Abstract
The textile industry uses high amounts of water and dyestuffs, and accordingly, wastewater containing significant amounts of dyestuffs is produced, which have to be treated before being discharged to the environment. The aim of this study is the treatment of synthetic wastewater containing 50 mg/L Acid Violet 90 dyestuff with catalytic ozonation process have been investigated by using n.CeO2, n.ZnO and n.CeO2/n.ZnO (1:1, m:m) nanocatalysts. The process optimization has been performed with the pH, ozone dose, catalyst dose and reaction time parameters. While the optimum conditions have been determined as pH 3, 600 mg/L ozone dose, 10 min reaction time, for all three catalysts, catalyst doses have differed as 50 mg/L for n. CeO2, 30 mg/L for n.ZnO and n.CeO2/n.ZnO. The removal efficiencies of AV 90 have been achieved as 97.04, 96.2 and 95.7% for n.CeO2, n.ZnO and n.CeO2/n.ZnO catalysts, respectively. Kinetic analyses showed that color removal of AV 90 dyestuff followed First order kinetics with R2 values of 0.9987 (n.CeO2), 0.9912 (n.ZnO) and 0.9923 (n.CeO2/n.ZnO) and reaction rate constant values have been calculated as 0,3435 (n.CeO2), 0,3551(n.ZnO) and 0,3226 (n.CeO2/n.ZnO) min−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Discharge of dyestuffs used in industries such as textile, paper, rubber, food and plastics into wastewater causes serious problems for receiving environments (Cheng et al., 2022). Generally, the amount discharged to wastewater from each step of the textile dyeing process is around 40–65 L/kg (Akti & Okur, 2018). The dyestuffs mixed with the water affect the photosynthetic activity by causing a decrease in light transmission due to the color change of the water (Qiu et al., 2017). In addition, dyestuffs cause an important environmental problem for human health and aquatic life even at very low concentrations, as they change the pH value and increase the chemical oxygen demand (COD) of the receiving water. Wastewater containing dyestuffs can be treated by physical, chemical and biological treatment processes in general (Holkar et al., 2016; Vakili et al., 2014).
Removal of azo dyes from wastewater is one of the most important environmental problems, as it is difficult to treat with conventional treatment systems. Therefore treatment systems such as coagulation (Yung-Tse et al., 2020), oxidation (Hassaan et al., 2017; Liu et al., 2020), filtration (Wen et al., 2022), adsorption (Islam & Mostafa, 2022; Sultana et al., 2022) and photo-degradation techniques (Chairungsri et al., 2022; Yang et al., 2022) has been researched for the removal of azo-dyes, in the litterature. Acid Violet 90 is a prototypical aromatic azo compound and generally stable crystalline species (Chiang & Wu, 2010). Aromatic azo compounds can be identified by the chromophore group (-N = N-) found in their structure. One of the carbon atoms attached to the azo group can be bounded with the aliphatic chain and the other can be an aromatic or heterocyclic ring. Aliphatic azo compounds have lower color intensity. Therefore, in most azo dyes, at least one of the nitrogen-bound groups is aromatic (Şarkaya, 2011).
One of the effective treatment methods used for dyestuff removal is ozonation (Fatimah & Wiharto, 2018; Turhan, 2021). As dyestuffs are transformed into biodegradable species as a result of ozonation, it can also assist biological treatment. However, despite providing effective treatment, ozone is not widely preferred because it is an expensive treatment system (Chaturvedi et al., 2021).
Catalytic ozonation systems (COPs), especially using heterogeneous catalysts, are more cost-effective wastewater treatment methods than ozonation. Since the catalyst is in a solid state in heterogeneous catalytic ozonation systems, the adsorption ability of the catalyst gains importance in wastewater treatment. Materials such as metal oxides and minerals has been used regularly as heterogeneous catalysts (Tanatti, 2021). There are 3 different scenarios for catalytic action; ozone is adsorbed on the catalyst surface, the pollutant molecule is adsorbed on the catalyst surface, or both ozone and the pollutant molecule are adsorbed on the catalyst surface (Kasprzyk-Hordern et al., 2003). It is also unfortunate that information from conventional catalytic reactions does not apply to catalytic ozonation, due to the unique self-degradation of ozone in aqueous solutions (Yong et al., 2005).
It has been determined in many studies that various metal oxides provide high efficiency in catalytic ozonation systems. The most common catalyst metal oxides in the literature are generally magnesium oxides (Mohammadi et al., 2017), zinc oxides (Yuan et al., 2017), ferric oxides (Wang et al., 2016), titanium oxides (Gupta et al., 2018), manganese oxides (Dong et al., 2009), nickel oxides (X. Zhang et al., 2009), and cerium oxides (Afzal et al., 2019).
