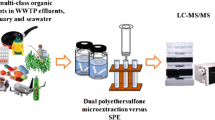
Abstract
Emerging contaminants (ECs) are substances that have no defined environmental quality standards or regulations, and have the potential to pose major adverse impacts on the environment and human health. The detection of contaminants in the natural environment is the key step for establishing precise environmental risk assessment approach for ECs. However, ECs come from different origins with various physicochemical properties, making their detection a complicated process. Moreover, their presence in the aquatic environment at trace concentration range (ng/L-µg/L), requires an accurate detection at low concentration levels. This study aims to develop an efficient analytical method for simultaneous determination of 5 different ECs in aqueous solution based on solid phase extraction technique (SPE) followed by liquid chromatography-mass spectrometry (LC–MS/MS). High recovery rates (72% to 114%) were achieved for all targeted compounds. Ciprofloxacin (CIP), diuron (DIU), terbutryn (TER) and diclofenac (DIC) had a limit of detection (LOD) of 5 µg/L and a limit of quantification (LOQ) of 10 ng/L, while LOD and LOQ for EE2 were 25 µg/L and 50 ng/L, respectively. These results confirm that the optimized method can be applied for extraction and analysis of ECs from different classes in the aquatic environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Emerging contaminants (ECs) are the pollutants for which no regulations are currently established (Tavengwa & Dalu, 2022). According to NORMAN association that is funded by the European Union (EU), the most frequently identified ECs include more than 4000 compounds which are categorized in 20 classes including pharmaceuticals, personal care products, steroid hormones, pesticides, plasticizers, surfactants etc. (NORMAN Database System, n.d.). Moreover, the EU is periodically issuing watch lists of priority substances that should be monitored for establishing environmental quality standards. Starting from the Directive 2013/39/EU that published the water quality limit for the first revised list of priority substances, there are three more EU watch lists that have been established for ECs including Decision (EU) 2015/495, Decision (EU) 2018/840 and Decision (EU) 2020/1161 (Alexa et al., 2022). Nonetheless, due to the continuous increase in daily use and/or consumption of the sources of ECs, the list of ECs is expected to constantly expand over time, while there are more than 500 thousand chemical substances currently identified in the Chemical Abstracts Service (CAS) (Jacobs et al., 2022).
These contaminants enter the environment from multiple sources, e.g., agricultural runoff, hospital wastewater, domestic and industrial effluent discharges, etc. ECs are most frequently detected in various aquatic environmental matrices such as seawater, freshwater and wastewater (AJIBOLA et al., 2021; Cipriani-avila et al., 2023; Klaic & Jirsa, 2022; Linke et al., 2021; Mostafa et al., 2023a, 2023b; Palma et al., 2021; ZIND et al., 2021). Moreover, ECs were also detected in drinking water and even the deep groundwater aquifers (Lapworth et al., 2018; López-Serna et al., 2013; Sui et al., 2015; Tijani et al., 2016; Wilkinson et al., 2017; G. Yang et al., 2014; Zainab et al., 2021). Although ECs are detected at trace concentration range of ng/L to µg/L (Chaturvedi et al., 2021; Gwenzi & Chaukura, 2018), they could pose detrimental effects on the living organisms due to their persistent nature in the environment, toxicity and bioaccumulation potential(T. H. Y. Lee et al., 2021; Parida et al., 2021; Saidulu et al., 2021; J. Yang et al., 2019). These adverse effects could span across various biological scopes, such as nephrotoxic, genotoxic, and hepatotoxic effects (T. H. Y. Lee et al., 2021). Therefore, the occurrence of these substances in the environment has become a matter of concern globally, and there are continues efforts to develop advanced analytical measurement techniques for taccurate monitoring and fate assessment of ECs in different environmental matrices (Angeles et al., 2021; Castillo-Zacarías et al., 2021; Hajeb et al., 2022; Martín-Pozo et al., 2019; Ofrydopoulou et al., 2021; Rao et al., 2023; Ryu et al., 2021).
The detection of multiple ECs in the environmental samples are mostly performed by liquid chromatography-mass spectrometry (LC–MS/MS) and gas chromatography-mass spectrometry (GC–MS) (Gimeno et al., 2014; Harati et al., 2020; Krupčík et al., 2013; Pitne et al., 2017; Rasheed et al., 2016; Vittoria et al., 2019; W. Wang et al., 2023a, 2023b). However, GC–MS can only deal with volatile and semi-volatile compounds, and a prolonged derivatization process is usually mandatory in order to ensure the volatility of the sample analytes (Bekele et al., 2014; Kanani et al., 2008; Sodré et al., 2010). Therefore, LC–MS/MS is the most preferred analytical technique for the measurement of the vast majority of ECs in environmental matrices due to its versatility and sensitivity (Mackeown et al., 2022; Martín-Pozo et al., 2019). LC/MS/MS is usually combined with selective extraction, clean-up and pre-concentration techniques for the accurate measurement of ECs at trace concentrations, and for the elimination of possible interferences from environmental matrices (Etteieb et al., 2020). Solid phase extraction (SPE) is the most widely utilized technique for the pre-treatment of environmental samples because of its simplicity and enhanced selectivity (Aalizadeh et al., 2022; Ajibola et al., 2020; Bain et al., 2021; Barbieri et al., 2019; Do & Stuckey, 2019; Junaid et al., 2018; Kovacs et al., 2021; Riva et al., 2021; Schmidt et al., 2018; Vittoria et al., 2019).
Most of the developed pre-treatment and measurement methods for the quantification of ECs have exclusively focused on one group of compounds (e.g., pesticides, pharmaceuticals) (X. Chen et al., 2022; Kunene & Mahlambi, 2020; Mostafa et al., 2023a, 2023b; Peleshok et al., 2021), while there are numerous number of ECs that belong to various classes and chemical groups such as pharmaceuticals, endocrine disrupters, pesticides, steroid hormones, plasticizers and surfactants with distinct physicochemical properties. Therefore, there is an urgent need for the development of new methods for the simultaneous detection of ECs in the natural environmental samples that is considered as the current challenge of monitoring the ECs in the environment (L. Chen et al., 2023). Thus, the aim of this study was to optimize a fast, selective and sensitive method for the simultaneous extraction and measurement of multiclasses of ECs in water matrices by SPE–LC–MS/MS analysis technique.
ECs for this study were chosen based on their distinct physicochemical properties such as different solubilities in water and organic solvents, as well as various chemical structures including phenylacetic acid (Diclofenac), carbonyl diamide (Diuron), heterocyclic N,S triazines (Terbutryn), quinolinecarboxylic acid (Ciprofloxacin) and steroids (EE2). These ECs also have diverse sources and applications (anti-inflammatory drugs, pesticides, herbicides, antibiotics and oestrogens). Moreover, these contaminanats are included in the EU watch list of substances that should be monitored (European Commission, 2013) and are among the most frequently used and detected ECs in the aqueous environment worldwide (Böger et al., 2021; Deich et al., 2021; Duan et al., 2020; Fahimi et al., 2020; Lagunas-Basave et al., 2022; Palma et al., 2021; Parolini, 2020; Rehberger et al., 2020; Sol et al., 2022; R. Wang et al., 2023a, 2023b; Zhang et al., 2021; ZIND et al., 2021).
2 Materials and Methods
Target compounds were obtained in powder form: diclofenac sodium salt (15307–79-6), terbutryn (886–50-0), ciprofloxacin (85721–33-1), 17α-ethynylestradiol (EE2) (57–63-6) (Sigma-Aldrich, China) and diuron (330–54-1) (Sigma-Aldrich, Germany). All chemicals were of high purity grade ≥ 97%. Methanol (99.9%), methyl tert–butyl ether (MTBE) (99.8%), acetonitrile (ACN, > 95%), ethylacetate (99.8%), acetone (98%), isopropyl (98%), dichloromethane (99.8%) and hexane (99.8%) were of gradient grade for liquid chromatography and purchased from Sigma Aldrich. Deuterium-labeled hydrogen isotopes, Diclofenac-d4 (CDN, purity 99.0%), Ciproflaxin-d8 (CDN, purity > 98%), 17-alpha -ethinylestradiol-d4 (CDN, purity 99.0%), Diuron-d6 (LGC, purity > 98%), Terbutrin-d5 (LGC, purity 99.0%) are used for LC/MS internal standard solution preparation (stored at 4 ℃) in order to quantify sensitive measurements with LC/MS–MS. Oasis HLB SPE cartridges (60 mg, 3 mL), Thermo Scientific SolEx C18 cartridges and HyperSep Retain PEP cartridges were purchased from Agilent Technologies.
