LC2W_2179, LC2W_2188, and LC2W_2189 in L. casei LC2W the EPS production increased 16, 10, and 18% compared to the wild-type strain. The first gene encodes Glucose-1-phosphate thymidyltransferase and two other ones produce EPS synthesis proteins. Díaz-Barrera et al. (2012), reported the relation between alg8 (encoding the catalytic subunit of alginate polymerase) expression and alginate polymerization in Azotobacter vinelandii. Higher alg8 expression generates higher molecular weight alginate. The mutant strain of A. vinelandii (ATCN4) with inactive nqrE gene produced alginate with higher yield and improved rheological properties. The product of nqrE is a subunit of Na+-translocating NADH:ubiquinone oxidoreductase complex (Gayta´n et al. 2012). In another study, by the coexpression of gumB and gumC (genes involved in xanthan biosynthesis) in Xanthomonas campestris the viscosity of xanthan was increased. It seems that GumB and GumC control xanthan chain length (Galván et al. 2013). Hassler et al. (1990), also found that mutant strains of X. campestris produced xanthan with various viscosity due to the variable acetylation and pyruvylation levels, and the presence of different sugar residues at terminal side chains.
Conclusion
Microbial EPSs display great diversity. They are multifunctional carbohydrates with considerable health-improving potential. Recent investigations have revealed the great health improving properties of microbial EPS in industries that may be related to their novel and distinct properties compared to polysaccharides obtained from other natural sources. Now, a large proportion of commercially-available EPSs are derived from microorganisms. The main benefit of microbial EPSs is the adjustable chemical composition and structure, which demonstrates their specific usage in pharmaceutical and medical fields. This review points that microbial EPSs can be considered promising alternatives to chemicals likes chemical antibiotics, antioxidants, anticancer, antiviral and antifungal drugs. Microbial EPSs are nontoxic, biocompatible, thermally stable and biodegradable molecules. By applying antibacterial or antifungal EPSs, the antagonistic activity of normal flora against pathogens which is likely to be lost in antibiotic treatment is maintained. Also, microbial EPSs owning to reduced adverse effects, and immune-stimulating activities may be considered safe alternatives to synthetic anticancer drugs. Moreover, EPSs are considered promising green substitutes for synthetic antioxidants because they participate in the removal of oxidative stress through scavenging various free radicals, suppression of lipid peroxidation, reducing metal ion chelating activity, and promoting enzymatic and nonenzymatic antioxidant activities. As it was presented physicochemical characteristics of EPSs, including molecular weight, branching degree, monosaccharide composition, glycosidic bonds, electric charge, and functional groups influence on their functional behavior.
The study of structure-function relationship could result in smart chemical modification of discovered EPSs to have improved bioactivities or targeted screening and isolation of the microbial EPSs with desired bioactivity in the near future.
Therefore, finding microbial EPSs with suitable chemical architecture through screening studies from unexplored ecosystems, imposing chemical modifications or genetic and metabolic engineering could facilitate obtaining a bioactive polymer to be applied in cosmetics, medical, food products, textiles, pharmaceutical, agricultural and other types of industrial sectors. This study reviewed the studies conducted on microbial EPSs along with their microbial sources, physicochemical properties with particular attention to bioactivities, and their mode actions to provide a platform for researchers to identify the relationship of structure properties to bioactivities.
However, there are contradictions about the effects of these properties on various EPS’s bioactivities. This could be related to different in vivo and in vitro models used to evaluate biological activities as well as the lack of comprehensive knowledge on all microbial EPS structures. Therefore, more studies should be performed to explore the mechanism behind EPS’s bioactivities.
References
Abarquero D, Renes E, Fresno JM, Tornadijo ME (2022) Study of exopolysaccharides from lactic acid bacteria and their industrial applications: a review. Int J Food Sci Technol 57:16–26
Abdalla AK, Ayyash MM, Olaimat AN, Osaili TM, Al-Nabulsi AA, Shah NP et al (2021) Exopolysaccharides as antimicrobial agents: mechanism and spectrum of activity. Front Microbiol 12:664395
Abinaya M, Vaseeharan B, Divya M, Vijayakumar S, Govindarajan M, Alharbi NS et al (2018) Structural characterization of Bacillus licheniformis Dahb1 exopolysaccharide—antimicrobial potential and larvicidal activity on malaria and Zika virus mosquito vectors. Environ Sci Pollut Res 25(19):18604–18619
Adebayo-Tayo B, Fashogbon R (2020) In vitro antioxidant, antibacterial, in vivo immunomodulatory, antitumor and hematological potential of exopolysaccharide produced by wild type and mutant Lactobacillus delbureckii subsp. bulgaricus. Heliyon 6(2):e03268
