Abstract
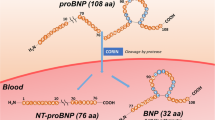
Molecular chaperones are a ubiquitous family of cellular proteins that mediate the correct folding of other target polypeptides. In our previous study, the recombinant anti-BNP scFv, which has promising applications for diagnostic, prognostic, and therapeutic monitoring of heart failure, was expressed in the cytoplasm of Escherichia coli. However, when the anti-BNP scFv was expressed, 73.4% of expressed antibodies formed insoluble inclusion bodies. In this study, molecular chaperones were coexpressed with anti-BNP scFv with the goal of improving the production of functional anti-BNP in the cytoplasm of E. coli. Five sets of molecular chaperones were assessed for their effects on the production of active anti-BNP scFv. These sets included the following: trigger factor (TF); groES/groEL; groES/groEL/TF; dnaK/dnaJ/grpE; groES/groEL/dnaK/dnaJ/grpE. Of these chaperones, the coexpression of anti-BNP scFv with the groES/groEL chaperones encoded in plasmid pGro7 exhibited the most efficient functional expression of anti-BNP scFv as an active form. Coexpressed with the groES/groEL chaperones, 64.9% of the total anti-BNP scFv was produced in soluble form, which is 2.4 times higher scFv than that of anti-BNP scFv expressed without molecular chaperones, and the relative binding activity was 1.5-fold higher. The optimal concentration of l-arabinose required for induction of the groES/groEL chaperone set was determined to be 1.0 mM and relative binding activity was 3.5 times higher compared with that of no induction with l-arabinose. In addition, soluble anti-BNP scFv was increased from 11.5 to 31.4 μg/ml with optimized inducer concentration (1.0 mM l-arabinose) for the coexpression of the groES/groEL chaperones. These results demonstrate that the functional expression of anti-BNP scFv can be improved by coexpression of molecular chaperones, as molecular chaperones can identify and help to refold improperly folded anti-BNP scFv.






Similar content being viewed by others
References
Cardarelli R, Lumicao TG (2003) B-type natriuretic peptide: a review of its diagnostic, prognostic, and therapeutic monitoring value in heart failure for primary care physicians. J Am Board Fam Pract 16:327–333
Choi GH, Lee DH, Min WK, Cho YJ, Kweon DH, Son DH, Park KM, Seo JH (2004) Cloning, expression, and characterization of against mycotoxin deoxynivalenol in recombinant Escherichia coli. Protein Expr Purif 35:84–92
Diamant S, Ben-Zvi AP, Bukau B, Goloubinoff P (2000) Size-dependent disaggregation for stable protein aggregates by the DnaK chaperone machinery. J Biol Chem 275:21107–21113
Donovan RS, Robinson CW, Glick BR (1996) Review: optimizing inducer and culture conditions for expression of foreign proteins under the control of the lac promoter. J Ind Microbiol 16:145–154
Grantham JA, Borgeson DD, Burnett JC (1997) BNP: pathophysiological and potential therapeutic roles in acute congestive heart failure. Am J Physiol Regul Integr Comp Physiol 92:1077–1083
Heo MA, Kim SH, Kim SY, Kim YJ, Chung JH, Oh MK, Lee SG (2006) Functional expression of single-chain variable fragment antibody against c-Met in the cytoplasm of Escherichia coli. Protein Expr Purif 47:203–209
Hu X, O’Hara L, White S, Magner E, Kane M, Wall JG (2007) Optimisation of production of a domoic acid-binding scFv antibody fragment in Escherichia coli. Using molecular chaperones and functional immobilisation on a mesoporous silicate support. Protein Expr Purif 52:194–201
Kim SG, Kweon DH, Lee DH, Park YC, Seo JH (2005) Coexpression of folding accessory proteins for production of active cyclodextrin glycosyltransferase of Bacillus macerans in recombinant Escherichia coli. Protein Expr Purif 41:426–432
Kondo A, Kohda J, Endo Y, Shiromizu T, Kurokawa Y, Nishihara K, Yanagi H, Yura T, Fukuda H (2000) Improvement of productivity of active horseradish peroxidase in Escherichia coli by coexpression of Dsb proteins. J Biosci Bioeng 90:600–606