2.1 SPE Method Development
A SPE method was optimized for the simultaneous extraction of five different ECs including diclofenac (anti-inflammatory drug), ciprofloxacin (antibiotic), EE2 (oestrogen), terbutryn (herbicide) and diuron (pesticide). Their logKow values ranges from 2.68 to 4.51 and their water solubility varies between 2 and 2000 mg/L (Table 1).
The extractions were carried out in duplicates for each sample containing 1 µg/L of the targeted analytes mixture and 50 µL of 1000 ppb internal standard in 500 mL. Solvents to be used for extaction, conditioning and elution were selected by a literature review to determine the possible appropriate solvent candidates of each contaminant. The selected solvents for the optimization of SPE method are limited to the low boiling point-having solvents to avoid losses of volatile analytes and to minimize the time required for evaporation step (HU et al., 2008). Dichloromethane, hexane, methanol, ethyl acetate, acetone, acetonitrile, isopropyl and MTBE were used for conditioning and eluting the SPE cartridges with a volume of 5 mL for each solvent. After conditioning, in order to remove any impurity coming from cartridge manufacturing and to activate the cartridge’s surface by wetting the sorbent material, 500 mL sample of contaminants mixture (1 µg/L) was percolated through the cartridges under vacuum by maintaining a flowrate of 5 mL/min. Afterward, the cartridges were rinsed with 5 mL ultrapure water (UPW) or a mixture of solvent:UPW in order to remove any undesired contaminants attached to the surface of the sorbent.
Then, the cartridges were left to dry under vacuum for 30 min in order to completely eliminate water because it could negatively affect the analytes recovery rates of the analytes. The analytes were eluted from the cartridges by successively adding the organic solvents and the final extract was dried under a gentle stream of nitrogen overnight. After complete dryness, the extracts were reconstituted to 1 mL by a mixture of UPW and organic solvent for LC–MS analysis.
2.2 Instrumental Analysis
After SPE, 100 µL of reconstituted extracts are directly injected in the LC–MS/MS instrument under the following optimized conditions.
2.2.1 LC/MS Optimization
LC–MS/MS measurements were conducted with Thermo TSQ Fortis Model Tandem Mass Spectrometer (MS–MS) connected to Thermo Ultimate 3000 Model Ultra Pressure Liquid Chromatograph (UPLC). Atmospheric Pressure Ionization (APCI) is used as the ionization source. First of all, MS parameters were optimized to insure that the targeted contaminants are recognized by the detector, then, the conditions that provide sufficiently high signals are determined and the appropriate peak integration for quantitative analysis was made. Afterwards, LC parameters were optimized.
2.2.2 Optimization of MS Parameters
Each analytical standard was prepared at a concentration of 1 mg/L by diluting from their stock solutions, and injected directly into the ionization source in the MS. 1:1 mixture of Methanol containing 5 mM Ammonium fluoride: UPW containing 5 mM Ammonium fluoride was used as the mobile phase solvent for screening after trials with ammonium formate, ammonium fluoride and formic acid as basic buffers. The mass/ion (m/z) ratios of the main substance and the ionization products were determined for each analytical standard. In this part, the ionization occurs in a triple quadrupole structure at a certain pressure and temperature. Determination of the mass weights require the optimization of the various pressure values with the "Compound Optimization Workspace".
Ammonium fluoride was chosen by considering the intensities of the electrical signals given by the ionization products. With MS/MS scanning, "spray voltage", "volatile temperature", "tube lens offset" parameters were also automatically optimized by considering the intensities of the received signals. “Nitrogen gas pressure”; “carrier gas pressure”, “ion stripping gas pressure”, and “additional gas pressure” were optimized by trial and error. The most effective parameter for the ionization process was the "collision energy" parameter.
2.2.3 Optimization of LC Parameters
Samples in the autosampler are passed through proper analytical columns to separate target compounds from the sample. Separation mechanism depends on the adsorption capability of the target compound on column package material and its solubility in the mobile phase that carries the sample from autosampler to LC–MS. Therefore, the type of column, including the characteristics of package material, types and gradient of mobile phase are optimized for the effective separation of the target compound from the sample. Moreover, temperature of the column and auto sampler, sample injection volume and mobile phase velocity should be considered for the high recovery of target compound from the LC column.
3 Results and Discussion
3.1 Optimization of SPE
3.1.1 Trial 1
OASIS HLB (Hydrophilic-lipophilic balanced N-vinylpyrrolidone-co-divinylbenzene) SPE cartridges were used for the first trial. As shown in Table 2, CIP and TER had the highest recovery percentages in the first method (88% and 114%, respectively), while the recovery rates of diuron, diclofenac and EE2 were at unacceptable levels (0%-44%). At first method, an elution mixture of UPW, methanol, and acetonitrile (70:20:10) were used as elution solvents. In the second method, replacing the methanol and acetonitrile with acetone led to a decrease in recoveries for all ECs except for EE2. Conditioning with MTBE and acetonitrile and eluting with isopropyl: UPW (15:85) resulted in low recoveries except for TER (Table 2). Changing the reconstitution solution with acetonitrile instead of UPW: isopropyl mixture did not increase the recoveries. The effect of the solution pH was also studied by changing it to 2 and 9. The recovery rates of targeted compounds except TER increased with decreasing pH value from 9 to 2 with using the same conditioning and elution solvents in method 6; however, they were still not in the acceptable range. The acidic pH could enhance the recovery rate of the targeted pollutants especially by avoiding the precipitation of DIC and improve the retention of EE2 compound on the SPE cartridge (Česen & Heath, 2017a; Kosjek et al., 2009; Valdés et al., 2015). Therefore, methanol and acetonitrile were further examined with other types of SPE cartridges and different elution solvents at acidic pH in the second trail.
3.1.2 Trial 2
In addition to OASIS HLB cartridge, the Thermo Scientific SolEx C18 (Silica) cartridge, which is a nonpolar cartridge and mainly chosen for its sufficient selectivity for the steroidal and nonpolar to moderately polar compounds (Kopperi et al., 2013; Riva et al., 2021; Valdés et al., 2015; Wu et al., 2014; Zuo et al., 2013) and HyperSep Retain PEP (HyperSep Urea-Modified Polystyrene DVB) cartridge, which is also a frequently used cartridge for the extraction of ECs were examined. In this trial, all three cartridges had relatively similar recovery rates. However, C18 and HLB are known to retain a wider range of structures compared to PEP which could prohibit the adsorption of highly polar compounds on its surface due to its low polarity (Vaudreuil et al., 2022). C18 and HLB cartridges have the tendency of increasing the retention of high polar substances by the special polar trapping groups that are generated in their sorbent material by the polymerization of hydrophilic N-vinylpyrrolidone and lipophilic divinylbenzene monomers (Liu et al., 2015). Thermo Scientific SolEx C18 and HLB cartridges were chosen for further examination in following trails. Adjusting the pH of solvent to 2 improved the recoveries for TER and DIU for all cartridges (Table 3), as these compounds are weakly acidic (pKa 4.4 and 3.7 respectively), their dissociation and solubility in water is expected to increase under acidic conditions, thus enhancing their adsorption to the sorbent material (Barriuso et al., 1992; Cosgrove et al., 2019). Elution with acetonitrile and methanol increased the recovery of CIP while the recoveries of TER and DIU were still high. The high recovery rate of CIP with acetonitrile and methanol compared to other solvents was also observed in other studies (H. B. Lee et al., 2007; Martínez Bueno et al., 2009; A. F. Martins et al., 2008; Pápai et al., 2010). This could be due to the higher capability of these solvents to disrupt the interactions between the functional groups of targeted analytes and the sorbent surface, which depend upon the retention mechanism of the analyte such as its polarity.