Adebayo-Tayo B, Ishola R, Oyewunmi T (2018) Characterization, antioxidant and immunomodulatory potential on exopolysaccharide produced by wild type and mutant Weissella confusa strains. Biotechnol Rep 19:e00271
Adesulu-Dahunsi A, Sanni A, Jeyaram K (2018) Production, characterization and in vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT 87:432–442
Al-Nabulsi AA, Jaradat ZW, Qudsi A, Elsalem FR, Osaili L, Olaimat TM et al (2022) Characterization and bioactive properties of exopolysaccharides produced by Streptococcus thermophilus and Lactobacillus bulgaricus isolated from labaneh. LWT 167:113817
Almasi F, Salimi F, Mohammadipanah F (2021) Microbial metabolites as promising anti-inflammatory resources in biomedicine. Adv Res Microb Metab Technol 4(1):1
Amer MN, Elgammal EW, Atwa NA, Eldiwany AI, Dawoud IE, Rashad FM (2021) Structure elucidation and in vitro biological evaluation of sulfated exopolysaccharide from LAB Weissella paramesenteroides MN2C2. J Appl Pharm Sci 11(5):022–031
Amini E, Salimi F, Imanparast S (2022) Optimization of lacticaseibacillus paracasei AS20 (1)-derived exopolysaccharide and its potential as an anti-biofilm and anticancer agent. BJM 11(44):105–118
Amini E, Salimi F, Imanparast S, Mansour FN (2022b) Isolation and characterization of exopolysaccharide derived from lacticaseibacillus paracasei AS20 (1) with probiotic potential and evaluation of its antibacterial activity. Lett Appl Microbiol 75(4):967–981
Andrew M, Jayaraman G (2020) Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr Res 487:107881
Andrew M, Jayaraman G (2022) Molecular characterization and biocompatibility of Exopolysaccharide produced by moderately Halophilic Bacterium Virgibacillus dokdonensis from the Saltern of Kumta Coast. Polymers 14(19):3986
Angelin J, Kavitha M (2020) Exopolysaccharides from probiotic bacteria and their health potential. Int J Biol Macromol 162:853–865
Arayes MA, Mabrouk ME, Sabry SA, Abdella B (2023) Exopolysaccharide production from Alkalibacillus sp. w3: statistical optimization and biological activity. Biologia 78(1):229–240
Arena A, Maugeri TL, Pavone B, Iannello D, Gugliandolo C, Bisignano G (2006) Antiviral and immunoregulatory effect of a novel exopolysaccharide from a marine thermotolerant Bacillus licheniformis. Int Immunopharmacol 6(1):8–13
Arena A, Gugliandolo C, Stassi G, Pavone B, Iannello D, Bisignano G et al (2009) An exopolysaccharide produced by Geobacillus thermodenitrificans strain B3-72: antiviral activity on immunocompetent cells. Immunol Lett 123(2):132–137
Arun J, Selvakumar S, Sathishkumar R, Moovendhan M, Ananthan G, Maruthiah T et al (2017) In vitro antioxidant activities of an exopolysaccharide from a salt pan bacterium Halolactibacillus miurensis. Carbohydr Polym 155:400–406
Ates O (2015) Systems biology of microbial exopolysaccharides production. Front Bioeng Biotechnol 3:200
Avci GA, Cagatay G, Cilak GO, Avci E (2020) Probable novel probiotics: EPS production, cholesterol removal and glycocholate deconjugation of Lactobacillus Plantarum GA06 and GA11 isolared from local handmade-cheese. J Microbiol Biotechnol Food Sci 10(1):83–86
Ayyash M, Abu-Jdayil B, Itsaranuwat P, Almazrouei N, Galiwango E, Esposito G et al (2020a) Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem 333:127418
Ayyash M, Abu-Jdayil B, Itsaranuwat P, Galiwango E, Tamiello-Rosa C, Abdullah H et al (2020b) Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int J Biol Macromol 144:938–946
Ayyash M, Abu-Jdayil B, Olaimat A, Esposito G, Itsaranuwat P, Osaili T et al (2020c) Physicochemical, bioactive and rheological properties of an exopolysaccharide produced by a probiotic Pediococcus pentosaceus M41. Carbohydr Polym 229:115462
Ayyash M, Stathopoulos C, Abu-Jdayil B, Esposito G, Baig M, Turner MS et al (2020d) Exopolysaccharide produced by potential probiotic Enterococcus faecium MS79: characterization, bioactivities and rheological properties influenced by salt and pH. LWT 131:109741
Bajpai VK, Rather IA, Majumder R, Shukla S, Aeron A, Kim K et al (2016) Exopolysaccharide and lactic acid bacteria: perception, functionality and prospects. Bangladesh J Pharmacol 11:1–23
Banerjee A, Rudra SG, Mazumder K, Nigam V, Bandopadhyay R (2018) Structural and functional properties of exopolysaccharide excreted by a novel Bacillus anthracis (strain PFAB2) of hot spring origin. Indian J Microbiol 58(1):39–50
Bhat B, Bajaj BK (2019) Hypocholesterolemic potential and bioactivity spectrum of an exopolysaccharide from a probiotic isolate Lactobacillus paracasei M7. Bioact Carbohydr Diet Fibre 19:100191
Biliavska L, Pankivska Y, Povnitsa O, Zagorodnya S (2019) Antiviral activity of exopolysaccharides produced by lactic acid bacteria of the genera Pediococcus, Leuconostoc and Lactobacillus against human adenovirus type 5. Medicina 55(9):519
Bleau C, Monges A, Rashidan K, Laverdure JP, Lacroix M, Van Calsteren MR et al (2010) Intermediate chains of exopolysaccharides from Lactobacillus rhamnosus RW-9595 M increase IL‐10 production by macrophages. J Appl Microbiol 108(2):666–675