ZnO is widely used in catalytic ozonation systems with its stable structures, environmentally friendly features, low toxicity, and catalytic properties. Zn, as a ZnO molecule, usually has only one stable valence, which allows it to remain in a stable structural state in catalytic ozonation systems (Jin et al., 2023). Composites of n.ZnO nano particles with other inorganic compounds provided effective catalytic ozone treatments (Kaur et al., 2016). It is seen in the literature that various composites such as TiO2, MgO, CuO, Bi2O3, FeO3 increase the efficiency of catalytic ozonation using Zn (Balachandran & Swaminathan, 2012; Lakshmi et al., 2012; Liu et al., 2012; Saravanan et al., 2013; William et al. 2008). CeO2 is a unique substance due to its redox properties and crystal structure. Because of the oxygen storage capacity and fast ion conductivity of CeO2, it can also be used in solid oxide fuel cells, apart from being effective as a catalyst (Liu et al., 2012; Sun et al., 2013). However, there are not many studies on the combination of ZnO and CeO2 composites. Since CeO2 alone has high catalytic activity, it is predicted that the CeO2/ZnO combination will provide an effective treatment (Ji et al., 2009; Kaur et al., 2016; Qin et al., 2016).
In this study, catalytic ozonation process of synthetic wastewater sample containing 50 mg/L AV 90 dyestuff has been investigated by using n.CeO2 and n.ZnO catalysts. Three different catalyst systems were optimized, with only n.CeO2, only n.ZnO and a 1:1 mixture of n.CeO2/n.ZnO catalysts. In order to optimize the processes, the effects of pH, ozone dose, catalyst dose and reaction time parameters on AV 90 removal were determined.
2 Materials and Methods
2.1 Materials
Acid Violet 90 (AV 90) dyestuff was provided by a textile factory in Sakarya. Hydrochloric acid (HCl) and sodium hydroxide (NaOH) (MERCK brand) were used to adjust the pH of the solutions, and pH measurements were made with a pH meter (HANNA brand HI-2211 model). Magnetic stirrer (MTOP brand MS 300 model) and ozone generator (SABO Electronics brand SL-10 model) were used in the reactor as devices. n.CeO2 and n.ZnO smaller than 50 nm in diameter were used as catalysts (SIGMA ALDRICH brand).
2.2 Dyestuff Preparation and Color Measurement Method
Synthetic wastewater with 50 mg/L AV90 dye concentration has been used for all experiments. λmax value of the AV90 dye has been identified by spectrum scanning to measure the concentrations of the AV 90 solution. 525 nm has been determined as the λmax value of AV 90 as a result of spectrum scanning. The calibration curve has been obtained with the solutions prepared between 0.5 mg/L and 50 mg/L at 525 nm. The regression coefficient (R2) of the calibration curve obtained has been calculated as 0.9999. The linear equation obtained from the calibration curve is given in Eq. (1).
To eliminate the adsorption effect of nanoparticles, adsorption percentages have been subtracted from all results and color removal efficiencies were given.
2.3 Experimental and Reactor Design
In this study, pH, catalyst dose, ozone dose, and reaction time parameters were examined to determine the effectiveness of n.CeO2 and n.ZnO nanoparticles in the catalytic ozonation process. The experiments were conducted with pH values ranging from 3 to 11, catalyst doses from 20 to 60 mg/L, ozone doses from 300 to 1500 mg/L, and reaction times from 1 to 12.5 min. Considering the adsorption properties of the nanoparticles, any color removals due to adsorption were identified and subtracted from the experimental results.
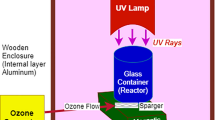
The reactor design of the COP processes used in color removal experimental has shown in Fig. 1. 300 ml glass reactor with a MTOP brand MS 300 model magnetic stirrer and SABO Electronics brand SL-10 model ozone generator (max 0.3 g/h capacity) has been combined for COP reactor. The experimental volume of wastewater containing AV 90 was 100 ml. Ozone was applied to the reactor containing 100 mL of wastewater at regular intervals. In COPs, the ozone dose has been calculated by multiplying the applied ozone time by the generator capacity and then dividing to the total solution volume (Tanatti, 2021). The residual ozone was eliminated with 5 min of aeration of the sample.
2.4 Nanoparticles Characterization
n.CeO2 and n.ZnO nanoparticles used as catalysts were characterized with the electronic scanning microscopy (SEM- FEI brand Quanta FEG 250 model), Surface area and porosity analysis (BET- FEI brand Quanta FEG 250 model), and zeta potential (Malvern brand-ZS90 model Zetasizer). In Fig. 2, the scanning electronic microscopy (SEM) images of CeO2 and ZnO has been shown. The particle sizes of n.CeO2 and n.ZnO nanoparticles are measured as 50 nm and show uniform distribution, as seen in Fig. 2.
The BET and adsorption surface areas of nanocatalysts are indicated in the Table 1. The BET results demonstrated that the surface areas and adsorption surface areas of n.CeO2 and n.ZnO nanoparticles are similar.
The zeta potential of n.CeO2 and n.ZnO nanoparticles can be seen in the Fig. 3. With the pH increase, the zeta potential values of both nanoparticles have been reduced.
2.5 Kinetic Analysis
The reaction rate constant (k) and regression coefficient (R2) values are calculated with the kinetic models depending on the reaction time. First order, second order, and pseudo second-order kinetic models were examined in the kinetic calculations of this study.