3.1.3 Trial 3
This trial was conducted to test the OASIS HLB and C18 cartridges to increase the recoveries of DIC and EE2 as shown in Table 4. C18 cartridge provided the highest recovery rates for all targeted compounds especially for EE2 which ranged from 56% to 74.6% with C18 while EE2 either was not recovered at all or had unacceptable recovery rates for most of the tested methods with HLB cartridge. Compared to Oasis HLB which is preferred for the extraction of high polarity compounds (Dias & Poole, 2002), silica C18 is a nonpolar cartridge and have sufficient selectivity for the steroidal and nonpolar to moderately polar compounds (Nodeh et al., 2016). Thus, it would be more suitable for the retention of oestrogens and aliphatic compounds such as EE2 by the strong interactions of electron lone pair (Ciofi et al., 2013).
The addition of ethyl acetate solvent for elution with C18 cartridge enhanced the recovery of DIC and EE2 from 16 to 32% and 66% to 77.2%, respectively (methods 2 and 4). Removing of methanol and acetone from the elution solvents and methanol and acetonitrile from the conditioning combination did not affect the recovery rate of any compound (methods 5, 6, 7 and 8). The presence of other solvents in the elution and conditioning steps such as MTBE could have a dominant influence on the recovery rates of analytes over other types of solvents due to polarity differences. Therefore, methanol and acetone had minor role in SPE and it were excluded from the next trials. However, the exclusion of ethyl acetate from conditioning and elution (5, 6, 7, 8) decreased the recoveries of EE2 significantly. As oestrogens are medium polarity-compounds, and the elution step depends mainly on the resemblance of solvents’ and analytes’ polarity levesl, ethyl acetate might be matching or approaching the polarity of EE2 compound. As a result of this, elution with ethyl acetate can provide high recovery rate for EE2 that is also observed in other studies such as Česen & Heath, 2017b; Isobe et al., 2003; Liu et al., 2015; Zuo et al., 2007, 2013.
The combination of MTBE, acetonitrile and ethyl acetate solvents had further enhanced the recovery rates of all targeted compounds without the need of acidifying the solvents. Therefore, these solvents with C18 cartridge were further examined in the next trial with different analytes concentration and re-solution mixtures.
3.1.4 Trial 4
In this trial, modifications were made to increase the recovery of DIC which was still at unacceptable levels. Combination of MTBE and ethyl acetate increased the recovery for DIC and EE2 as seen in Table 5 (Method 3). The elution with the reverse order of conditioning solvent combination gave the best results for the recoveries of all ECs. The selected solvents from Trial 4 had been further examined with lower concentration (50 µg/L) of ECs and two different re-solution mixtures. As shown in Tables 5 and 6, all the extracted compounds had acceptable recovery rates at 50 µg/L and 500 µg/L. The reconstitution with the mixture of methanol: UPW (30:70 v/v %) had given higher recovery for EE2 (65% to 90%) and the relatively similar recovery rates for other analytes.
Based on the findings of four experimental trials conducted for the SPE of selected compounds, the method that was determined as the best possible method for the simultaneous recovery of all the targeted analytes from the aqueous phase by SPE is presented in Table 7.
3.2 Optimization of LC Parameters
The auto sampler temperature in the developed method has been optimized to 10 ℃. In order to reduce the measurement limit for EE2, the injection volume was kept as high as 100 µL. For this reason, both different material structures and volumes have been tried in order to achieve the highest recovery efficiency from the column.
The following columns are used in the trials;
-
Thermo Accucore C18 brand 2.6 µm pore diameter 100*2.1 mm size
-
Thermo Acclaim Polar Advantage 2.2 µm pore diameter 100*2.1 mm size
-
Terra C18 3.5 µm pore diameter 100*2.1 mm size
-
X-Bridge C18 3.5 µm pore diameter 50*4.6 mm size
-
Waters Symetry C18 3.5 µm pore diameter 50*4.6 mm size
The columns showed the necessary selectivity for the separation of substances, and attention was paid to the tolerance of high sample volume, especially due to their different pore diameters. Thermo Accucore C18 brand column with a medium pore size of 100*2.1 mm and a pore diameter of 2.6 µm was selected.
Mobile phase solutions were selected in accordance with the polarities of the substances measured. Methonal and UPW were preferred as mobile phase solvents due to their common usage in different methods. As multiple measurements are made in a single injection, an optimization has been made to obtain a polarity gradient that will allow the separation of the substances, taking into account the degree of polarity of the substances to be separated. Since the mobile phase solvent also carries the substances to the MS where ionization takes place, a buffer solution is added to the mobile phase. The concentration for ammonium fluoride, which provides the most effective ionization during the optimization of MS parameters, was determined as 5 mM by trial and error, taking into account the pH levels in which the substance ionized. The mobile phase gradient, in which the measured substances are separated efficiently from each other, is shown in Table 8.
Accordingly, the mobile phase gradient starting with methanol containing 10% of 5 Mm ammonium fluoride is kept constant for 0.2 min, then increased to 70% within 2 min, increased to 90% between 2 and 5 min, and it remained stable at 90% for 2 min, and it then returned to its initial conditions for 2 min. The other mobile phase was ultrapure water containing 1 mM ammonium fluoride. Therefore, the developed method takes 7 min. Then, chromatographic conditions such as injection volume, mobile phase velocity and column temperature were tested and optimized so that all the substances to be measured were efficiently separated and formed non-intermingled peaks (Table 8). The optimized injection volume was 100 µL and the mobile phase velocity was 0.300 mL/min. While the column temperature was determined as 40 ℃.
3.3 Optimized MS Parameters
The optimized parameter values are summarized in Table 9. In MS/MS scanning systems, attention has been paid to using at least two ionization products in order to make precise measurements. The higher signal ion is used to quantify, and the other is used to verify the substance (Bester & Schlu, 2005). The ionization products detected in LC–MS/MS belonging to the selected contaminants are shown in Table 10. After the ionization products are detected, the parameters of the peak width, scan width, scan time, micro scan number, which determine the quality of the peak, have been tested and optimized and are summarized in Table 9.
3.4 Method Validation
In order to ensure the suitability of the optimized method for applications in routine analysis, the analytical quality parameters were evaluated including selectivity, stability, robustness, linearity, sensitivity, accuracy and precision.
3.4.1 Selectivity
Selectivity is an essential qualitative assessment for the analytical method which test the capability of the extraction method and it represents to which extent the interferences were avoided and the targeted analytes were distinguished among other organic compounds in the matrix. For this reason, the extraction of blank samples was performed using UPW, synthetic surface water and surface water matrices. The results were compared to the UPW matrice that was spiked with the targeted analytes. The recovery rates of the blank samples (0%—0.05%) and samples spiked with targeted analytes were (90%—114%) in an acceptable range. Moreover, there was no interference peaks in the chromatogram of blanks which indicates a good selectivity for the separation and extraction of the targeted analytes by the developed method.
3.4.2 Stability and Robustness
Stability and robustness parameters measure the capacity of the optimized method to remain unaffected by little but dynamic alterations in different factors or experimental conditions such as sample storage time and water matrix. The stability was evaluated by applying the optimized SPE method on 4 different sets including the targeted analytes at 50 µg/L. The first set was extracted immediately, while the other three sets were stored in dark at 4 °C and extracted after 24 h, 48 h and 72 h. The robustness of the method, which test the effect of the optimized method in different matrix, was examined by extracting 3 different sets including UPW and two synthetic surface water matrices with different TOC concentration (4.5 mg/L and 13.6 mg/L). The recovery percentages of all the analytes in the stability and robustness experiments did not exhibit any significant variation and were in the acceptable range (85%—115%) which confirm the robustness and stability of the optimized method.
3.4.3 Linearity
The linearity of the method tests the ability of the optimized method to provide directly proportional results to the real concentration of the analytes in samples. The linearity was examined by extraction of six spiked samples in the range of 10 µg/L – 750 µg/L (10 µg/L, 50 µg/L, 100 µg/L, 200 µg/L, 500 µg/L and750 µg/L). The correlation coefficients ( r2 values) for the targeted analytes ranged between 0.988–0.999, which is an evidence for a good linearity of the optimized analytical method within the studied range of concentrations (Figure S1 in Supplementary Information (SI)).