Chaisuwan W, Jantanasakulwong K, Wangtueai S, Phimolsiripol Y, Chaiyaso T, Techapun C et al (2020) Microbial exopolysaccharides for immune enhancement: fermentation, modifications and bioactivities. Food Biosci 35:100564
Chen G, Qian W, Li J, Xu Y, Chen K (2015) Exopolysaccharide of Antarctic bacterium Pseudoaltermonas sp. S-5 induces apoptosis in K562 cells. Carbohydr Polym 121:107–114
Ciszek-Lenda M, Nowak B, Śróttek M, Gamian A, Marcinkiewicz J (2011) Immunoregulatory potential of exopolysaccharide from Lactobacillus rhamnosus KL37. Effects on the production of inflammatory mediators by mouse macrophages. Int J Exp Pathol 92(6):382–391
Dahech I, Belghith KS, Hamden K, Feki A, Belghith H, Mejdoub H (2011) Antidiabetic activity of levan polysaccharide in alloxan-induced diabetic rats. Int J Biol Macromol 49(4):742–746
Dertli E, Mayer MJ, Narbad A (2015) Impact of the exopolysaccharide layer on biofilms, adhesion and resistance to stress in Lactobacillus johnsonii FI9785. BMC microbiol 15(1):1–9
Di W, Zhang L, Wang S, Yi H, Han X, Fan R et al (2017) Physicochemical characterization and antitumour activity of exopolysaccharides produced by Lactobacillus casei SB27 from yak milk. Carbohydr Polym 171:307–315
Dilna SV, Surya H, Aswathy RG, Varsha KK, Sakthikumar DN, Pandey A et al (2015) Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT 64(2):1179–1186
Ding X, Zhang J, Jiang P, Xu X, Liu Z (2004) Structural features and hypoglycaemic activity of an exopolysaccharide produced by Sorangium cellulosum. Lett Appl Microbiol 38(3):223–228
Dinić M, Pecikoza U, Djokić J, Stepanović-Petrović R, Milenković M, Stevanović M et al (2018) Exopolysaccharide produced by probiotic strain Lactobacillus paraplantarum BGCG11 reduces inflammatory hyperalgesia in rats. Front Pharmacol 9:1
Dı´az-Barrera A, Soto E, Altamirano C (2012) Alginate production and alg8 gene expression by Azotobacter vinelandii in continuous cultures. J Ind Microbiol Biotechnol 39(4):613–621
Domingos-Lopes M, Nagy A, Stanton C, Ross P, Gelencsér E, Silva C (2017) Immunomodulatory activity of exopolysaccharide producing Leuconostoc citreum strain isolated from Pico cheese. J Funct Foods 33:235–243
Du R, Qiao X, Zhao F, Song Q, Zhou Q, Wang Y et al (2018) Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr Polym 198:529–536
El Awady ME, Eldin MAN, Ibrahim HM, Al Bahnasy ME, Aziz SHA (2019) In vitro evaluation of antioxidant, anticancer, and antiviral activities of exopolysaccharide from Streptomyces hirsutus NRC2018. J Appl Pharm Sci 9(11):010–018
El-Deeb NM, Yassin AM, Al-Madboly LA, El-Hawiet A (2018) A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-κB inflammatory pathways in human colon cancer. Microb Cell Factories 17(1):1–15
Fanning S, Hall LJ, van Sinderen D (2012) Bifidobacterium breve UCC2003 surface exopolysaccharide production is a beneficial trait mediating commensal-host interaction through immune modulation and pathogen protection. Gut Microbes 3(5):420–425
Farag MM, Moghannem SA, Shehabeldine AM, Azab MS (2020) Antitumor effect of exopolysaccharide produced by Bacillus mycoides. Microb Pathog 140:103947
Farinazzo FS, Valente LJ, Almeida MB, Simionato AS, Fernandes MTC, Mauro CSI et al (2020) Characterization and antioxidant activity of an exopolysaccharide produced by Leuconostoc pseudomesenteroides JF17 from juçara fruits (Euterpe edulis Martius). Process Biochem 91:141–148
Freitas F, Alves VD, Reis MA (2011) Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol 29(8):388–398
Galván EM, Ielmini MV, Patel Y, Bianco MI, Franceschini EA, Schneider JC et al (2013) Xanthan chain length is modulated by increasing the availability of the polysaccharide copolymerase protein GumC and the outer membrane polysaccharide export protein GumB. Glycobiol 23(2):259–272
Gawande K, Kolhekar M, Kumari M, Kapila S, Sharma P, Ali SA et al (2021) Lactic acid bacteria based purified exopolysaccharide showed viscofying and hypercholesterolemic capabilites. FHFH 1:100042
Gayta´n I, Pen˜a C, Nu´n˜ez C, Co´rdova MaS, Espı´n G, Galindo E (2012) Azotobacter vinelandii lacking the Na+-NQR activity: a potential source for producing alginates with improved properties and at high yield. World J Microbiol Biotechnol 28(8):2731–2740
Ghoneim MAM, Hassan AI, Mahmoud MG, Asker MS (2016) Effect of polysaccharide from Bacillus subtilis sp. on cardiovascular diseases and atherogenic indices in diabetic rats. BMC Complement Altern Med 16:112
González CG, García MdSC, García JM, Alfaro-Rodriguez M-C (2019) A comparison of the Effect of temperature on the rheological properties of Diutan and Rhamsan gum aqueous solutions. Fluids 4(1):22
Guo MQ, Hu X, Wang C, Ai L (2017) Polysaccharides: structure and solubility. In: Xu Z (ed) Solubility of polysaccharides. IntechOpen, London, pp 1–17
Hasheminya S-M, Dehghannya J (2020) Novel ultrasound-assisted extraction of kefiran biomaterial, a prebiotic exopolysaccharide, and investigation of its physicochemical, antioxidant and antimicrobial properties. Mater Chem Phys 243:122645
Hassler RA, Doherty DH (1990) Genetic engineering of polysaccharide structure: production of variants of Xanthan Gum in Xanthomonas campestris. Biotechnol Prog 6(3):182–187