The kinetic equations are as follows (Zhang et al., 2017):
In these equations, the Co value is the initial concentration of the dyestuff, the C value is the dyestuff concentration at the t time, the Cmax value is the maximum percent removal efficiency, and the k value is the reaction rate constant.
3 Results and Discussion
3.1 Effect of pH on Dyestuff Removal
The solution pH is one of the major parameters in catalytic ozonation processes, which directly affects the formation of radicals, degradable pollutants charge states and the surface properties of the catalyst (Bai et al., 2016; Sajjad et al., 2021).
In this study, the initial concentration of 50 mg/L Acid Violet 90 (AV 90) dyestuff removal has been studied by catalytic ozonation method with different nanocatalysts (n.CeO2, n.ZnO, and n.CeO2/n.ZnO) in synthetic wastewater. For the examination of pH on AV 90 color removal for each nanocatalyst, experiments have been conducted at pH 3, 5, 7, 9, and 11 and shown in Fig. 4. For the determination of pH effect on color removal, the O3 dose was 600 mg/L, the nanocatalyst dose was 50 mg/L and the time was kept constant as 10 min.
Ozone has a higher oxidation capacity for anionic surfaces than cationic surfaces because of ozone's electrophilic properties. In the catalytic ozonation processes, the catalytic reactions between ozone and catalyst are affected by pH, which can be explained with electrostatic interactions (Jung & Choi, 2006). Surface hydroxyl groups control the chemistry of catalyst surface.
Maximum removal efficiencies for AV 90 dyestuff with n.CeO2, n.ZnO and n.CeO2/n.ZnO catalysts have been obtained as 97.04%, 95.95% and 94.38%, respectively at pH 3. With the pH increase, the color removal efficiency of synthetic wastewater decreases. The decrease in color removal efficiencies with pH increase has been around 1%, furthermore higher removal efficiency reductions (8–13%) have been occurred at different pHs depending on the catalyst type. These decreases have been observed after pH 9 for n.CeO2, pH 7 for n.ZnO and pH 3 for n.CeO2/n.ZnO mixture. The sudden decreases mentioned here has been observed as 88.15% color removal at pH 7 in the process with n.ZnO nanocatalyst, 78.90% at pH 11 in CeO2 nanocatalyst, and 88.57% at pH 5 in n.CeO2/n.ZnO nanocatalyst. The results indicate that the n. CeO2 catalyzed ozonation process for AV 90 removal achieved high removal efficiency at acidic and neutral pHs. On the other hand, it has been determined that the ozonation process with n.ZnO and n.CeO2/n.ZnO catalysis is more effective at acidic pH. AV 90 removal efficiencies for n.CeO2, n.ZnO and n.CeO2/n.ZnO catalysts in the catalyzed ozone process have been decreased as the pH increases.
Since the point of zero charges (PZC) is around 9–9,3 (Bahnemann et al., 1987; Jung & Choi, 2006) for n.ZnO nanoparticles and 6.2–6,7 (Aponte et al., 2020; Wang et al., 2019; Zheng et al., 2018) for CeO2 nanoparticles, below the pHpzc the surface of ZnO and CeO2 metal oxide catalysts are charged positively (Kasprzyk-Hordern et al., 2003; Zhai et al., 2010). Due to the catalysed oxidation principles, AV 90, which is an anionic dyestuff (Chattopadhyay, 2011), is absorbed on the catalysed surface and then oxidated by ozone (Khataee et al., 2016). Ozone has a higher radical production capacity on a negatively charged surface than positively or uncharged surfaces because of electrophilic properties (Zhai et al., 2010). As the pH increases (pH > pHpzc), the reactivity of ozone increases, so the removal of AV 90 is still higher, but not higher than at pH 3 (Zhai et al., 2010).
3.2 Effect of catalyst dose on dyestuff removal
In catalytic ozonation processes, the degradation rates of the organic pollutants increase and their conversion to inorganic materials provide compared to ozonation processes (Hu et al., 2016). In COPs the catalysts supply active sites for the oxidation reactions between water, ozone and organic matters, so removal of organic matters advances with the increase in the amount of catalysts generally (Tanatti, 2021).
In this study, the removal of AV 90 dyestuff from wastewater has been investigated with the catalytic ozone processes, by using n.CeO2, n.ZnO and n.CeO2/n.ZnO nanocatalysts separately.
The heterogonous n.CeO2/n.ZnO catalyst mix has been prepared gravimetrically using equal amounts of n.CeO2 and n.ZnO. In order to investigate the effect of catalyst dose on AV 90 removal, nanocatalyst dose has been studied at five different doses as 20, 30, 40, 50, and 60 mg/L. For all nanocatalysts, the effect of catalyst dose has been researched on the removal efficiency of AV 90 dyestuff at pH 3, 600 mg/L ozone dose and 10 min reaction time. The results of the studies indicate that over 93% removal efficiencies of AV 90 dyestuff have been achieved separately for n.CeO2, n.ZnO and n.CeO2/n.ZnO at all catalyst doses as seen in Fig. 5.
