Abstract
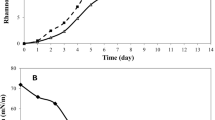
The present study aims to evaluate the growth potential of the P. aeruginosa ATCC9027 strain with molasses as the sole carbon source to produce rhamnolipids. The influence of the cultivation time and substrate concentration on biosurfactant production was investigated by using a complete 3-level factorial design, with the rhamnolipid concentration as the variable response. The strain was able to produce the biosurfactant in all design conditions tested, producing 758.04 mg/L rhamnolipids with 7% v/v substrate concentration in a cultivation time of 120 h. The substrate concentration used in the cultivation step directly influenced the biosurfactant production, and, even with the decrease in biomass growth, the biosurfactant production continued to increase. High Performance Liquid Chromatography (HPLC) revealed the presence of 62.3% mono- (RL1) and 37.6% di-rhamnolipids (RL3). The stability tests showed that the biosurfactant has good performance in extreme conditions of temperature, pH and saline concentration. The emulsification index was also evaluated for several oils and hydrocarbons, obtaining emulsification rates of up to 84.9% for the burnt motor oil. In addition, rhamnolipid showed a good ability to remove spilled oil from the sand, removing 58.51% of burnt motor oil and 70.09% of post-frying soybean oil. The results indicate that molasses, an agro-industrial residue abundant in Brazil, can be used as the only carbon source for quality rhamnolipid production when under optimized conditions, therefore presenting itself as a management option for this residue and, at the same time, providing the production product with high added value.
Graphical abstract






Similar content being viewed by others
Data availability
We declare that the datasets generated during and/or analyzed during the current study are available by the corresponding author, on reasonable request.
References
Abdel-Mawgoud MA, Lépine F, Déziel E (2010) Rhamnolipids diversity of structures, microbial origins and roles. Appl Environ Microbiol 86:1323–1326
Amani H, Muller MM, Syldatk CX, Hausmann R (2013) Production of microbial rhamnolipid by Pseudomonas aeruginosa MM1011 for ex situ enhanced oil recovery. Appl Biochem Biotechnol 170:1080–1093
Anaukwu CG, Ogbukagu CM, Ekwealor IA (2020) Optimized biosurfactant production by Pseudomonas aeruginosa strain CGA1 using agro-industrial waste as sole carbon source. Adv Appl Microbiol 10(10):543–562. https://doi.org/10.4236/aim.2020.1010040
Banat IM, Satpute SK, Cameotra SS, Patil R, Nyayanit NV (2014) Cost effective technologies and renewable substrates for biosurfactants’ production. Front Microbiol 5:1–18. https://doi.org/10.3389/fmicb.2014.00697
Barros FFC, Quadros CP, Pastore GM (2008) Propriedades emulsificantes e estabilidade do biossurfactante produzido por Bacillus subtilis em manipueira. Ciên Tecnol Aliment 24:979–985
Behring JL, Lucas M, Machado C, Barcellos IO (2004) Adaptação no método do peso da gota para determinação da tensão superficial: um método simplificado para a quantificação da CMC de surfactantes no ensino da química. Química nova 27:492–495
Bezerra KG, Gomes UV, Silva RO, Sarubbo LA, Ribeiro E (2019) The potential application of biosurfactant produced by Pseudomonas aeruginosa TGC01 using crude glycerol on the enzymatic hydrolysis of lignocellulosic material. Biodegradation 30(4):351–361
Bueno SM, Silva AN, Garcia-Cruz CH (2010) Estudo da produção de biossurfactante em caldo de fermentação. Química Nova 33:1572–7
Câmara JM, Sousa MA, Barros Neto EL (2019) Optimization and characterization of biosurfactant rhamnolipid production by Pseudomonas aeruginosa isolated from an artificially contaminated soil. J Surfactants Deterg 22(4):711–9