Hou C, Yin M, Lan P, Wang H, Nie H, Ji X (2021) Recent progress in the research of Angelica sinensis (Oliv.) Diels polysaccharides: extraction, purification, structure and bioactivities. Chem Biol Technol Agric 8:1–14
Hu Y-Q, Wei W, Gao M, Zhou Y, Wang G-X, Zhang Y (2019) Effect of pure oxygen aeration on extracellular polymeric substances (EPS) of activated sludge treating saline wastewater. Process Saf Environ Prot 123:344–350
Imran MYM, Reehana N, Jayaraj KA, Ahamed AAP, Dhanasekaran D, Thajuddin N et al (2016) Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. Int J Biol Macromol 93:731–745
Ismail B, Nampoothiri KM (2013) Exposition of antitumour activity of a chemically characterized exopolysaccharide from a probiotic Lactobacillus plantarum MTCC 9510. Biologia 68(6):1041–1047
Jeong D, Kim D-H, Kang I-B, Kim H, Song K-Y, Kim H-S et al (2017) Characterization and antibacterial activity of a novel exopolysaccharide produced by Lactobacillus kefiranofaciens DN1 isolated from kefir. Food Control 78:436–442
Ji X, Peng B, Ding H, Cui B, Nie H, Yan Y (2021) Purification, structure and biological activity of pumpkin polysaccharides: a review. Food Rev Int 1:1–13
Ji X, Hou C, Shi M, Yan Y, Liu Y (2022) An insight into the research concerning Panax ginseng CA Meyer polysaccharides: a review. Food Rev Int 38(6):1149–1165
Jin M, Lu Z, Huang M, Wang Y, Wang Y (2012) Effects of Se-enriched polysaccharides produced by Enterobacter cloacae Z0206 on alloxan-induced diabetic mice. Int J Biol Macromol 50(2):348–352
Jouault SC, Chevolot L, Helley D, Ratiskol J, Bros A, Sinquin C et al (2001) Characterization, chemical modifications and in vitro anticoagulant properties of an exopolysaccharide produced by Alteromonas infernus. Biochim Biophys Acta 1528(2–3):141–151
Jurášková D, Ribeiro SC, Silva CC (2022) Exopolysaccharides produced by lactic acid bacteria: from biosynthesis to health-promoting properties. Foods 11(2):156
Kanmani P, Albarracin L, Kobayashi H, Hebert EM, Saavedra L, Komatsu R et al (2018a) Genomic characterization of Lactobacillus delbrueckii TUA4408L and evaluation of the antiviral activities of its extracellular polysaccharides in porcine intestinal epithelial cells. Front Immunol 9:2178
Kanmani P, Albarracin L, Kobayashi H, Iida H, Komatsu R, Kober AH et al (2018b) Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells. Mol Immunol 93:253–265
Kenyon W, Buller C (2002) Structural analysis of the curdlan-like exopolysaccharide produced by Cellulomonas flavigena KU. J Ind Microbiol Biotechnol 29(4):200–203
Kim J-U, Kim Y-H, Han K-S, Oh S-J, Whang K-Y, Kim J-N et al (2006) Function of cell-bound and released exopolysaccharides produced by Lactobacillus rhamnosus ATCC 9595. J Microbiol Biotechnol 16(6):939–945
Kim Y, Oh S, Yun H, Oh S, Kim S (2010) Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. Lett Appl Microbiol 51(2):123–130
Kim K, Lee G, Thanh HD, Kim J-H, Konkit M, Yoon S et al (2018) Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response. J Dairy Sci 101(7):5702–5712
Kitazawa H, Itoh T, Tomioka Y, Mizugaki M, Yamaguchi T (1996) Induction of IFN-γ and IL-1α production in macrophages stimulated with phosphopolysaccharide produced by Lactococcus lactis ssp. cremoris. Int J Food Microbiol 31(1–3):99–106
Kodali VP, Sen R (2008) Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol J: Healthc Nutr Technol 3(2):245–251
Kodali VP, Perali RS, Sen R (2011) Purification and partial elucidation of the structure of an antioxidant carbohydrate biopolymer from the probiotic bacterium Bacillus coagulans RK-02. J Nat Prod 74(8):1692–1697
Koller VJ, Marian B, Stidl R, Nersesyan A, Winter H, Simić T et al (2008) Impact of lactic acid bacteria on oxidative DNA damage in human derived colon cells. Food Chem Toxicol 46(4):1221–1229
Kumar R, Bansal P, Singh J, Dhanda S (2020) Purification, partial structural characterization and health benefits of exopolysaccharides from potential probiotic Pediococcus acidilactici NCDC 252. Process Biochem 99:79–86
Lee K, Kim HJ, Kim SA, Park S-D, Shim J-J, Lee J-L (2021) Exopolysaccharide from Lactobacillus plantarum HY7714 protects against skin aging through skin–gut Axis Communication. Molecules 26(6):1651
Leivers S, Hidalgo-Cantabrana C, Robinson G, Margolles A, Ruas-Madiedo P, Laws AP (2011) Structure of the high molecular weight exopolysaccharide produced by Bifidobacterium animalis subsp. lactis IPLA-R1 and sequence analysis of its putative eps cluster. Carbohydr Res 346(17):2710–2717
Levander F, Svensson M, Rådström P (2002) Enhanced exopolysaccharide production by metabolic engineering of Streptococcus thermophilus. Appl Environ Microbiol 68(2):784–790
Li B, Huang G (2022) Preparation, structure-function relationship and application of Grifola umbellate polysaccharides. Ind Crops Prod 186:115282
Li S, Shah NP (2016) Characterization, anti-inflammatory and antiproliferative activities of natural and sulfonated exo‐polysaccharides from Streptococcus thermophilus ASCC 1275. J Food Sci 81(5):M1167–M1176