Due to the color removal efficiency has been obtained as 97.041%, 50 mg/L n.CeO2 catalyst dose has been chosen as the optimum dose.
Also, with the 30 mg/L catalyst dose for n.ZnO and n.CeO2/n.ZnO AV 90 removal efficiencies have been obtained as 96.20 and 95.70%, respectively. At all catalysts doses, the AV 90 removal efficiencies fluctuate around ± 1% in this study.
Similarly, Hu et al. (2016) investigated the removal of dye-containing wastewater by catalytic ozonation and found that a high amount of catalysts doses are not required for color removal (Hu et al., 2016). With the increase of the catalyst dose, the catalyst residues produce on the catalyst active region, which makes the adsorption of the organic substances difficult on the catalyst surface (Nakhate et al., 2019).
3.3 Effect of O3 Dose on Dyestuff Removal
In the catalytic ozone processes, the ozone dose is a key parameter. With the increase of ozone dose, the decomposition of ozone enhances and the formation of OH· increases which cause the rise of pollutants degradation (Bai et al., 2016).
In order to investigate the effect of ozone dose on the removal of AV 90 dyestuff, 300, 600, 900, 1200, and 1500 mg/L ozone doses were applied for all three COPs. The effect of ozone dose on the removal of AV 90 dyestuff at pH 3, catalyst concentrations of 50 mg/L for n.CeO2, 30 mg/L for n.ZnO and n.CeO2/n.ZnO (15mg/L for each) have been investigated for 10 min. The AV 90 removal efficiencies obtained depending on the ozone dose are given in Fig. 6. In all processes, AV90 dyestuff removal efficiencies have been obtained between 65 and 76% at 300 mg/L ozone dose. At 600 mg/L ozone dose, n.CeO2, n.ZnO and n.CeO2/n.ZnO removal efficiencies have been found as 97.04%, 96.20 and 95.70%, respectively. Above 600 mg/L ozone dose, the dye removal efficiencies have a tendency to increase slowly. For this reason, the optimum ozone dose has been chosen as 600 mg/L.
In COPS, with the increase of ozone dose, the reactions based on radicals and pollutants accelerate on the catalyst surface, and pollutant removal efficiencies rise up, which can also be seen in some studies (Bai et al., 2016; Boysan, 2021; Wang et al., 2019; Zhai et al., 2010).
3.4 Effect of Reaction Time on Dyestuff Removal
The reaction time is one of the important parameters effecting the treatment efficiency in the catalytic ozone process. In order to observe the effect of reaction time for AV90 removal for 1, 3, 5, 7.5, 10, and 12.5 min have been studied with the COPs. The effect of the reaction time on AV 90 removal has been investigated at pH 3, 600 mg/L O3 doses, 50 mg/L catalyst dose for n.CeO2, 30 mg/L for n.ZnO and n.CeO2/n.ZnO. The results has shown in Fig. 7.
The AV 90 removal efficiencies, which were around 20% at the first minute, tended to increase rapidly for all three COPs up to the 10th minute. The AV 90 removal efficiencies have been found for n.CeO2, n.ZnO and n.CeO2/n.ZnO as 97.04, 96.20, and 95.70%, respectively, at the 10th minute. Furthermore, in 12.5 min, AV 90 removal has been tended to be fixed and removal efficiencies of around 98% have been obtained for all catalysts. For this reason, the optimum reaction time has been decided as 10 min.
AV 90 removal with catalytic ozonation process studies has not existed in the literature. However, in the adsorption study carried out in water containing 50 mg/L AV 90 dyestuff using PANI and PANI/Clinoptilolite adsorbent, 97.8% and 78.6% color removal was obtained in a long period of 340 min (Akti & Okur, 2018).
There are some studies for different acid violet dyes in the literature, especially AV 7 studies. In the electrooxidation study of acid violet 7 with BDD anode, color removal occurs in less than 80 min at current densities above 30 mA/cm2 (Brito et al., 2018). In the study about the degradation of acid violet 7 with the 5 ppm concentration in visible light with ZnO/PPy photocatalysis. Color removal has been reported after 120 min (González-Casamachin et al., 2019). Again, in a study with acid violet 7, the adsorption process was carried out using MnO2, and maximum color removal was achieved in 20 mg adsorbent amount at pH 4 and in 20 min adsorption time (Jamshidi & Shemirani, 2020). The other research has investigated the treatability of Acid violet 7 with 20 ppm initial concentration with hydrogen peroxide, Fenton and persulfate processes with the combination of ultrasound and the removals are given as the COD parameter. COD removal efficiencies have been determined around 23.1% in 120 min in the US/H2O2 process, around 80.9% in the US/Fenton process in 60 min, and around 81% in the US/persulfate process in 60 min (Kodavatiganti et al., 2021).
