Abstract
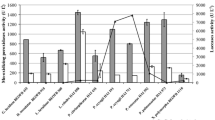
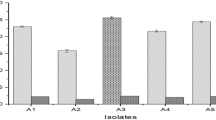
In the present study Lenzites elegans, Schizophyllum commune, Ganoderma applanatum and Pycnoporus sanguineus (wood-degrading fungi) were assayed for their tannase producing potential in culture media containing plant residues or/and tannic acid as carbon source. Aspergillus niger was used as positive control for tannase production. We also carried out the isolation, purification and characterization of the enzyme from the fungi selected as the major productor. The highest fungal growth was observed in A. niger and L. elegans in the media containing tannic acid + glucose + plant residues (Fabiana densa). A. niger and L. elegans reached the highest extracellular tannase production in a medium containing tannic acid + F. densa and in a medium supplemented with glucose + tannic acid + F. densa. The produced enzyme by L. elegans was purified by DEAE-Sepharose. Km value was 5.5 mM and relative molecular mass was about 163,000. Tannase was stable at a pH range 3.0–6.0 and its optimum pH was 5.5. The enzyme showed an optimum temperature of 60°C and was stable between 40 and 60°C. This paper is the first communication of tannase production by wood-degrading fungi. Fermentation technology to produce tannase using plant residues and xylophagous fungi could be very important in order to take advantage of plant industrial waste.






Similar content being viewed by others
References
Aguilar CN, Gutiérrez-Sánchez G (2001) Review: sources, properties, applications and potencial uses of tannin acyl hydrolase. Food Sci Technol Int 7:373–382
Aguilar CN, Rodríguez R, Gutiérrez-Sánchez G, Augur C, Favela-Torres E, Prado-Barragan L, Ramírez-Coronel A, Contreras-Esquivel J (2007) Microbial tannases: advances and perspectives. Appl Microbiol Biotechnol 76:47–59
Andrews P (1964) Estimation of molecular weights of proteins by sephadex gel filtration. Biochem J 91:222–233
Aoki K, Shinke R, Nishira H (1976) Purification and some properties of yeast tannase. Agric Biol Chem 40:79–85
Banerjee D, Mondal KC (2001) Production and characterization of extracellular and intracellular tannase from newly isolated Aspergillus auleatus DBF 9. J Basic Microbiol 41:313–318
Banerjee R, Mukherjee G, Patra KCh (2005) Microbial transformation of tannin-rich substrate to gallic acid through co-culture method. Bioresour Technol 96:949–953
Batra A, Saxena RK (2005) Potential tannase producers from the genera Aspergillus and Penicillium. Process Biochem 40:1553–1557
Belmares R, Reyes-Vega ML, Contreras-Esquivel JC, Rodriguez-Herrera R, Aguilar N (2003) Effect of carbon source on tannase production by two strains of Aspergillus niger. Rev Mex Ing Quím 2:95–100
Belmares R, Contreras-Esquivel JC, Rodríguez-Herrera R, Ramírez Coronel A, Aguilar CN (2004) Microbial production of tannase: an enzyme with potential use in food industry. LWT Food Sci Technol 37:857–864
Bradoo S, Gupta R, Saxena RK (1996) Screening of extracellular tannase producing fungi: development of a rapid and simple plate assay. J Gen Appl Microbiol 42:325–329
Cabrera AL (1978) Flora de la Provincia de Jujuy. Colección Científica del INTA. Buenos Aires, Argentina. Parte X—Compositae
Coggon P, Graham NH, Sanderson GW (1975) UK Patent 1, 380, 135
Curiel JA, Betancor L, de las Rivas B, Muñoz R, Guisan JM, Fernández-Lorente G (2010) Hydrolysis of tannic acid catalyzed by immobilized-stabilized derivatives of Tannase from Lactobacillus plantarum. J Agric Food Chem 58:6403–6409
Deschamps AM (1989) Microbial degradation of tannins and related compounds. In: Lewis NG, Paice MG (eds) Plant cell wall polymers biogenesis and biodegradation. American Chemical Society, Washington, DC, pp 559–566