Cameotra SS, Makkar R (1998) Synthesis of biosurfactant in extreme conditions. Appl Microbiol Biotechnol 50:520–529
Chen C, Sun N, Li D, Long S, Tang X, Xiao G, Wang L (2018) Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ Sci Pollut Res 25:14934–14943. https://doi.org/10.1007/S11356-018-1691-1
Cooper DG, Goldenberg BG (1987) Surface-active agents from two Bacillus species. Appl Environ Microbiol 53:224–229
Costa WA, Padilha CE, Oliveira Júnior SD, Silva FL, Silva J, Ancântara MA, Ferrari M, Santos ES (2020) Oil-lipids, carotenoids and fatty acids simultaneous production by Rhodotorula mucilaginosa Cct3892 using sugarcane molasses as carbon source. Braz J Food Technol 23:E2019064
Da Rosa CFC, Michelon M, de Medeiros Burkert J, Kalil SJ, Burkert CAV (2010) Production of a rhamnolipid-type biosurfactant by Pseudomonas aeruginosa LBM10 grown on glycerol. Afr J Biotechnol 9(53):9012–9017
de Oliveira Schmidt VK, de SouzaCarvalho J, de Oliveira D, de Andrade CJ (2021) Biosurfactant inducers for enhanced production of surfactin and rhamnolipids: an overview. World J Microbiol Biotechnol 37(2):1–5
De Rienzo MD, Kamalanathan ID, Martin PJ (2016) Comparative study of the production of rhamnolipid biosurfactants by B. thailandensis E264 and P. aeruginosa ATCC 9027 using foam fractionation. Process Biochem 51(7):820–7
Goswami D, Sn Borah J, Lahkar PH, Deka S (2015) Propriedades antifúngicas de ramnolipídeos produzidos por Pseudomonas aeruginosa Ds9 contra colletotrichum falcatum. j Microbiol Básico 55:1265–1274. https://doi.org/10.1002/Jobm.201500220
Gouveia ER (2003) Bactérias produtoras de biossurfactantes. Rev Biotecnol Ciênc E Desenvolv 30:39–45
Gudiña EJ, Rodrigues AI, De Freitas V, Azevedo Z, Teixeira JA, Rodrigues LR (2016) Valorization of agro-industrial wastes toward the production of rhamnolipids. Bioresour Technol 212:144–150
Haba E, Espuny MJ, Busquets M, Manresa A (2000) Screening and production of rhamnolipids by Pseudomonas aeruginosa 47t2 Ncib 40044 from waste frying oils. J Appl Microbiol 88:379–387
Haba E, Pinazo A, Jauregui O, Espuny MJ, Infante MR, Manresa A (2003) Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47t2 Ncbim 40044. Biotechnol Bioeng 81:316–322
Hamza RA, Iorhemen OT, Tay JH (2016) Occurrence, impacts and removal of emerging substances of concern from wastewater. Environ Technol Innov 1:161–175
Ji F, Li F, Wang J, Ba Y (2016) Production of rhamnolipids with a high specificity by Pseudomonas aeruginosa M408 isolated from petroleum-contaminated soil using olive oil as sole carbon source. Ann Microbiol 66:1145–1156
Jimenez AP, Requiso PJ, Maloles JS, Alcantara EP, Alcantara VA (2020) Biosurfactant production by Streptomyces sp. CGS B11 using molasses and spent yeast medium. Philipp J Sci 149(4):1227–1241
Joshi S, Bharucha C, Jha S, Yadav S, Nerurkar A, Desai AJ (2008) Biosurfactant production using molasses and whey under thermophilic conditions. Bioresour Technol 99(1):195–199. https://doi.org/10.1016/j.biortech.2006.12.010
Kanna R (2017) Biological surfactant production by Pseudomonas aeruginosa Atcc 9027 and probable application in microbial enhanced oil recovery (Meor). Int J Civ Eng Technol 8:619–626
Karlapudi AP, Venkateswarulu TC, Tammineedi J, Kanumuri L, Ravuru BK, Dirisala VR, Kodali VP (2018) Role of biosurfactants in bioremediation of oil pollution—a review. Petrol 3:241–249
Kaskatepe B, Yildiz S, Gumustas M, Ozkan SA (2015) Biosurfactant production by Pseudomonas aeruginosain Kefir and fish meal. Braz J Microbiol 46:855–859. https://doi.org/10.1590/S1517-838246320140727