3.4.4 Accuracy and Precision
The accuracy of the method was tested at four different concentrations representing low, medium and high levels (10 µg/L, 50 µg/L, 100 µg/L and 500 µg/L) within the dynamic linear range. The recovery rates of the various concentrations of targeted analytes were in the range of 71%—117%, The precision was demonstrated by the replicate injection of the samples with a relative standard deviation of (RSD) less than 20%, which shows that the developed method has good repeatability and precision.
3.4.5 Sensitivity
Sensitivity parameter includes the determination of the limit of detection (LOD) taking three times value of the signal-to-noise ratio (S/N) for LOD and limit of quantification (LOQ) taking ten times S/N value after the application of SPE that provides the concentration factor for the calculations. SPE was applied for 500 mL of samples and the final volume is 1 mL after complete evaporation; therefore, the concentration factor is 500. The LODs values were 5 µg/L for ciprofloxacin, diuron, terbutryn and diclofenac, and 25 µg/L for EE2, while LOQs values for the targeted analytes were 0.01 µg/L for ciprofloxacin, diuron, terbutryn and diclofenac and 0.05 µg/L for EE2.
4 Conclusion
An analytical method was optimized for the simultaneous extraction and measurement of 5 different ECs in aqueous solutions based on a SPE-LC/MS/MS technique. The method optimization was achieved by examining the efficiency of different SPE cartridges and extraction solvents in multiple experimental trials. C18 cartridges along with acetonitrile, ethyl acetate and MTBE as extraction solvents have provided the highest recovery rate for all of the targeted compounds (90%—114%). The validity of the optimized method was inspected by the analytical quality parameter tests and the results confirmed the suitability, efficiency and sensitivity of this method for accurate and simultaneous detection of various ECs.
Data Availability
Data is available upon request to corresponding author.
References
Aalizadeh, R., Nikolopoulou, V., Alygizakis, N., Slobodnik, J., & Thomaidis, N. S. (2022). A novel workflow for semi-quantification of emerging contaminants in environmental samples analyzed by LC-HRMS. Analytical and Bioanalytical Chemistry, 414(25), 7435–7450. https://doi.org/10.1007/s00216-022-04084-6
Ajibola, A., Adebiyi, A., Nwaeke, D., Ajibola, F., & Adewuyi, G. (2021). Analysis, Occurrence and Ecological Risk Assessment of Diclofenac and Ibuprofen Residues in Wastewater from Three Wastewater Treatment Plants in South-Western Nigeria. Journal of Applied Sciences and Environmental Management, 25(3), 330–340. https://doi.org/10.4314/jasem.v25i3.5
Ajibola AS, Tisler S Zwiener C (2020). Simultaneous determination of multiclass antibiotics in sewage sludge based on QuEChERS extraction and liquid chromatography-tandem mass spectrometry. 12(4), 576–586https://doi.org/10.1039/c9ay02188d
Alexa ET, Bueno M de los ÁBR del H, González, R., Sánchez, AV, García H, Prats, D. (2022). Occurrence and Removal of Priority Substances and Contaminants of Emerging Concern at the WWTP of Benidorm (Spain). Water, 14(24), 4129https://doi.org/10.3390/w14244129
Angeles, L. F., Singh, R. R., Vikesland, P. J., & Aga, D. S. (2021). Increased coverage and high confidence in suspect screening of emerging contaminants in global environmental samples. Journal of Hazardous Materials, 414, 125369. https://doi.org/10.1016/j.jhazmat.2021.125369
Aris, A. Z., Shamsuddin, A. S., & Praveena, S. M. (2014). Occurrence of 17α-ethynylestradiol (EE2) in the environment and effect on exposed biota: A review. Environment International, 69, 104–119. https://doi.org/10.1016/j.envint.2014.04.011
Bain, P. A., Gregg, A., Pandey, A. K., Mudiam, M. K. R., Neale, P. A., & Kumar, A. (2021). Using bioanalytical tools to detect and track organic micropollutants in the Ganga River near two major cities. Journal of Hazardous Materials, 404, 124135. https://doi.org/10.1016/j.jhazmat.2020.124135
Barbieri, M. V., Postigo, C., Guillem-Argiles, N., Monllor-Alcaraz, L. S., Simionato, J. I., Stella, E., Barceló, D., & López de Alda, M. (2019). Analysis of 52 pesticides in fresh fish muscle by QuEChERS extraction followed by LC-MS/MS determination. Science of the Total Environment, 653, 958–967. https://doi.org/10.1016/j.scitotenv.2018.10.289
Barriuso, E., & Calvet, R. (1992). Soil Type and Herbicides Adsorption. International Journal of Environmental Analytical Chemistry, 46(1–3), 117–128. https://doi.org/10.1080/03067319208027003
Barriuso, E., Feller, C., Calvet, R., & Cerri, C. (1992). Sorption of atrazine, terbutryn and 2,4-D herbicides in two Brazilian Oxisols. Geoderma, 53(1–2), 155–167. https://doi.org/10.1016/0016-7061(92)90028-6
Bekele, E. A., Annaratone, C. E. P., Hertog, M. L. A. T. M., Nicolai, B. M., & Geeraerd, A. H. (2014). Analytica Chimica Acta Multi-response optimization of the extraction and derivatization protocol of selected polar metabolites from apple fruit tissue for GC – MS analysis. Analytica Chimica Acta, 824, 42–56. https://doi.org/10.1016/j.aca.2014.03.030
Ben Fredj, S., Nobbs, J., Tizaoui, C., & Monser, L. (2015). Removal of estrone (E1), 17β-estradiol (E2), and 17α-ethinylestradiol (EE2) from wastewater by liquid-liquid extraction. Chemical Engineering Journal, 262, 417–426. https://doi.org/10.1016/j.cej.2014.10.007
Bester, K., & Schlu, M. P. (2005). Determination of steroid hormones, hormone conjugates and macrolide antibiotics in influents and effluents of sewage treatment plants utilising high-performance liquid chromatography / tandem mass spectrometry with electrospray and atmospheric pressure c. Rapid Communications in Mass Spectrometry: An International Journal Devoted to the Rapid Dissemination of up-to-the-Minute Research in Mass Spectrometry, 19(22), 3269–3278. https://doi.org/10.1002/rcm.2189
Böger, B., Surek, M., de Vilhena, R. O., Fachi, M. M., Junkert, A. M., Santos, J. M., Domingos, E. L., de Cobre, A. F., Momade, D. R., & Pontarolo, R. (2021). Occurrence of antibiotics and antibiotic resistant bacteria in subtropical urban rivers in Brazil. Journal of Hazardous Materials, 402, 123448. https://doi.org/10.1016/j.jhazmat.2020.123448
Cardoza, L. A., Knapp, C. W., Larive, C. K., Belden, J. B., Lydy, M., & Graham, D. W. (2005). Factors affecting the fate of ciprofloxacin in aquatic field systems. Water, Air, and Soil Pollution, 161(1–4), 383–398. https://doi.org/10.1007/s11270-005-5550-6
Castillo-Zacarías, C., Barocio, M. E., Hidalgo-Vázquez, E., Sosa-Hernández, J. E., Parra-Arroyo, L., López-Pacheco, I. Y., Barceló, D., Iqbal, H. N. M., & Parra-Saldívar, R. (2021). Antidepressant drugs as emerging contaminants: Occurrence in urban and non-urban waters and analytical methods for their detection. Science of the Total Environment, 757, 143722. https://doi.org/10.1016/j.scitotenv.2020.143722
Česen, M., & Heath, E. (2017a). Disk-based solid phase extraction for the determination of diclofenac and steroidal estrogens E1, E2 and EE2 listed in the WFD watch list by GC–MS. Science of the Total Environment, 590–591, 832–837. https://doi.org/10.1016/j.scitotenv.2017.02.222