3.5 Kinetic Analysis of AV 90 Removal Efficiencies
First order, second order, and pseudo second-order kinetic models were examined in the kinetic calculations of this study, in which the investigation of the removal of AV 90 dyestuff by the catalytic ozone process using n.CeO2, n.ZnO, and n.CeO2/n.ZnO nanocatalysts. The k and R2 values were calculated for all three kinetic models.
The k and R2 values obtained from the kinetic calculations are given in Table 2 and the most appropriate kinetic model for all three nanocatalysts has been found as the first order. R2 values for n.CeO2, n.ZnO and n.CeO2/ZnO catalysts have been calculated as 0.9987, 0.9912, 0.9923, and k values calculated as 0.3435, 0.3551, 0.3226 1/min, respectively.
The obtained reaction rate constants have been used to determine the theoretical results depending on the first order kinetic model. According to the calculations, theoretical results are similar to the experimental results as shown in Fig. 8.
4 Conclusions
The treatment of AV 90 containing synthetic wastewater have been investigated in this study by COP systems with three different n.CeO2, n.ZnO and 1:1 mixture of n.CeO2/n.ZnO nanocatalysts. pH, catalysts dose, ozone dose and reaction time has been investigated as operational parameters to obtain the effects on AV 90 removal efficiency. Optimum parameters have been determined separately for each catalyst type. pH 3, catalyst dose 30 mg/L, ozone dose 600 mg/L and reaction time 10 min have been obtained for n.ZnO and n.CeO2/n.ZnO Besides, For n.CeO2, pH 3, catalyst dose 50 mg/L, ozone dose 600 mg/L and reaction time 10 min were achieved for AV 90 removal.
Under optimum conditions, color removal efficiencies have been obtained as 96.2%, 97.04%, and 95.7% at the n.ZnO, n.CeO2, and n.CeO2/n.ZnO respectively. AV 90 removal efficiencies have been investigated under the same conditions in all three processes. As a result of the studies, high colour removal efficiencies were achieved in all three processes used for AV 90 removal. However, it was observed that the best results among the processes were achieved with n.CeO2. However, studies have shown that all these processes show an effective performance for AV 90 removal.
The results revealed that the first order kinetic model was in concordance with the experimental results for all processes.
Data Availability
All the results is original, availability of data and materials has not been used in this manuscript.
References
Afzal, S., Quan, X., & Lu, S. (2019). Catalytic performance and an insight into the mechanism of CeO2 nanocrystals with different exposed facets in catalytic ozonation of p-nitrophenol. Applied Catalysis b: Environmental, 248(January), 526–537. https://doi.org/10.1016/j.apcatb.2019.02.010
Akti, F., & Okur, M. (2018). The Removal of Acid Violet 90 from Aqueous Solutions Using PANI and PANI/Clinoptilolite Composites: Isotherm and Kinetics. Journal of Polymers and the Environment, 26(11), 4233–4242. https://doi.org/10.1007/s10924-018-1297-1
Aponte, Á. G., RamÍrez, M. A. L., Mora, Y. C., Santa MarÍn, J. F., & Sierra, R. B. (2020). Cerium oxide nanoparticles for color removal of indigo carmine and methylene blue solutions. AIMS Materials Science, 7(4), 468–485. https://doi.org/10.3934/matersci.2020.4.468
Bahnemann, D. W., Kormann, C., & Hoffmann, M. R. (1987). Preparation and characterization of quantum size zinc oxide: A detailed spectroscopic study. Journal of Physical Chemistry, 91(14), 3789–3798. https://doi.org/10.1021/j100298a015
Bai, Z., Yang, Q., & Wang, J. (2016). Catalytic ozonation of sulfamethazine using Ce0.1Fe0.9OOH as catalyst: Mineralization and catalytic mechanisms. Chemical Engineering Journal, 300, 169–176. https://doi.org/10.1016/j.cej.2016.04.129
Balachandran, S., & Swaminathan, M. (2012). Facile fabrication of heterostructured Bi2O3–ZnO photocatalyst and its enhanced photocatalytic activity. The Journal of Physical Chemistry C, 116(50), 26306–26312.
Boysan, F. (2021). Removal of Acid Violet 90 Dyestuffs in Aqueous Solutions by Ozonation Method. Sakarya University Journal of Science, 25(1), 200–211.