Lee K, Kim H, Jeong H, Lee Y (2002) Chaperone groELS mediates the protein folding of human liver mitochondrial aldehyde dehydrogenase in Escherichia coli. Biochem Biophys Res Commun 298:216–224
Lee DH, Kim MD, Lee WH, Kweon DH, Seo JH (2004) Consortium of fold-catalyzing proteins increase soluble expression of cyclohexanone monooxygenase in recombinant Escherichia coli. Appl Microbiol Biotechnol 63:549–552
Li T, Cheng J, Hu B, Liu Y, Qian G, Liu F (2008) Construction, production, and characterization of recombinant scFv antibody against methamidophos expressed in Pichia pastoris. World J Microbiol Biotechnol 24:867–874
Longenecker KL, Ruan Q, Fry EH, Saldana SC, Brophy SE, Richardson PL, Tetin SY (2009) Crystal structure and thermodynamic analysis of diagnostic mAb 1063 complexed with BNP 5–13 (C10A). Proteins 76:536–547
Maeng BH, Nam DH, Kim YH (2009) Functional expression of anti-BNP scFv in E. coli cytoplasm for the detection of B-type natriuretic peptide. KSBB J 24:591–597
Martineau P, Jones P, Winter G (1998) Expression of an antibody fragment at high levels in the bacterial cytoplasm. J Mol Biol 208:117–127
Morrison LK, Krishnaswamy P, Nowak RM (2002) Utility of a rapid B-Natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J Am Coll Cardiol 39:202–209
Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, Pfisterer M, Perruchoud AP (2004) Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med 350:647–654
Nishihara K, Kanemori M, Kitakawa M, Yanaki H, Yura T (1998) Chaperone coexpression plasmids: differential and synergenic roles of dnaK-dnaJ-grpE and groEL-groES in assisting folding of an allergen of Japanese cedar pollen, Cryj2, in E. coli. Appl Environ Microbiol 64:1694–1699
Nishihara K, Kanemori M, Kitakawa M, Yanaki H, Yura T (2000) Overexpression of trigger factor prevents aggregation of recombinant proteins in E. coli. Appl Environ Microbiol 66:884–889
Rippmann JF, Klein M, Hoischen C, Brocks B, Rettig WJ, Gumpert J, Pfizenmaier K, Mattes R, Moosmayer D (1998) Prokaryotic expression of single-chain variable-fragment (scFv) antibodies: secretion in L-form cells of Proteus mirabilis leads to active product and overcomes the limitations of periplasmic expression in Escherichia coli. Appl Environ Microbiol 64:4862–4869
Shibui T, Nagahari K (1992) Secretion of a functional Fab fragment in Escherichia coli and the inXuence of culture conditions. Appl Microbiol Biotechnol 37:352–357
Shin EJ, Park SL, Jeon SJ, Lee JW, Kim YT, Kim YH (2006) Effect of molecular chaperones on the soluble expression of alginate lyase in E. coli. Biotechnol Bioprocess Eng 11:414–419
Wallen T, Landahl S, Hedner T, Nako K, Saito Y (1997) Brain natriuretic peptide predicts mortality in the elderly. Heart 77:264–267
Whittington HA, Ashworth LJ, Hawkins RE (1998) Recombinant adenoviral delivery for in vivo expression of scFv antibody fusion proteins. Gene Ther 5:770–777
Zhanh Z, Li ZH, Wang F, Fang M, Yin CC, Zhou ZY, Lin Q, Huang HL (2002) Overexpression of DsbC and DsbG markedly improves soluble and functional expression of single-chain Fv antibodies in Escherichia coli. Protein Expr Purif 26:218–228
Acknowledgments
This research was financially supported by the Ministry of Knowledge Economy(MKE) and Korea Institute for Advancement of Technology(KIAT) through the Research and Development for Regional Industry. We are grateful for the financial support of i-SENS and the Ministry of Knowledge Economy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeng, B.H., Nam, D.H. & Kim, Y.H. Coexpression of molecular chaperones to enhance functional expression of anti-BNP scFv in the cytoplasm of Escherichia coli for the detection of B-type natriuretic peptide. World J Microbiol Biotechnol 27, 1391–1398 (2011). https://doi.org/10.1007/s11274-010-0590-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0590-5