Česen, M., & Heath, E. (2017b). Disk-based solid phase extraction for the determination of diclofenac and steroidal estrogens E1, E2 and EE2 listed in the WFD watch list by GC–MS. Science of the Total Environment, 590, 832–837. https://doi.org/10.1016/j.scitotenv.2017.02.222
Chaturvedi, P., Shukla, P., Shekher, B., Chowdhary, P., Chandra, R., Gupta, P., & Pandey, A. (2021). Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment : A review on emerging contaminants. Environmental Research, 194, 110664. https://doi.org/10.1016/j.envres.2020.110664
Chen, X., Liu, S., Jiang, R., Luan, T., & Ouyang, G. (2022). Rapid detection and speciation of illicit drugs via a thin-film microextraction approach for wastewater-based epidemiology study. Science of the Total Environment, 842, 156888. https://doi.org/10.1016/j.scitotenv.2022.156888
Chen, L., Yan, X., Zhou, X., Peng, P., Sun, Q., & Zhao, F. (2023). Trends in Analytical Chemistry Advances in the on-line solid-phase extraction-liquid chromatography-mass spectrometry analysis of emerging organic contaminants. Trends in Analytical Chemistry, 160, 116976. https://doi.org/10.1016/j.trac.2023.116976
Ciofi, L., Fibbi, D., Chiuminatto, U., Coppini, E., Checchini, L., & Del Bubba, M. (2013). Fully-automated on-line solid phase extraction coupled to high-performance liquid chromatography-tandem mass spectrometric analysis at sub-ng/L levels of selected estrogens in surface water and wastewater. Journal of Chromatography A, 1283, 53–61. https://doi.org/10.1016/j.chroma.2013.01.084
Cipriani-avila, I., Molinero, J., Cabrera, M., Medina-villamizar, E. J., Capparelli, M. V., Jara-negrete, E., Pinos-velez, V., Leiva, D., Barrado, M., & Mogollón, N. G. S. (2023). Occurrence of emerging contaminants in surface water bodies of a coastal province in Ecuador and possible in fl uence of tourism decline caused by COVID-19 lockdown. Science of the Total Environment Journal, 866, 161340. https://doi.org/10.1016/j.scitotenv.2022.161340
Commission, E. (2013). Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Official Journal of the European Union, 226, 1–17. https://doi.org/10.5040/9781782258674.0032
Cosgrove, S., Jefferson, B., & Jarvis, P. (2019). Pesticide removal from drinking water sources by adsorption: A review. Environmental Technology Reviews, 8(1), 1–24. https://doi.org/10.1080/21622515.2019.1593514
Czyrski, A. (2022). The spectrophotometric determination of lipophilicity and dissociation constants of ciprofloxacin and levofloxacin. Spectrochimica Acta - Part a: Molecular and Biomolecular Spectroscopy, 265, 120343. https://doi.org/10.1016/j.saa.2021.120343
NORMAN Database System. (n.d.). NORMAN organises the development and maintenance of various web-based databases for the collection & evaluation of data / information on emerging substances in the environment. Retrieved March 14, 2023, from https://www.norman-network.com/nds/.
Deich, C., Frazão, H. C., Appelt, J. S., Li, W., Pohlmann, T., & Waniek, J. J. (2021). Occurrence and distribution of estrogenic substances in the northern South China Sea. Science of the Total Environment, 770, 145239. https://doi.org/10.1016/j.scitotenv.2021.145239
Dias, N. C., & Poole, C. F. (2002). Mechanistic study of the sorption properties of Oasis® HLB and its use in solid-phase extraction. Chromatographia, 56(5–6), 269–275. https://doi.org/10.1007/BF02491931
Do, M. T., & Stuckey, D. C. (2019). Fate and removal of Ciprofloxacin in an anaerobic membrane bioreactor (AnMBR). Bioresource Technology, 289(June), 121683. https://doi.org/10.1016/j.biortech.2019.121683
Duan, Q., Duan, L., Liu, Y., Naidu, R., Zhang, H., & Lei, Y. (2020). A novel in-situ passive sampling technique in the application of monitoring diuron in the aquatic environment. Environmental Technology and Innovation, 20, 101073. https://doi.org/10.1016/j.eti.2020.101073
Etteieb, S., Magdouli, S., Zolfaghari, M., & Brar, S. (2020). Monitoring and analysis of selenium as an emerging contaminant in mining industry : A critical review. Science of the Total Environment, 698, 134339. https://doi.org/10.1016/j.scitotenv.2019.134339. Contents.
Fahimi, A., Zanoletti, A., Federici, S., Assi, A., Bilo, F., Depero, L. E., & Bontempi, E. (2020). New eco-materials derived fromwaste for emerging pollutants adsorption: The case of diclofenac. Materials, 13(18), 3964. https://doi.org/10.3390/ma13183964
Giacomazzi, S., & Cochet, N. (2004). Environmental impact of diuron transformation: A review. Chemosphere, 56(11), 1021–1032. https://doi.org/10.1016/j.chemosphere.2004.04.061
Gimeno, P., Thomas, S., Bousquet, C., Maggio, A. F., Civade, C., Brenier, C., & Bonnet, P. A. (2014). Identification and quantification of 14 phthalates and 5 non-phthalate plasticizers in PVC medical devices by GC-MS. Journal of Chromatography b: Analytical Technologies in the Biomedical and Life Sciences, 949–950, 99–108. https://doi.org/10.1016/j.jchromb.2013.12.037
González, S., Müller, J., Petrovic, M., Barceló, D., & Knepper, T. P. (2006). Biodegradation studies of selected priority acidic pesticides and diclofenac in different bioreactors. Environmental Pollution, 144(3), 926–932. https://doi.org/10.1016/j.envpol.2006.02.021
Guimarães, A. C. D., Mendes, K. F., dos Reis, F. C., Campion, T. F., Christoffoleti, P. J., & Tornisielo, V. L. (2018). Role of soil physicochemical properties in quantifying the fate of diuron, hexazinone, and metribuzin. Environmental Science and Pollution Research, 25(13), 12419–12433. https://doi.org/10.1007/s11356-018-1469-5
Gwenzi, W., & Chaukura, N. (2018). Organic contaminants in African aquatic systems: Current knowledge, health risks, and future research directions. Science of the Total Environment, 619, 1493–1514. https://doi.org/10.1016/j.scitotenv.2017.11.121
Hajeb, P., Zhu, L., Bossi, R., & Vorkamp, K. (2022). Sample preparation techniques for suspect and non-target screening of emerging contaminants. Chemosphere, 287, 132306. https://doi.org/10.1016/j.chemosphere.2021.132306
Halling-Sorensen, B., Holten Lutzhoft, H. C., Andersen, H. R., & Ingerslev, F. (2000). Environmental risk assessment of antibiotics: Comparison of mecillinam, trimethoprim and ciprofloxacin. Journal of Antimicrobial Chemotherapy, 46, 53–58. https://doi.org/10.1093/jac/46.suppl_1.53
Han, J., Qiu, W., Cao, Z., Hu, J., & Gao, W. (2013). Adsorption of ethinylestradiol (EE2) on polyamide 612: Molecular modeling and effects of water chemistry. Water Research, 47(7), 2273–2284. https://doi.org/10.1016/j.watres.2013.01.046
Harati, F., Ghiasvand, A., Dalvand, K., & Haddad, P. R. (2020). Fused-silica capillary internally modified with nanostructured octadecyl silica for dynamic in-tube solid-phase microextraction of polycyclic aromatic hydrocarbons from aqueous media. Microchemical Journal, 155, 104672. https://doi.org/10.1016/j.microc.2020.104672
Hildebrand, C., Londry, K. L., & Farenhorst, A. (2006). Sorption and desorption of three endocrine disrupters in soils. Journal of Environmental Science and Health - Part B Pesticides, Food Contaminants, and Agricultural Wastes, 41(6), 907–921. https://doi.org/10.1080/03601230600806020
Hu, B., Song, W., Xie, L., & Shao, T. (2008). Determination of 33 pesticides in tea using accelerated solvent extraction/gel permeation chromatography and solid phase extraction/gas chromatography-mass spectrometry. Chinese Journal of Chromatography, 26(1), 22–28.