Li W, Ji J, Chen X, Jiang M, Rui X, Dong M (2014) Structural elucidation and antioxidant activities of exopolysaccharides from Lactobacillus helveticus MB2-1. Carbohydr Polym 102:351–359
Li N, Huang Y, Liu Z, You C, Guo G (2015) Regulation of EPS production in Lactobacillus casei LC2W through metabolic engineering. Lett Appl Microbiol 61(6):555–561
Li X, Liu Z, Zhu L, Miao S, Fang Z, Zhao L et al (2020) Carboxylic modification of welan gum. J Appl Polym Sci 137(3):48301
Li J, Shi H, Yu J, Lei Y, Huang G, Huang H (2022) Extraction and properties of Ginkgo biloba leaf polysaccharide and its phosphorylated derivative. Ind Crops Prod 189:115822
Li J, Chen Z, Shi H, Yu J, Huang G, Huang H (2023) Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason Sonochem. https://doi.org/10.1016/j.ultsonch.2023.106295
Lin B, Huang G (2022) An important polysaccharide from fermentum. Food Chem 10:100388
Liu C-T, Chu F-J, Chou C-C, Yu R-C (2011a) Antiproliferative and anticytotoxic effects of cell fractions and exopolysaccharides from Lactobacillus casei 01. Mutat Res Genet Toxicol Environ Mutagen 721(2):157–162
Liu CF, Tseng KC, Chiang SS, Lee BH, Hsu WH, Pan TM (2011b) Immunomodulatory and antioxidant potential of Lactobacillus exopolysaccharides. J Sci Food Agric 91(12):2284–2291
London LE, Kumar AH, Wall R, Casey PG, O’Sullivan O, Shanahan F et al (2014) Exopolysaccharide-producing probiotic Lactobacilli reduce serum cholesterol and modify enteric microbiota in ApoE-deficient mice. J Nutr Dis 144(12):1956–1962
Mahdhi A, Leban N, Chakroun I, Chaouch MA, Hafsa J, Fdhila K et al (2017) Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb Pathog 109:214–220
Mahmoud MG, Mohamed SS, Asker MS, Hassan AI (2016) The chemical structure and gastroprotective effect of Pseudomonas-exopolysaccharide in rats. Der Pharm Lett 8(5):243–259
Makino S, Ikegami S, Kano H, Sashihara T, Sugano H, Horiuchi H et al (2006) Immunomodulatory effects of polysaccharides produced by Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J Dairy Sci 89(8):2873–2881
Matsuzaki C, Takagaki C, Tomabechi Y, Forsberg LS, Heiss C, Azadi P et al (2017) Structural characterization of the immunostimulatory exopolysaccharide produced by Leuconostoc mesenteroides strain NTM048. Carbohydr Res 448:95–102
Mazmanian SK, Round JL, Kasper DL (2008) A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453(7195):620–625
Mizuno H, Tomotsune K, Islam MA, Funabashi R, Albarracin L, Ikeda-Ohtsubo W et al (2020) Exopolysaccharides from Streptococcus thermophilus ST538 modulate the antiviral innate immune response in porcine intestinal epitheliocytes. Front Microbiol 11:894
Moradi Z, Kalanpour N (2019) Kefiran, a branched polysaccharide: preparation, properties and applications: a review. Carbohydr Polym 223:115100
Nácher-Vázquez M, Iturria I, Zarour K, Mohedano ML, Aznar R, Pardo M et al (2017) Dextran production by Lactobacillus sakei MN1 coincides with reduced autoagglutination, biofilm formation and epithelial cell adhesion. Carbohydr Polym 168:22–31
Nadzir MM, Nurhayati RW, Idris FN, Nguyen MH (2021) Biomedical applications of bacterial exopolysaccharides: a review. Polymers 13(4):530
Nagaoka M, Hashimoto S, Watanabe T, Yokokura T, Mori Y (1994) Anti-ulcer effects of lactic acid bacteria and their cell wall polysaccharides. Biol Pharm Bull 17(8):1012–1017
Najdenski HM, Gigova LG, Iliev II, Pilarski PS, Lukavsky J, Tsvetkova IV et al (2013) Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int J Food Sci Technol 48(7):1533–1540
Nehal F, Sahnoun M, Smaoui S, Jaouadi B, Bejar S, Mohammed S (2019) Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb Pathog 132:10–19
Noda M, Danshiitsoodol N, Sakaguchi T, Kanno K, Sugiyama M (2021) Exopolysaccharide produced by plant-derived Lactobacillus plantarum SN35N exhibits antiviral activity. Biol Pharm Bull 44(12):1886–1890
Notararigo S, de Las Casas-Engel M, de Palencia PF, Corbí AL, López P (2014) Immunomodulation of human macrophages and myeloid cells by 2-substituted (1–3)-β-d-glucan from P. parvulus 2.6. Carbohydr Polym 112:109–113
Nwodo UU, Green E, Okoh AI (2012) Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci 13(11):14002–14015
Pan D, Liu J, Zeng X, Liu L, Li H, Guo Y (2015) Immunomodulatory activity of selenium exopolysaccharide produced by Lactococcus lactis subsp. Lactis Food Agric Immunol 26(2):248–259
Panchal R, Prajapati K, Prajapati M, Sharma S, Saraf S, M (2022) Bacterial exopolysaccharides: types, its biosynthesis and their application in different Fields. ASBT 3(2):3–11
Patten DA, Leivers S, Chadha MJ, Maqsood M, Humphreys PN, Laws AP et al (2014) The structure and immunomodulatory activity on intestinal epithelial cells of the EPSs isolated from Lactobacillus helveticus sp. Rosyjski and Lactobacillus acidophilus sp. 5e2. Carbohydr Res 384:119–127
Paynich ML, Jones-Burrage SE, Knight KL (2017) Exopolysaccharide from Bacillus subtilis induces anti-inflammatory M2 macrophages that prevent T cell–mediated disease. J Immun 198(7):2689–2698
Prete R, Alam MK, Perpetuini G, Perla C, Pittia P, Corsetti A (2021) Lactic acid bacteria exopolysaccharides producers: a sustainable tool for functional foods. Foods 10(7):1653