Kim D-J, Chung S, Lee S, Choi J (2012) Relation of microbial biomass to counting units for Pseudomonas aeruginosa. Afr J Microbiol Res 6:4620–4622
Kumar AP, Janardhan A, Radha S, Viswanath B, Narasimha G (2014) Statistical approach to optimize production of biosurfactant by Pseudomonas aeruginosa 2297. 3 Biotech 5:71–79
Luna JM, Rufino RD, Albuquerque CDC, Sarubbo LA, Campos-Takaki GM (2011) Economic optimized medium for tensio-active agent production by Candida sphaerica UCP0995 and application in the removal of hydrophobic contaminant from sand. Int J Mol Sci 12:2463–2476. https://doi.org/10.3390/ijms12042463
Moya Ramírez I, Altmajer Vaz D, Banat IM, Marchant R, Jurado Alameda E, García Román M (2016) Hydrolysis of olive mill waste to enhance rhamnolipids and surfactin production. Bioresour Technol 205:1–6. https://doi.org/10.1016/j.biortech.2016.01.016
Müller MM, Hörmann B, Syldatk C, Hausmann R (2010) Pseudomonas aeruginosa Pao1 as a model for rhamnolipid production in bioreactor systems. Appl Microbiol Biotechnol 87(1):167–174
Nitschke M, Pastore GM (2002) Biosurfactantes: propriedades e aplicações. Quím Nova, São Paulo 25:772–776
Nitschke M, Costa SGVA, Contiero J (2005) Rhamnolipid surfactants: an update on the general aspects of these remarkable biomolecules. Biotechnol Prog 21:1595–1600
Oliveira ACSM, Bezerra MS, Padilha CEA, Melchuna AM, Macedo GR, Santos ES (2013) Recovery of rhamnolipids produced bypseudomonas aeruginosausing acidic precipitation, extraction, and adsorption on activated carbon. Sep Sci Technol 48(18):2852–2859. https://doi.org/10.1080/01496395.2013.809107
Pacheco GJ, Reis RS, Fernandes ACLB, Da Rocha SLG, Pereira MD, Perales J, Freire DMG (2012) Rhamnolipid production: effect of oxidative stress on virulence factors and proteome of Pseudomonas aeruginosa Pa1. Appl Microbiol Biotechnol 95:1519–1529. https://doi.org/10.1007/S00253-012-4258-Y
Palmonari A, Cavallini D, Sniffen CJ, Fernandes L, Holder P, Fagioli L, Fusaro I, Biagi G, Formigoni A, Mammi L (2020) Short communication: characterization of molasses chemical composition. J Dairy Sci 103(7):6244–6249. https://doi.org/10.3168/Jds.2019-17644
Patel RM, Desai AJ (1997) Biosurfactant production by Pseudomonas aeruginosa GS3 from molasses. Lett Appl Microbiol 25:91–94
Phulpoto IA, Wang Y, Qazi MA, Hu B, Ndayisenga F, Yu Z (2021) Bioprospecting of rhamnolipids production and optimization by an oil-degrading Pseudomonas Sp. S2 we isolated from freshwater lake. Bioresour Technol 323:124601. https://doi.org/10.1016/J.Biortech.2020.124601
Radzuan MN, Banat IM, Winterburn J (2017) Production and characterization of rhamnolipid using palm oil agricultural refinery waste. Bioresour Technol 225:99–105
Rahman KSM, Rahman TJ, Mcclean S, Marchant R, Banat IM (2002) Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw materials. Biotechnol Prog 18:1277–1281
Ramnani P, Kumar SS, Gupta R (2005) Concomitant production and downstream processing of alkaline protease and biosurfactant from Bacillus licheniformis Rg1: bioformulation as detergent additive. Process Biochem 40:3352–3359
Ray M, Kumar V, Banerjee C, Gupta P, Singh S, Singh A (2021) Investigation of biosurfactants produced by three indigenous bacterial strains, their growth kinetics and their anthracene and fluorene tolerance. Ecotoxicol Environ Safe 208:111621
Raza ZA, Khan MS, Khalid ZM (2007a) Physicochemical and surface-active properties of biosurtactant produced using molasses by a Pseudomonas aeruginosas mutant. J Environ Sci Health Part A 42:73–80
Raza ZA, Khan MS, Khalid ZM (2007b) Evaluation of distant carbon sources in biosurfactant production by a gamma ray-induced Pseudomonas putida mutant. Process Biochem 42:686–692