Huang, Y., Guo, J., Yan, P., Gong, H., & Fang, F. (2019). Sorption-desorption behavior of sulfamethoxazole, carbamazepine, bisphenol A and 17Α-ethinylestradiol in sewage sludge. Journal of Hazardous Materials, 368, 739–745. https://doi.org/10.1016/j.jhazmat.2019.01.063
Isobe, T., Shiraishi, H., Yasuda, M., Shinoda, A., Suzuki, H., & Morita, M. (2003). Determination of estrogens and their conjugates in water using solid-phase extraction followed by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 984(2), 195–202. https://doi.org/10.1016/S0021-9673(02)01851-4
Jacobs, A., Williams, D., Hickey, K., Patrick, N., Williams, A. J., Chalk, S., McEwen, L., Willighagen, E., Walker, M., Bolton, E., Sinclair, G., & Sanford, A. (2022). CAS Common Chemistry in 2021: Expanding Access to Trusted Chemical Information for the Scientific Community. Journal of Chemical Information and Modeling, 62(11), 2737–2743. https://doi.org/10.1021/acs.jcim.2c00268
Jiménez-Bambague, E. M., Florez-Castillo, J. S., Gómez-Angulo, R. D., Morales-Acosta, P. A., Peña-Salamanca, E. J., Machuca-Martínez, F., & Madera-Parra, C. A. (2021). Cell growth and removal capacity of ibuprofen and diclofenac by Parachlorella kessleri at bench scale. Journal of Chemical Technology and Biotechnology, 97(6), 1416–1423. https://doi.org/10.1002/jctb.6911
Junaid, M., Jia, P. P., Tang, Y. M., Xiong, W. X., Huang, H. Y., Strauss, P. R., Li, W. G., & Pei, D. S. (2018). Mechanistic toxicity of DEHP at environmentally relevant concentrations (ERCs) and ecological risk assessment in the Three Gorges Reservoir Area, China. Environmental Pollution, 242, 1939–1949. https://doi.org/10.1016/j.envpol.2018.07.067
Kanani, H., Chrysanthopoulos, P. K., & Klapa, M. I. (2008). Standardizing GC-MS metabolomics. Journal of Chromatography b: Analytical Technologies in the Biomedical and Life Sciences, 871(2), 191–201. https://doi.org/10.1016/j.jchromb.2008.04.049
Klaic, M., & Jirsa, F. (2022). 17α-Ethinylestradiol (EE2): Concentrations in the environment and methods for wastewater treatment - an update. RSC Advances, 12(20), 12794–12805. https://doi.org/10.1039/d2ra00915c
Kopperi, M., Ruiz-jiménez, J., Hukkinen, J. I., & Riekkola, M. (2013). Analytica Chimica Acta New way to quantify multiple steroidal compounds in wastewater by comprehensive two-dimensional gas chromatography – time-of-flight mass spectrometry. Analytica Chimica Acta, 761, 217–226. https://doi.org/10.1016/j.aca.2012.11.059
Kosjek, T., Heath, E., Pérez, S., Petrović, M., & Barceló, D. (2009). Metabolism studies of diclofenac and clofibric acid in activated sludge bioreactors using liquid chromatography with quadrupole - time-of-flight mass spectrometry. Journal of Hydrology, 372(1–4), 109–117. https://doi.org/10.1016/j.jhydrol.2009.04.006
Kovacs, E. D., Silaghi-Dumitrescu, L., Kovacs, M. H., & Roman, C. (2021). Determination of the Uptake of Ibuprofen, Ketoprofen, and Diclofenac by Tomatoes, Radishes, and Lettuce by Gas Chromatography-Mass Spectrometry (GC–MS). Analytical Letters, 54(1–2), 314–330. https://doi.org/10.1080/00032719.2020.1779278
Krupčík, J., Gorovenko, R., Špánik, I., Bočková, I., Sandra, P., & Armstrong, D. W. (2013). On the use of ionic liquid capillary columns for analysis of aromatic hydrocarbons in low-boiling petrochemical products by one-dimensional and comprehensive two-dimensional gas chromatography. Journal of Chromatography A, 1301, 225–236. https://doi.org/10.1016/j.chroma.2013.05.075
Kunene, P. N., & Mahlambi, P. N. (2020). Optimization and application of ultrasonic extraction and Soxhlet extraction followed by solid phase extraction for the determination of triazine pesticides in soil and sediment. Journal of Environmental Chemical Engineering, 8(2), 103665. https://doi.org/10.1016/j.jece.2020.103665
Lagunas-Basave, B., Brito-Hernández, A., Saldarriaga-Noreña, H. A., Romero-Aguilar, M., Vergara-Sánchez, J., Moeller-Chávez, G. E., de Díaz-Torres, J. J., Rosales-Rivera, M., & Murillo-Tovar, M. A. (2022). Occurrence and Risk Assessment of Atrazine and Diuron in Well and Surface Water of a Cornfield Rural Region. Water, 14(22), 1–14. https://doi.org/10.3390/w14223790
Lapworth, D. J., Das, P., Shaw, A., Mukherjee, A., Civil, W., Petersen, J. O., Gooddy, D. C., Wakefield, O., Finlayson, A., Krishan, G., Sengupta, P., & MacDonald, A. M. (2018). Deep urban groundwater vulnerability in India revealed through the use of emerging organic contaminants and residence time tracers. Environmental Pollution, 240, 938–949. https://doi.org/10.1016/j.envpol.2018.04.053
Lee, H. B., Peart, T. E., & Svoboda, M. L. (2007). Determination of ofloxacin, norfloxacin, and ciprofloxacin in sewage by selective solid-phase extraction, liquid chromatography with fluorescence detection, and liquid chromatography-tandem mass spectrometry. Journal of Chromatography A, 1139(1), 45–52. https://doi.org/10.1016/j.chroma.2006.11.068
Lee, T. H. Y., Chuah, J., & Snyder, S. A. (2021). Occurrence of Emerging Contaminants in Southeast Asian Environments: Present Status, Challenges, and Future Prospects. ACS ES and T Water, 2(6), 907–931. https://doi.org/10.1021/acsestwater.1c00453
Letzel, M., Metzner, G., & Letzel, T. (2009). Exposure assessment of the pharmaceutical diclofenac based on long-term measurements of the aquatic input. Environment International, 35(2), 363–368. https://doi.org/10.1016/j.envint.2008.09.002
Linke, F., Olsson, O., Preusser, F., Kümmerer, K., Schnarr, L., Bork, M., & Lange, J. (2021). Sources and pathways of biocides and their transformation products in urban storm water infrastructure of a 2 ha urban district. Hydrology and Earth System Sciences, 25(8), 4495–4512. https://doi.org/10.5194/hess-25-4495-2021
Liu, N., Shi, Y. E., Li, M., Zhang, T. D., & Gao, S. (2015). Simultaneous determination of four trace estrogens in feces, leachate, tap and groundwater using solid-liquid extraction/auto solid-phase extraction and high-performance liquid chromatography with fluorescence detection. Journal of Separation Science, 38(20), 3494–3501. https://doi.org/10.1002/jssc.201500443
López-Serna, R., Jurado, A., Vázquez-Suñé, E., Carrera, J., Petrović, M., & Barceló, D. (2013). Occurrence of 95 pharmaceuticals and transformation products in urban groundwaters underlying the metropolis of Barcelona, Spain. Environmental Pollution, 174, 305–315. https://doi.org/10.1016/j.envpol.2012.11.022
Mackeown, H., Benedetti, B., Carro, M. D., & Magi, E. (2022). The study of polar emerging contaminants in seawater by passive sampling : A review. Chemosphere, 299, 134448. https://doi.org/10.1016/j.chemosphere.2022.134448
Marín-benito, J. M., Sánchez-martín, M. J., Ordax, J. M., Draoui, K., Azejjel, H., & Rodríguez-cruz, M. S. (2018). Organic sorbents as barriers to decrease the mobility of herbicides in soils. Modelling of the Leaching Process. Geoderma, 313, 205–216. https://doi.org/10.1016/j.geoderma.2017.10.033
Martínez Bueno, M. J., Hernando, M. D., Agüera, A., & Fernández-Alba, A. R. (2009). Application of passive sampling devices for screening of micro-pollutants in marine aquaculture using LC-MS/MS. Talanta, 77(4), 1518–1527. https://doi.org/10.1016/j.talanta.2008.09.047
Martín-Pozo, L., de Alarcón-Gómez, B., Rodríguez-Gómez, R., García-Córcoles, M. T., Çipa, M., & Zafra-Gómez, A. (2019). Analytical methods for the determination of emerging contaminants in sewage sludge samples. A Review. Talanta, 192, 508–533. https://doi.org/10.1016/j.talanta.2018.09.056