Brito, C. N., Ferreira, M. B., de Moura Santos, E. C. M., Léon, J. J. L., Ganiyu, S. O., & Martínez-Huitle, C. A. (2018). Electrochemical degradation of Azo-dye Acid Violet 7 using BDD anode: Effect of flow reactor configuration on cell hydrodynamics and dye removal efficiency. Journal of Applied Electrochemistry, 48(12), 1321–1330. https://doi.org/10.1007/s10800-018-1257-4
Chairungsri, W., Subkomkaew, A., Kijjanapanich, P., & Chimupala, Y. (2022). Direct dye wastewater photocatalysis using immobilized titanium dioxide on fixed substrate. Chemosphere, 286(P2), 131762. https://doi.org/10.1016/j.chemosphere.2021.131762
Chattopadhyay, D. P. (2011). Chemistry of dyeing. Handbook of Textile and Industrial Dyeing: Principles, Processes and Types of Dyes (Vol. 1 pp. 150–183). Woodhead Publishing Limited. https://doi.org/10.1533/9780857093974.1.150
Chaturvedi, A., Rai, B. N., Singh, R. S., & Jaiswal, R. P. (2021). A comprehensive review on the integration of advanced oxidation processes with biodegradation for the treatment of textile wastewater containing azo dyes. Reviews in Chemical Engineering. https://doi.org/10.1515/revce-2020-0010
Cheng, Y., Cao, T., Xiao, Z., Zhu, H., & Yu, M. (2022). Photocatalytic Treatment of Methyl Orange Dye Wastewater by Porous Floating Ceramsite Loaded with Cuprous Oxide. Coatings, 12(2), 286. https://doi.org/10.3390/coatings12020286
Chiang, M.-F., & Wu, T.-M. (2010). Synthesis and characterization of biodegradable poly (L-lactide)/layered double hydroxide nanocomposites. Composites Science and Technology, 70(1), 110–115.
Dong, Y., Yang, H., He, K., Song, S., & Zhang, A. (2009). β-MnO2 nanowires: A novel ozonation catalyst for water treatment. Applied Catalysis B: Environmental, 85(3–4), 155–161. https://doi.org/10.1016/j.apcatb.2008.07.007
Fatimah, S., & Wiharto, W. (2018). Multiple linear regression for the analysis of the parameters used in dyes decolourisation by ozonation techniques. MATEC Web of Conferences, 154, 2–6. https://doi.org/10.1051/matecconf/201815401003
González-Casamachin, D. A., Rivera De la Rosa, J., Lucio-Ortiz, C. J., De Haro De Rio, D. A., Martínez-Vargas, D. X., Flores-Escamilla, G. A., et al. (2019). Visible-light photocatalytic degradation of acid violet 7 dye in a continuous annular reactor using ZnO/PPy photocatalyst: Synthesis, characterization, mass transfer effect evaluation and kinetic analysis. Chemical Engineering Journal, 373(March), 325–337. https://doi.org/10.1016/j.cej.2019.05.032
Gupta, V. K., Fakhri, A., Agarwal, S., Bharti, A. K., Naji, M., & Tkachev, A. G. (2018). Preparation and characterization of TiO2 nanofibers by hydrothermal method for removal of Benzodiazepines (Diazepam) from liquids as catalytic ozonation and adsorption processes. Journal of Molecular Liquids, 249, 1033–1038. https://doi.org/10.1016/j.molliq.2017.11.144
Hassaan, M. A., El Nemr, A., & Madkour, F. F. (2017). Advanced oxidation processes of Mordant Violet 40 dye in freshwater and seawater. The Egyptian Journal of Aquatic Research, 43(1), 1–9. https://doi.org/10.1016/J.EJAR.2016.09.004
Holkar, C. R., Jadhav, A. J., Pinjari, D. V., Mahamuni, N. M., & Pandit, A. B. (2016). A critical review on textile wastewater treatments: Possible approaches. Journal of Environmental Management, 182, 351–366. https://doi.org/10.1016/j.jenvman.2016.07.090
Hu, E., Wu, X., Shang, S., Tao, X. M., Jiang, S. X., & Gan, L. (2016). Catalytic ozonation of simulated textile dyeing wastewater using mesoporous carbon aerogel supported copper oxide catalyst. Journal of Cleaner Production, 112, 4710–4718. https://doi.org/10.1016/j.jclepro.2015.06.127
Islam, M. R., & Mostafa, M. G. (2022). Adsorption kinetics, isotherms and thermodynamic studies of methyl blue in textile dye effluent on natural clay adsorbent. Sustainable Water Resources Management, 8(2), 1–12. https://doi.org/10.1007/s40899-022-00640-1
Jamshidi, P., & Shemirani, F. (2020). Adsorption/desorption of acid violet-7 onto magnetic MnO2 prior to its quantification by UV–visible spectroscopy: Optimized by fractional factorial design. Research on Chemical Intermediates, 46(10), 4403–4422. https://doi.org/10.1007/s11164-020-04211-x
Ji, P., Zhang, J., Chen, F., & Anpo, M. (2009). Study of adsorption and degradation of acid orange 7 on the surface of CeO2 under visible light irradiation. Applied Catalysis B: Environmental, 85(3–4), 148–154.
Jin, X., Wu, C., Fu, L., Tian, X., Wang, P., Zhou, Y., & Zuo, J. (2023). Development, dilemma and potential strategies for the application of nanocatalysts in wastewater catalytic ozonation: A review. Journal of Environmental Sciences, 124, 330–349. https://doi.org/10.1016/j.jes.2021.09.041
Jung, H., & Choi, H. (2006). Catalytic decomposition of ozone and para-Chlorobenzoic acid (pCBA) in the presence of nanosized ZnO. Applied Catalysis B: Environmental, 66(3–4), 288–294. https://doi.org/10.1016/j.apcatb.2006.03.009
Kasprzyk-Hordern, B., Ziółek, M., & Nawrocki, J. (2003). Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Applied Catalysis B: Environmental, 46(4), 639–669. https://doi.org/10.1016/S0926-3373(03)00326-6
Kaur, J., Bhukal, S., Gupta, K., Tripathy, M., Bansal, S., & Singhal, S. (2016). Nanocomposite of CeO2 and ZnO: An active material for the treatment of contaminated water. Materials Chemistry and Physics, 177, 512–520.