Priatni S, Budiwati TA, Ratnaningrum D, Kosasih W, Andryani R, Susanti H et al (2016) Antidiabetic screening of some indonesian marine cyanobacteria collection. Biodivers J Biol Divers 17(2):642–646
Radonić A, Thulke S, Achenbach J, Kurth A, Vreemann A, König T et al (2011) Anionic polysaccharides from phototrophic microorganisms exhibit antiviral activities to Vaccinia virus. J Antivir Antiretrovir 2:51–55
Rajoka MSR, Jin M, Haobin Z, Li Q, Shao D, Jiang C et al (2018) Functional characterization and biotechnological potential of exopolysaccharide produced by Lactobacillus rhamnosus strains isolated from human breast milk. LWT 89:638–647
Rajoka MSR, Mehwish HM, Fang H, Padhiar AA, Zeng X, Khurshid M et al (2019) Characterization and anti-tumor activity of exopolysaccharide produced by Lactobacillus kefiri isolated from chinese kefir grains. J Funct Foods 63:103588
Rajoka MSR, Wu Y, Mehwish HM, Bansal M, Zhao L (2020) Lactobacillus exopolysaccharides: new perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci Technol 103:36–48
Ramezanzadeh M, Saeedi N, Mesbahfar E, Farrokh P, Salimi F, Rezaei A (2021) Design and characterization of new antimicrobial peptides derived from aurein 1.2 with enhanced antibacterial activity. Biochimie 181:42–51
Rana S, Upadhyay LSB (2020) Microbial exopolysaccharides: synthesis pathways, types and their commercial applications. Int J Biol Macromol 157:577–583
Rani RP, Anandharaj M, Ravindran AD (2018) Characterization of a novel exopolysaccharide produced by Lactobacillus gasseri FR4 and demonstration of its in vitro biological properties. Int J Biol Macromol 109:772–783
Reichert M, Bergmann S, Hwang J, Buchholz R, Lindenberger C (2017) Antiviral activity of exopolysaccharides from Arthrospira platensis against koi herpesvirus. J Fish Dis 40(10):1441–1450
Rodríguez C, Medici M, Rodríguez AV, Mozzi F, de Font G (2009) Prevention of chronic gastritis by fermented milks made with exopolysaccharide-producing Streptococcus thermophilus strains. J Dairy Sci 92(6):2423–2434
Ruffing AM, Chen RR (2012) Transcriptome profiling of a curdlan-producing Agrobacterium reveals conserved regulatory mechanisms of exopolysaccharide biosynthesis. Microb Cell Factories 11:17
Tripathi SM (2020) Optimization and characterization of alginic acid synthesized from a novel strain of Pseudomonas stutzeri. Biotechnol Rep 27:e00517
Saadat YR, Khosroushahi AY, Gargari BP (2019) A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr Polym 217:79–89
Sahana TG, Rekha PD (2019) A bioactive exopolysaccharide from marine bacteria Alteromonas sp. PRIM-28 and its role in cell proliferation and wound healing in vitro. Int J Biol Macromol 131:10–18
Sahana TG, Rekha PD (2020) A novel exopolysaccharide from marine bacterium Pantoea sp. YU16-S3 accelerates cutaneous wound healing through Wnt/β-catenin pathway. Carbohydr Polym 238:116191
Salazar N, López P, Garrido P, Moran J, Cabello E, Gueimonde M et al (2014) Immune modulating capability of two exopolysaccharide-producing Bifidobacterium strains in a Wistar rat model. Biomed Res Int. https://doi.org/10.1155/2014/106290
Salimi F, Imanparast S (2022) Characterization of probiotic Pichia sp. DU2-derived exopolysaccharide with oil-in-water emulsifying and anti-biofilm activities. Appl Biochem Biotechnol 1:1–21
Salimi F, Mohammadipanah F (2021) Nanomaterials versus the microbial compounds with wound healing property. Front nanotechnol 2:584489
Salimi F, Almasi F, Mohammadipanah F, Abdalla MA (2022) A comparative review of plant and microbial antioxidant secondary metabolites. Appl Food Biotechnol 9(2):173–194
Sarikaya H, Aslim B, Yuksekdag Z (2017) Assessment of anti-biofilm activity and bifidogenic growth stimulator (BGS) effect of lyophilized exopolysaccharides (l-EPSs) from Lactobacilli strains. Int J Food Prop 20(2):362–371
Sasikumar K, Vaikkath DK, Devendra L, Nampoothiri KM (2017) An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Bioresour Technol 241:1152–1156
Sato T, Nishimura-Uemura J, Shimosato T, Kawai Y, Kitazawa H, Saito T (2004) Dextran from Leuconostoc mesenteroides augments immunostimulatory effects by the introduction of phosphate groups. J Food Prot 67(8):1719–1724
Schmid J, Sieber V, Rehm B (2015) Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol 6:496
Seo B-J, Bajpai VK, Rather IA, Park Y-H (2015) Partially purified exopolysaccharide from Lactobacillus plantarum YML009 with total phenolic content, antioxidant and free radical scavenging efficacy. Indian J Pharm Educ Res 49(4):282–292
Shang N, Xu R, Li P (2013) Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydr Polym 91(1):128–134
Shin JS, Jung JY, Lee SG, Shin KS, Rhee YK, Lee MK et al (2016) Exopolysaccharide fraction from Pediococcus pentosaceus KFT 18 induces immunostimulatory activity in macrophages and immunosuppressed mice. J Appl Microbioly 120(5):1390–1402
Shirzad M, Hamedi J, Motevaseli E, Modarressi MH (2018) Anti-elastase and anti-collagenase potential of Lactobacilli exopolysaccharides on human fibroblast. Artif Cells Nanomed Biotechnol 46(S1):S1051–S1061