Martins, A. F., Vasconcelos, T. G., Henriques, D. M., da Frank, C. S. S., König, A., & Kümmerer, K. (2008). Concentration of ciprofloxacin in Brazilian hospital effluent and preliminary risk assessment: A case study. Clean - Soil, Air, Water, 36(3), 264–269. https://doi.org/10.1002/clen.200700171
Martins, N., Pereira, R., Abrantes, N., Pereira, J., Gonçalves, F., & Marques, C. R. (2012). Ecotoxicological effects of ciprofloxacin on freshwater species: Data integration and derivation of toxicity thresholds for risk assessment. Ecotoxicology, 21(4), 1167–1176. https://doi.org/10.1007/s10646-012-0871-x
Mostafa, A., Shaaban, H., Alqarni, A., Al-ansari, R., Alrashidi, A., Al-sultan, F., Alsulaiman, M., Alsaif, F., & Aga, O. (2023). Multi-class determination of pharmaceuticals as emerging contaminants in wastewater from Eastern Province, Saudi Arabia using eco-friendly SPE-UHPLC-MS / MS : Occurrence, removal and environmental risk assessment. Microchemical Journal, 187, 108453. https://doi.org/10.1016/j.microc.2023.108453
Mostafa, A., Shaaban, H., Alqarni, A., Al-Ansari, R., Alrashidi, A., Al-Sultan, F., Alsulaiman, M., Alsaif, F., & Aga, O. (2023b). Multi-class determination of pharmaceuticals as emerging contaminants in wastewater from Eastern Province, Saudi Arabia using eco-friendly SPE-UHPLC-MS/MS: Occurrence, removal and environmental risk assessment. Microchemical Journal, 187, 108453. https://doi.org/10.1016/j.microc.2023.108453
Nodeh, H. R., Wan Ibrahim, W. A., Sanagi, M. M., & Aboul-Enein, H. Y. (2016). Magnetic graphene-based cyanopropyltriethoxysilane as an adsorbent for simultaneous determination of polar and non-polar organophosphorus pesticides in cow’s milk. RSC Advances, 6(30), 24853–24864. https://doi.org/10.1039/c5ra26742k
Ofrydopoulou, A., Nannou, C., Evgenidou, E., & Lambropoulou, D. (2021). Sample preparation optimization by central composite design for multi class determination of 172 emerging contaminants in wastewaters and tap water using liquid chromatography high-resolution mass spectrometry. Journal of Chromatography A, 1652, 462369. https://doi.org/10.1016/j.chroma.2021.462369
Palma, P., Fialho, S., Lima, A., Catarino, A., Costa, M. J., Barbieri, M. V., Monllor-Alcaraz, L. S., Postigo, C., & de Alda, M. L. (2021). Occurrence and risk assessment of pesticides in a Mediterranean Basin with strong agricultural pressure (Guadiana Basin: Southern of Portugal). Science of the Total Environment, 794, 148703. https://doi.org/10.1016/j.scitotenv.2021.148703
Pápai, K., Budai, M., Ludányi, K., Antal, I., & Klebovich, I. (2010). Analysis of ciprofloxacin in low- and high-fat milk by high-performance liquid chromatography-mass spectrometry. Acta Chromatographica, 22(1), 117–131. https://doi.org/10.1556/AChrom.22.2010.1.9
Parida, V. K., Saidulu, D., Majumder, A., Srivastava, A., Gupta, B., & Gupta, A. K. (2021). Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. Journal of Environmental Chemical Engineering, 9(5), 105966. https://doi.org/10.1016/j.jece.2021.105966
Parolini, M. (2020). Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen towards freshwater invertebrates: A review. Science of the Total Environment, 740, 140043. https://doi.org/10.1016/j.scitotenv.2020.140043
Peleshok, K., Piponski, M., Kovalenko, S., Ahmed, H., Abdel-Megied, A., Ezike, O. F., & Logoyda, L. (2021). New liquid chromatography assays for simultaneous quantification of antihypertensives atenolol and valsartan in their dosage forms. Journal of Separation Science, 44(2), 565–575. https://doi.org/10.1002/jssc.202000859
Pitne, W., Sposoby, I. ŚC., & Usuwania, I. C. H. (2017). FATE AND REMOVAL OF PHARMACEUTICALS AND ILLEGAL DRUGS PRESENT IN DRINKING WATER AND WASTEWATER The most common pharmaceuticals in treated wastewater and potential methods for removal. Ecological Chemistry and Engineering, 24(1), 65–85. https://doi.org/10.1515/eces-2017-0006
Prete, M. C., De Oliveira, F. M., & Tarley, C. R. T. (2017). Assessment on the performance of nano-carbon black as an alternative material for extraction of carbendazim, tebuthiuron, hexazinone, diuron and ametryn. Journal of Environmental Chemical Engineering, 5(1), 93–102. https://doi.org/10.1016/j.jece.2016.11.022
Rao, R., Ku, Y., Tsai, T., Hua, H., Lin, Y., Shiea, J., Huang, P., Andaluri, G., & Kumar, V. (2023). Novel fast pesticides extraction ( FaPEx ) strategy coupled with UHPLC-MS / MS for rapid monitoring of emerging pollutant fipronil and its metabolite in food and environmental samples. Environmental Research, 217, 114823. https://doi.org/10.1016/j.envres.2022.114823
Rasheed, A., Farooq, F., Rafique, U., Nasreen, S., & Aqeel Ashraf, M. (2016). Analysis of sorption efficiency of activated carbon for removal of anthracene and pyrene for wastewater treatment. Desalination and Water Treatment, 57(1), 145–150. https://doi.org/10.1080/19443994.2015.1015304
Rehberger, K., Wernicke von Siebenthal, E., Bailey, C., Bregy, P., Fasel, M., Herzog, E. L., Neumann, S., Schmidt-Posthaus, H., & Segner, H. (2020). Long-term exposure to low 17α-ethinylestradiol (EE2) concentrations disrupts both the reproductive and the immune system of juvenile rainbow trout. Oncorhynchus Mykiss. Environment International, 142, 105836. https://doi.org/10.1016/j.envint.2020.105836
Riva, F., Zuccato, E., Pacciani, C., Colombo, A., & Castiglioni, S. (2021). A multi-residue analytical method for extraction and analysis of pharmaceuticals and other selected emerging contaminants in sewage sludge. Analytical Methods, 13(4), 526–535. https://doi.org/10.1039/d0ay02027c
Ros, O., Vallejo, A., Blanco-Zubiaguirre, L., Olivares, M., Delgado, A., Etxebarria, N., & Prieto, A. (2015). Microextraction with polyethersulfone for bisphenol-A, alkylphenols and hormones determination in water samples by means of gas chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry analysis. Talanta, 134, 247–255. https://doi.org/10.1016/j.talanta.2014.11.015
Ryu, H., Li, B., De Guise, S., McCutcheon, J., & Lei, Y. (2021). Recent progress in the detection of emerging contaminants PFASs. Journal of Hazardous Materials, 408, 124437. https://doi.org/10.1016/j.jhazmat.2020.124437
Saidulu, D., Gupta, B., Kumar, A., & Sarathi, P. (2021). Journal of Environmental Chemical Engineering A review on occurrences, eco-toxic effects, and remediation of emerging contaminants from wastewater : Special emphasis on biological treatment based hybrid systems. Journal of Environmental Chemical Engineering, 9(4), 105282. https://doi.org/10.1016/j.jece.2021.105282
Scheytt, T., Mersmann, P., Lindstädt, R., & Heberer, T. (2005). 1-Octanol/water partition coefficients of 5 pharmaceuticals from human medical care: Carbamazepine, clofibric acid, diclofenac, ibuprofen, and propyphenazone. Water, Air, and Soil Pollution, 165(1–4), 3–11. https://doi.org/10.1007/s11270-005-3539-9
Schmidt, S., Hoffmann, H., Garbe, L. A., & Schneider, R. J. (2018). Liquid chromatography–tandem mass spectrometry detection of diclofenac and related compounds in water samples. Journal of Chromatography A, 1538, 112–116. https://doi.org/10.1016/j.chroma.2018.01.037
Sodré, F. F., Locatelli, M. A. F., & Jardim, W. F. (2010). Occurrence of emerging contaminants in Brazilian drinking waters: A sewage-to-tap issue. Water, Air, and Soil Pollution, 206(1–4), 57–67. https://doi.org/10.1007/s11270-009-0086-9