Khataee, A., Kiranşan, M., Karaca, S., & Arefi-Oskoui, S. (2016). Preparation and characterization of ZnO/MMT nanocomposite for photocatalytic ozonation of a disperse dye. Turkish Journal of Chemistry, 40(4), 546–564. https://doi.org/10.3906/kim-1507-77
Kodavatiganti, S., Bhat, A. P., & Gogate, P. R. (2021). Intensified degradation of Acid Violet 7 dye using ultrasound combined with hydrogen peroxide, Fenton, and persulfate. Separation and Purification Technology, 279(September), 119673. https://doi.org/10.1016/j.seppur.2021.119673
Lakshmi, G. C., Ananda, S., Somashekar, R., & Ranganathaiah, C. (2012). Synthesis of ZnO/MgO nanocomposites by electrochemical method for photocatalytic degradation kinetics of eosin yellow dye. International Journal of Nano-Science and Nanotechnology, 3, 47–63.
Liu, Y., Yu, L., Hu, Y., Guo, C., Zhang, F., & Lou, X. W. D. (2012). A magnetically separable photocatalyst based on nest-like γ-Fe 2 O 3/ZnO double-shelled hollow structures with enhanced photocatalytic activity. Nanoscale, 4(1), 183–187.
Liu, J., Li, J., He, S., Sun, L., Yuan, X., & Xia, D. (2020). Heterogeneous catalytic ozonation of oxalic acid with an effective catalyst based on copper oxide modified g-C3N4. Separation and Purification Technology, 234(September 2019), 116120. https://doi.org/10.1016/j.seppur.2019.116120
Mohammadi, L., Bazrafshan, E., Ansari-Moghaddam, A., Balarak, D., Noroozifar, M., & Barahuie, F. (2017). Removing 2,4-dichlorophenol from aqueous environments by heterogeneous catalytic ozonation using synthesized MgO nanoparticles. Water Science and Technology, 76(11), 3054–3068. https://doi.org/10.2166/wst.2017.479
Nakhate, P. H., Gadipelly, C. R., Joshi, N. T., & Marathe, K. V. (2019). Engineering aspects of catalytic ozonation for purification of real textile industry wastewater at the pilot scale. Journal of Industrial and Engineering Chemistry, 69, 77–89. https://doi.org/10.1016/j.jiec.2018.09.010
Qin, L., Wang, Z., Sun, Y., Shu, Q., Feng, P., Zhu, L., et al. (2016). Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environmental Science and Pollution Research, 23(9), 8379–8387. https://doi.org/10.1007/s11356-015-6004-3
Qiu, J., Feng, Y., Zhang, X., Jia, M., & Yao, J. (2017). Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. Journal of Colloid and Interface Science, 499, 151–158. https://doi.org/10.1016/j.jcis.2017.03.101
Sajjad, S., Ikhlaq, A., Javed, F., Ahmad, S. W., & Qi, F. (2021). A study on the influence of pH changes during catalytic ozonation process on alumina, zeolites and activated carbons for the decolorization of reactive red-241. Water Science and Technology, 83(3), 727–738. https://doi.org/10.2166/wst.2020.609
Saravanan, R., Karthikeyan, S., Gupta, V. K., Sekaran, G., Narayanan, V., & Stephen, A. (2013). Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Materials Science and Engineering: C, 33(1), 91–98.
Şarkaya, K. (2011). Rezorsinaren bazli azo boyarmaddeleri̇n sentezi̇ ve absorpsi̇yon özelli̇kleri̇ni̇n i̇ncelenmesi̇. Pamukkale University.
Sultana, S., Islam, K., Hasan, M. A., Khan, H. M. J., Khan, M. A. R., Deb, A., et al. (2022). Adsorption of crystal violet dye by coconut husk powder: Isotherm, kinetics and thermodynamics perspectives. Environmental Nanotechnology, Monitoring and Management, 17(May 2021), 100651. https://doi.org/10.1016/j.enmm.2022.100651
Sun, D., Gu, M., Li, R., Yin, S., Song, X., Zhao, B., et al. (2013). Effects of nitrogen content in monocrystalline nano-CeO2 on the degradation of dye in indoor lighting. Applied Surface Science, 280, 693–697.
Tanatti, N. P. (2021). Treatability and Kinetic Analysis of BPA-containing Wastewater by Catalytic Ozone Processes Using ZnSO4 and ZnO Catalysts. Ozone: Science and Engineering, 44(2), 227–233. https://doi.org/10.1080/01919512.2021.1951160
Turhan, K. (2021). Determination of optimal conditions in decolorization of disperse dyes in aqueous solution by ozonation. Global Nest Journal, 23(1), 143–151. https://doi.org/10.30955/gnj.003096
Vakili, M., Rafatullah, M., Salamatinia, B., Abdullah, A. Z., Ibrahim, M. H., Tan, K. B., et al. (2014). Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydrate Polymers, 113, 115–130.