Dubois M, Gilles KA, Hamilton JK, Ribers P, Smith F (1956) Colorimetric methods for determination of sugar and related substances. Anal Chem 28:350–356
Hadi TA, Banerjee R, Bhattacharya BC (1994) Optimization of tannase biosynthesis by a newly isolated Rizopus oryzae. Bioprocess Eng 11:239–243
Hagerman AE, Rice ME, Ritchard NT (1998) Mechanisms of protein precipitation for two tannins, pentagalloyl glucose and epicatechin16 (4–8) catechin (procyanidin). J Agric Food Chem 46:2590–2595
Kumar R, Sing M (1984) Tannins: their adverse role in ruminant nutrition. J Agric Food Chem 32:447–453
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leal DP, Isla MI, Vattuone MA, Sampietro AR (1994) Production of plant protoplasts by enzymes from selected wood-destroying fungi. Appl Microbiol Biotechnol 41:58–61
Lekha PK, Lonsane BK (1997) Production and application of tannin acyl hydrolase; state of the art. Adv Appl Microbiol 44:215–260
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Lowry OH, Rosembrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Macedo GA, Matsuda LK, Battestin V (2005) Selezâo de fungos productores de tanase em residuos vegetais ricos em taninos. Ciência e Agrotecnologia 29:833–838
Mukharjee G, Banerjee R (2003) Production of gallic acid: biotechnological routes (Part 1). Chim Oggi 21:59–62
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Porter L, Hrstich L, Chan B (1986) The conversion of procyanidins and prodelphinidins to cyanidin and del-phinidin. Phytochem 25:223–230
Pourrat H, Regerat F, Pourrat A, Daniel J (1985) Production of gallic acid from tara by a strain of Aspergillus niger. J Ferment Technol 63:401–403
Rajakumar GS, Nandy SC (1983) Isolation, purification and some properties of Penicillum chrysogenum tannase. Appl Environ Microbiol 46:525–527
Sabu A, Kiran GS, Pandey A (2005) Purification and characterization of tannin acyl hydrolase from Aspergillus niger ATCC 16620. Food Technol Biotech 43:133–138
Salunkhe DK, Chavan JK, Kadam SS (1989) Dietary tannins: consequences and remedies. CRC Press, Boca Raton
Sharma S, Bhat TK, Dawra RK (2000) A spectrophotometric method for assay of tannase using rhodanine. Anal Biochem 279:85–89
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteau reagent. Method Enzymol 299:152–178
Somogyi NA (1945) A new reagent for the determination of sugar. J Biol Chem 160:61–63
Treviño-Cueto B, Luis M, Contreras-Esquivel JC, Rodríguez R, Aguilera A, Aguilar CN (2007) Gallic acid and tannase accumulation during fungal solid state cultura of a tannin-rich desert plant (Larrea tridentata Cov.). Bioresour Technol 98:721–724
Weetall HH (1985) Enzymatic gallic acid esterification. Biotechnol Bioeng 28:124–127
Zampini IC, Cuello S, Alberto MR, Ordoñez RM, D’ Almeida R, Solorzano E, Isla MI (2009) Antimicrobial activity of selected plant species from “the Argentine Puna” against sensitive and multi-resistant bacteria. J Ethnopharmacol 124:499–505
Acknowledgments
The authors acknowledge the cooperation from the inhabitants of the areas of study and the financial support from Consejo de Investigación de la Universidad Nacional de Tucumán (CIUNT), Argentina and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. M. Ordoñez and M. I. Isla have equally contributed.
Rights and permissions
About this article
Cite this article
Ordoñez, R.M., Colombo, I., Alberto, M.R. et al. Production of tannase from wood-degrading fungus using as substrate plant residues: purification and characterization. World J Microbiol Biotechnol 27, 2325–2333 (2011). https://doi.org/10.1007/s11274-011-0699-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0699-1