Sol, D., Menéndez-Manjón, A., Arias-García, P., Laca, A., Laca, A., Rancaño, A., & Díaz, M. (2022). Occurrence of Selected Emerging Contaminants in Southern Europe WWTPs: Comparison of Simulations and Real Data. Processes, 10(12), 1–16. https://doi.org/10.3390/pr10122491
Sui, Q., Cao, X., Lu, S., Zhao, W., Qiu, Z., & Yu, G. (2015). Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerging Contaminants, 1(1), 14–24. https://doi.org/10.1016/j.emcon.2015.07.001
Talja, K. M., Kaukonen, S., Kilpi-Koski, J., Malin, I., Kairesalo, T., Romantschuk, M., Tuominen, J., & Kontro, M. H. (2008). Atrazine and terbutryn degradation in deposits from groundwater environment within the boreal region in Lahti, Finland. Journal of Agricultural and Food Chemistry, 56(24), 11962–11968. https://doi.org/10.1021/jf802528a
Tavengwa, N. T. & Dalu, T. (2022). Introduction to emerging freshwater pollutants. In Emerging Freshwater Pollutants (pp. 1–6). Elsevier. https://doi.org/10.1016/b978-0-12-822850-0.00029-6
Tijani, J. O., Fatoba, O. O., Babajide, O. O., & Petrik, L. F. (2016). Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review. Environmental Chemistry Letters, 14(1), 27–49. https://doi.org/10.1007/s10311-015-0537-z
Valdés, M. E., Marino, D. J., Wunderlin, D. A., & lbert., Somoza, G. M. anue., Ronco, A. E. stel. & Carriquiriborde, P. (2015). Screening concentration of E1, E2 and EE2 in sewage effluents and surface waters of the “Pampas” region and the “Río de la Plata” estuary (Argentina). Bulletin of Environmental Contamination and Toxicology, 94(1), 29–33. https://doi.org/10.1007/s00128-014-1417-0
Vaudreuil, M. A., Vo Duy, S., Munoz, G., & Sauvé, S. (2022). Pharmaceutical pollution of hospital effluents and municipal wastewaters of Eastern Canada. Science of the Total Environment, 846, 157353. https://doi.org/10.1016/j.scitotenv.2022.157353
Vittoria, M., Postigo, C., Guillem-argiles, N., Monllor-alcaraz, L. S., Izabelle, J., Stella, E., Barceló, D., López, M., & Alda, D. (2019). Science of the Total Environment Analysis of 52 pesticides in fresh fi sh muscle by QuEChERS extraction followed by LC-MS / MS determination. Science of the Total Environment, 653, 958–967. https://doi.org/10.1016/j.scitotenv.2018.10.289
Wang, R., Zhang, S., Cai, M., Zhu, J., Xu, Z., Zheng, H., Xiao, K., & Wang, F. (2023). Screening triazine herbicides in drinking water in the Yangtze River Delta, China: Occurrence and health risk. Journal of Hazardous Materials Advances, 10, 100277. https://doi.org/10.1016/j.hazadv.2023.100277
Wang, W., Cao, G., Zhang, J., Qiao, H., Wang, F., & Cai, Z. (2023). Recent applications of mass spectrometry in the analysis of transformation products of emerging contaminants in PM 2.5. Analytical Science Advances, 4(3–4), 49–59. https://doi.org/10.1002/ansa.202200038
Wilkinson, J., Hooda, P. S., Barker, J., Barton, S., & Swinden, J. (2017). Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environmental Pollution, 231, 954–970. https://doi.org/10.1016/j.envpol.2017.08.032
Wu, P. G., Pan, X. D., Ma, B. J., Wang, L. Y., & Zhang, J. (2014). Determination of phthalate esters in non-alcoholic beverages by GC-MS and optimization of the extraction conditions. European Food Research and Technology, 238(4), 607–612. https://doi.org/10.1007/s00217-013-2139-y
Yang, J., Zhao, Y., Li, M., Du, M., Li, X., & Li, Y. (2019). A Review of a Class of Emerging Contaminants: The Classification, Distribution, Intensity of Consumption, Synthesis Routes, Environmental Effects and Expectation of Pollution Abatement to Organophosphate Flame Retardants ( OPFRs ). International Journal of Molecular Sciences, 20(12), 2874. https://doi.org/10.3390/ijms20122874
Yang, G., Fan, M. & Zhang, G. (2014). Emerging contaminants in surface waters in China - A short review. Environmental Research Letters, 9(7). https://doi.org/10.1088/1748-9326/9/7/074018
Zainab, S. M., Junaid, M., Rehman, M. Y. A., Lv, M., Yue, L., Xu, N., & Malik, R. N. (2021). First insight into the occurrence, spatial distribution, sources, and risks assessment of antibiotics in groundwater from major urban-rural settings of Pakistan. Science of the Total Environment, 791, 148298. https://doi.org/10.1016/j.scitotenv.2021.148298
Zhang, R., Du, J., Dong, X., Huang, Y., Xie, H., Chen, J., Li, X., & Kadokami, K. (2021). Occurrence and ecological risks of 156 pharmaceuticals and 296 pesticides in seawater from mariculture areas of Northeast China. Science of the Total Environment, 792, 148375. https://doi.org/10.1016/j.scitotenv.2021.148375
Zind, H., Mondamert, L., Remaury, Q. B., Cleon, A., Leitner, N. K. V., & Labanowski, J. (2021). Occurrence of carbamazepine, diclofenac, and their related metabolites and transformation products in a French aquatic environment and preliminary risk assessment. Water Research, 196, 117052. https://doi.org/10.1016/j.watres.2021.117052
Zou, J., Yao, B., Yan, S., & Song, W. (2022). Determination of trace organic contaminants by a novel mixed-mode online solid-phase extraction coupled to liquid chromatography–tandem mass spectrometry. Environmental Pollution, 303, 119112. https://doi.org/10.1016/j.envpol.2022.119112
Zuo, Y., Zhang, K., & Lin, Y. (2007). Microwave-accelerated derivatization for the simultaneous gas chromatographic-mass spectrometric analysis of natural and synthetic estrogenic steroids. Journal of Chromatography A, 1148(2), 211–218. https://doi.org/10.1016/j.chroma.2007.03.037
Zuo, Y., Zhang, K., & Zhou, S. (2013). Determination of estrogenic steroids and microbial and photochemical degradation of 17α-ethinylestradiol (EE2) in lake surface water, a case study. Environmental Sciences: Processes and Impacts, 15(8), 1529–1535. https://doi.org/10.1039/c3em00239j
Acknowledgements
Authors would like to thank to Fahrettin Duyum and SUMER Laboratory at Gebze Technical University for their support measuring with LC-MS/MS.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work was supported by generous grants from the Scientific and Technological Research Council of Turkey under the TUBITAK 2247-A Fellowship for Outstanding Researchers (no. 120C147). However, all scientific contributions made in this project are owned and approved solely by the author.
Author information
Authors and Affiliations
Contributions
Lama Ramadan: Conception and design, material preparation, data collection, and analysis, original draft manuscript preparation.
Irem Ozturk-Ufuk: Conception and design, material preparation, investigation, manuscript review and editing.
Ebubekir Yuksel: Conception and design, instrumentation, manuscript review and editing.
Emel Topuz: Conception and design, data analysis, supervision, grant owner, manuscript review and editing.
Ebubekir Yüksel. Emel Topuz provided supervision, contributed to writing, conceptualized the research, and conducted the investigation. Lama Ramadan initiated the original draft preparation and was involved in reviewing and editing. İrem Öztürk, H. Cengiz Yatmaz, Ebubekir Yüksel and Emel Topuz contributed to reviewing and editing. Lama Ramadan authored the first manuscript draft, and subsequent versions were collectively reviewed and commented on by all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramadan, L., Ozturk-Ufuk, I., Yuksel, E. et al. Development of LC–MS/MS Method for the Simultaneous Detection of Emerging Contaminants in Aquatic Matrices. Water Air Soil Pollut 235, 537 (2024). https://doi.org/10.1007/s11270-024-07342-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07342-8