Wang, Y., Yang, W., Yin, X., & Liu, Y. (2016). The role of Mn-doping for catalytic ozonation of phenol using Mn/γ-Al2O3 nanocatalyst: Performance and mechanism. Journal of Environmental Chemical Engineering, 4(3), 3415–3425. https://doi.org/10.1016/j.jece.2016.07.016
Wang, J., Quan, X., Chen, S., Yu, H., & Liu, G. (2019). Enhanced catalytic ozonation by highly dispersed CeO2 on carbon nanotubes for mineralization of organic pollutants. Journal of Hazardous Materials, 368(January), 621–629. https://doi.org/10.1016/j.jhazmat.2019.01.095
Wen, H., Huang, W., & Liu, C. (2022). Double-barrier forward osmosis membrane for rejection and destruction of bacteria and removal of dyes. Desalination, 529(November 2021), 115609. https://doi.org/10.1016/j.desal.2022.115609
William, L., IV., Kostedt, I. V., Ismail, A. A., & Mazyck, D. W. (2008). Impact of heat treatment and composition of ZnO− TiO2 nanoparticles for photocatalytic oxidation of an azo dye. Industrial & Engineering Chemistry Research, 47(5), 1483–1487.
Yang, C., Xue, Z., Yin, H., Lu, K., & Liu, W. (2022). Aqueous foam loaded TiO2 nano-catalysts for promoting photodegradation of methylene blue. Journal of Nanoparticle Research, 24(3), 1–22. https://doi.org/10.1007/s11051-022-05441-3
Yong, K., Wu, J., & Andrews, S. (2005). Heterogeneous catalytic ozonation of aqueous reactive dye. Ozone: Science and Engineering, 27(4), 257–263.
Yuan, X., Yan, X., Xu, H., Li, D., Sun, L., Cao, G., & Xia, D. (2017). Enhanced ozonation degradation of atrazine in the presence of nano-ZnO: Performance, kinetics and effects. Journal of Environmental Sciences, 61, 3–13. https://doi.org/10.1016/j.jes.2017.04.037
Yung-Tse, H. U. N. G., Howard, H. P. A. U. L., & B. M. A. (2020). Coagulation-flocculation treatment for naphthol green band flour wastewater. International Journal for Modern Trends in Science and Technology, 6(12), 190–197. https://doi.org/10.46501/ijmtst061237
Zhai, X., Chen, Z., Zhao, S., Wang, H., & Yang, L. (2010). Enhanced ozonation of dichloroacetic acid in aqueous solution using nanometer ZnO powders. Journal of Environmental Sciences, 22(10), 1527–1533. https://doi.org/10.1016/S1001-0742(09)60284-9
Zhang, X., Li, X., & Qin, W. (2009). Investigation of the catalytic activity for ozonation on the surface of NiO nanoparticles. Chemical Physics Letters, 479(4–6), 310–315. https://doi.org/10.1016/j.cplett.2009.08.029
Zhang, H., Liu, D., Ren, S., & Zhang, H. (2017). Kinetic studies of direct blue photodegradation over flower-like TiO2. Research on Chemical Intermediates, 43(3), 1529–1542. https://doi.org/10.1007/s11164-016-2713-6
Zheng, N. C., Wang, Z., Long, J. Y., Kong, L. J., Chen, D. Y., & Liu, Z. Q. (2018). Shape-dependent adsorption of CeO2 nanostructures for superior organic dye removal. Journal of Colloid and Interface Science, 525, 225–233. https://doi.org/10.1016/j.jcis.2018.03.087
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). There is no any funding that supports this manuscript.
Author information
Authors and Affiliations
Contributions
B. ERDEN conducted treatment experiment, wrote and revised manuscript. I read and approved the final manuscript.
G. KATIRCIOĞLU SINMAZ conducted treatment experiment, wrote and revised manuscript. I read and approved the final manuscript.
M. AKSU conducted treatment experiment, wrote and revised manuscript. I read and approved the final manuscript.
N.P.TANATTI conducted treatment experiment, wrote and revised manuscript. I read and approved the final manuscript.
M. HAS conducted treatment experiment, wrote and revised manuscript. I read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
There is no ethical approval about this manuscript.
Consent to Participate
There is no consent to participate about this manuscript.
Consent to Publish
There is no constent to publish about this manuscript.
Competing interests/Conflict of interest
There is no competing interests/ conflict of interest about this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erden, B., Katırcıoğlu Sınmaz, G., Aksu, M. et al. Comparative Study on Removal of Acid Violet 90 dye by using Catalytic Ozonation Processes with n.CeO2, n.ZnO and n.CeO2/n.ZnO Nanocatalysts and Kinetic Examination. Water Air Soil Pollut 235, 581 (2024). https://doi.org/10.1007/s11270-024-07397-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07397-7