Sims IM, Frese SA, Walter J, Loach D, Wilson M, Appleyard K et al (2011) Structure and functions of exopolysaccharide produced by gut commensal Lactobacillus reuteri. ISME J 5(7):1115–1124
Soliemani O, Salimi F, Rezaei A (2022) Characterization of exopolysaccharide produced by probiotic Enterococcus durans DU1 and evaluation of its anti-biofilm activity. Arch Microbiol 204(7):419
Song X, Xiong Z, Kong L, Wang G, Ai L (2018) Relationship between putative eps genes and production of Exopolysaccharide in Lactobacillus casei LC2W. Front Microbiol 9:1882
Spanò A, Laganà P, Visalli G, Maugeri TL, Gugliandolo C (2016) In vitro antibiofilm activity of an exopolysaccharide from the marine thermophilic Bacillus licheniformis T14. Curr Microbiol 72:518–528
Sun X, Zhang J (2021) Bacterial exopolysaccharides: chemical structures, gene clusters and genetic engineering. Int J Biol Macromol 173:481–490
Sun M-L, Zhao F, Chen X-L, Zhang X-Y, Zhang Y-Z, Song X-Y et al (2020) Promotion of wound healing and prevention of frostbite injury in rat skin by exopolysaccharide from the arctic marine bacterium Polaribacter sp. SM1127. Mar Drugs 18(1):48
Sungur T, Aslim B, Karaaslan C, Aktas B (2017) Impact of Exopolysaccharides (EPSs) of Lactobacillus gasseri strains isolated from human vagina on cervical tumor cells (HeLa). Anaerobe 47:137–144
Sze JH, Brownlie JC, Love CA (2016) Biotechnological production of hyaluronic acid: a mini review. 3 Biotech 6(1):67
Tang Q, Huang G (2022) Improving method, properties and application of polysaccharide as emulsifier. Food Chem 376:131937
Taylan O, Yilmaz MT, Dertli E (2019) Partial characterization of a levan type exopolysaccharide (EPS) produced by Leuconostoc mesenteroides showing immunostimulatory and antioxidant activities. Int J Biol Macromol 136:436–444
Thapa D, Zhang H (2009) Lactobacillus rhamnosus exopolysaccharide reduces mutagenic potential of genotoxins. Int J Probiotics Prebiotics 4:79–82
Tok E, Aslim B (2010) Cholesterol removal by some lactic acid bacteria that can be used as probiotic. Microbiol Immunol 54(5):257–264
Trabelsi I, Ktari N, Slima SB, Triki M, Bardaa S, Mnif H et al (2017) Evaluation of dermal wound healing activity and in vitro antibacterial and antioxidant activities of a new exopolysaccharide produced by Lactobacillus sp. Ca6. Int J Biol Macromol 103:194–201
Tsuda H, Hara K, Miyamoto T (2008) Binding of mutagens to exopolysaccharide produced by Lactobacillus plantarum mutant strain 301102S. J Dairy Sci 91(8):2960–2966
Tukenmez U, Aktas B, Aslim B, Yavuz S (2019) The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci Rep 9(1):1–14
Uyen PTT, An NH, Hai PT, Ha BTV (2021) Cholesterol-lowering potential and exopolysaccharide biosynthesis of Lactobacillus spp. isolated from human milk. VNU J Sci: Nat Sci Technol 37(4):48–56
Vinderola G, Perdigón G, Duarte J, Farnworth E, Matar C (2006) Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 36(5–6):254–260
Vitlic A, Sadiq S, Ahmed HI, Ale EC, Binetti AG, Collett A et al (2019) Isolation and characterization of a high molecular mass β-glucan from Lactobacillus fermentum Lf2 and evaluation of its immunomodulatory activity. Carbohydr Res 476:44–52
Volk R-B, Venzke K, Blaschek W, Alban S (2006) Complement modulating and anticoagulant effects of a sulfated exopolysaccharide released by the cyanobacterium Synechocystis aquatilis. Planta Med 72(15):1424–1427
Vu B, Chen M, Crawford RJ, Ivanova EP (2009) Bacterial extracellular polysaccharides involved in Biofilm formation. Molecules 14(7):2535–2554
Wang K, Li W, Rui X, Chen X, Jiang M, Dong M (2014a) Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int J Biol Macromol 63:133–139
Wang K, Li W, Rui X, Chen X, Jiang M, Dong M (2014b) Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int J Biol Macromol 67:71–78
Wang J, Zhao X, Tian Z, Yang Y, Yang Z (2015a) Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohydr Polym 125:16–25
Wang J, Zhao X, Yang Y, Zhao A, Yang Z (2015b) Characterization and bioactivities of an exopolysaccharide produced by Lactobacillus plantarum YW32. Int J Biol Macromol 74:119–126
Wang X, Shao C, Liu L, Guo X, Xu Y, Lü X (2017) Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int J Biol Macromol 103:1173–1184
Wang J, Fang X, Wu T, Min W, Yang Z (2018a) Exopolysaccharide producing Lactobacillus plantarum SKT109 as adjunct culture in Cheddar cheese production. LWT 97:419–426
Wang J, Wu T, Fang X, Min W, Yang Z (2018b) Characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus plantarum JLK0142 isolated from fermented dairy tofu. Int J Biol Macromol 115:985–993
Wang J, Fang X, Wu T, Fang L, Liu C, Min W (2020a) In vitro immunomodulatory effects of acidic exopolysaccharide produced by Lactobacillus planetarium JLAU103 on RAW264. 7 macrophages. Int J Biol Macromol 156:1308–1315
Wang K, Niu M, Song D, Song X, Zhao J, Wu Y et al (2020b) Preparation, partial characterization and biological activity of exopolysaccharides produced from Lactobacillus fermentum S1. J Biosci Bioeng 129(2):206–214
Wei Y, Li F, Li L, Huang L, Li Q (2019) Genetic and biochemical characterization of an exopolysaccharide with in vitro antitumoral activity produced by Lactobacillus fermentum YL-11. Front Microbiol 10:2898