Rocha VA, de Castilho LV, de Castro RP, Teixeira DB, Magalhães AV, Gomez JG, Freire DM (2020) Comparison of mono-rhamnolipids and di-rhamnolipids on microbial enhanced oil recovery (MEOR) applications. Biotechnol Prog 36(4):e2981
Sana S, Datta S, Biswas D, Bhattacharya M (2017) Production kinetics of rhamnolipid using fish fat: a step toward environmental hazard control of sewage. Environ Technol Innov 8:299–308
Schenk T, Schuphan I, Schmidt B (1995) High-performance liquid chromatographic determination of the rhamnolipids produced by Pseudomonas aeruginosa. J Chromatogr A 693(1):7–13
Sharma R, Singh J, Verma N (2017) Optimization of rhamnolipid production from Pseudomonas aeruginosa Pbs toward application for microbial enhanced oil recovery. 3 Biotech 8(1):1–15. https://doi.org/10.1007/S13205-017-1022-0
Silva RCFS, Rufino RD, Luna JM, Farias CBB, Santos VA, Filho HJBL, Sarubbo LA (2013) Enhancing biosurfactant production from Pseudomonas cepacia Cct6659 by optimizing nutritional parameters using a response surface methodology. Tenside Surfactant Deterg 50(2):137–142
Silva RCFS, Almeida DG, Meira HM, Silva EJ, Farias CBB, Rufino RD, Luna JM, Sarubbo LA (2017) Production and characterization of a new biosurfactant from Pseudomonas cepacia grown in low-cost fermentative medium and its application in the oil industry. Biocatal Agric Biotechnol 12:206–215
Thio CW, Lim WH, Md. Shah UK, Phang LY (2022) Palm kernel fatty acid distillate as substrate for rhamnolipids production using Pseudomonas sp. LM19. Green Chem Lett Rev 15(1):83–92
Varjani Sj, Upasani Vn (2016) Core Flood study for enhanced oil recovery through ex situ bioaugmentation with thermo and halo-tolerant rhamnolipid produced by Pseudomonas aeruginosa NCIM 5514. Bioresour Technol 220:175–182
Zhang L, Pemberton JE, Maier RM (2014) Effect of fatty acid substrate chain length on Pseudomonas aeruginosa ATCC 9027 monorhamnolipid yield and congener distribution. Process Biochem 49:989–995
Zhao F, Jiang H, Sun H, Liu C, Han S, Zhang Y (2019) Production of rhamnolipids with different proportions of mono-rhamnolipids using crude glycerol and a comparison of their application potential for oil recovery from oily sludge. RSC Adv 9(6):2885–2891
Zhao F, Han S, Zhang Y (2020) Comparative studies on the structural composition, surface/interface activity and application potential of rhamnolipids produced by Pseudomonas aeruginosa using hydrophobic or hydrophilic substrates. Bioresour Technol 295:122269
Acknowledgements
The authors are grateful for the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for funding the research grant, according to the 88882.329713/2019-01 process.
Funding
This study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) according to the 88882.329713/2019-01 process.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. LMB conducted the experiments, wrote the manuscript and analyzed the data. AMS-B contributed with new reagents and analytical tools. GSSA wrote the manuscript and analyzed the data. EBT conceived the research. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Letícia Martini Braz, Ana María Salazar-Bryam, Grazielle Santos Silva Andrade, and Elias Basille Tambourgi declares that they have no conflict of interest. All authors have no relevant financial or nonfinancial interests to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Braz, L.M., Salazar-Bryam, A.M., Andrade, G.S.S. et al. Optimization and characterization of rhamnolipids produced by Pseudomonas aeruginosa ATCC 9027 using molasses as a substrate. World J Microbiol Biotechnol 39, 51 (2023). https://doi.org/10.1007/s11274-022-03494-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03494-z