Werning ML, Hernández-Alcántara AM, Ruiz MJ, Soto LP, Dueñas MT, López P et al (2022) Biological functions of exopolysaccharides from lactic acid bacteria and their potential benefits for humans and farmed animals. Foods 11(9):1284
Wu M-H, Pan T-M, Wu Y-J, Chang S-J, Chang M-S, Hu C-Y (2010) Exopolysaccharide activities from probiotic bifidobacterium: immunomodulatory effects (on J774A. 1 macrophages) and antimicrobial properties. Int J Food Microbiol 144(1):104–110
Wu Z, Wang G, Pan D, Guo Y, Zeng X, Sun Y et al (2016) Inflammation-related pro-apoptotic activity of exopolysaccharides isolated from Lactococcus lactis subsp. lactis. Benef Microbes 7(5):761–768
Xu R, Shang N, Li P (2011) In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 17(5):226–231
Xu X, Qiao Y, Peng Q, Shi B, Dia VP (2022a) Antioxidant and immunomodulatory properties of partially purified exopolysaccharide from lactobacillus casei isolated from chinese Northeast Sauerkraut. Immunol Invest 51(4):748–765
Xu Y, Li Y, You X, Pei C, Wang Z, Jiao S et al (2022) Novel insights into the sulfated glucuronic acid-based anti-SARS-CoV-2 mechanism of exopolysaccharides from halophilic archaeon Haloarcula hispanica. Front Chem. https://doi.org/10.3389/fchem.2022.871509
Yasuhara-Bell J, Lu Y (2010) Marine compounds and their antiviral activities. Antiviral Res 86(3):231–240
Ye G, Li G, Wang C, Ling B, Yang R, Huang S (2019) Extraction and characterization of dextran from Leuconostoc pseudomesenteroides YB-2 isolated from mango juice. Carbohydr Polym 207:218–223
You X, Li Z, Ma K, Zhang C, Chen X, Wang G et al (2020) Structural characterization and immunomodulatory activity of an exopolysaccharide produced by Lactobacillus helveticus LZ-R-5. Carbohydr Polym 235:115977
Yu Y-J, Chen Z, Chen PT, Ng I-S (2018) Production, characterization and antibacterial activity of exopolysaccharide from a newly isolated Weissella cibaria under sucrose effect. J Biosci Bioeng 126(6):769–777
Zaghloul EH, Ibrahim MIA (2022) Production and characterization of exopolysaccharide from newly isolated marine probiotic Lactiplantibacillus plantarum EI6 with in vitro wound healing activity. Front Microbiol 13:903363
Zhang L, Liu C, Li D, Zhao Y, Zhang X, Zeng X et al (2013) Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int J Biol Macromol 54:270–275
Zhang J, Cao Y, Wang J, Guo X, Zheng Y, Zhao W et al (2016) Physicochemical characteristics and bioactivities of the exopolysaccharide and its sulphated polymer from Streptococcus thermophilus GST-6. Carbohydr Polym 146:368–375
Zhang H, Ren W, Guo Q, Xiong Z, Wang G, Xia Y et al (2018) Characterization of a yogurt-quality improving exopolysaccharide from Streptococcus thermophilus AR333. Food Hydrocoll 81:220–228
Zhang G, Zhang W, Sun L, Sadiq FA, Yang Y, Gao J et al (2019) Preparation screening, production optimization and characterization of exopolysaccharides produced by Lactobacillus sanfranciscensis Ls-1001 isolated from chinese traditional sourdough. Int J Biol Macromol 139:1295–1303
Zhang Q, Wang J, Sun Q, Zhang S-M, Sun X-Y, Li C-Y et al (2021a) Characterization and antioxidant activity of released exopolysaccharide from potential probiotic Leuconostoc mesenteroides LM187. J Microbiol Biotechnol 31(8):1144–1153
Zhang Y, Chen X, Hu P, Liao Q, Luo Y, Li J et al (2021b) Extraction, purification, and antioxidant activity of exopolysaccharides produced by Lactobacillus kimchi SR8 from sour meat in vitro and in vivo. CYTA J Food 19(1):228–237
Zhou S, Huang G (2023) Extraction, structural analysis and antioxidant activity of aloe polysaccharide. J Mol Struct 1273:134379
Zhou X, Hong T, Yu Q, Nie S, Gong D, Xiong T et al (2017) Exopolysaccharides from Lactobacillus plantarum NCU116 induce c-Jun dependent Fas/Fasl-mediated apoptosis via TLR2 in mouse intestinal epithelial cancer cells. Sci Rep 7(1):1–13
Zhou S, Huang G, Huang H (2022) Extraction, derivatization and antioxidant activities of onion polysaccharide. Food Chem 388:133000
Zhu Y, Wang C, Jia S, Wang B, Zhou K, Chen S et al (2018) Purification, characterization and antioxidant activity of the exopolysaccharide from Weissella cibaria SJ14 isolated from Sichuan paocai. Int J Biol Macromol 115:820–828
Zhu Y, Wang X, Pan W, Shen X, He Y, Yin H et al (2019) Exopolysaccharides produced by yogurt-texture improving Lactobacillus plantarum RS20D and the immunoregulatory activity. Int J Biol Macromol 121:342–349
Acknowledgements
The authors acknowledge Damghan University.
Funding
Authors state no funding involved.
Author information
Authors and Affiliations
Contributions
FS and PF wrote the main manuscript text and FS. prepared Figs. 1–5. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
If the article is accepted for publication, the transfer of copyright from the author to this journal.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salimi, F., Farrokh, P. Recent advances in the biological activities of microbial exopolysaccharides. World J Microbiol Biotechnol 39, 213 (2023). https://doi.org/10.1007/s11274-023-03660-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03660-x