Abstract
Mercury (Hg) remediation across contaminated environments in the United States is an ongoing project. As part of the Hg cleanup strategy at East Fork Poplar Creek (EFPC), located in Oak Ridge, TN, the deployment of sorbents is considered. However, the impact of sorbents on soil microorganisms is poorly understood. In this study, we investigated the effect of sorbents on soil microorganism viability and biofilm formation to assess soil health during sorbent application for Hg remediation. We specifically investigated the effect of two engineered sorbents, Organoclay PM-199 and Organoclay MRM (which are manufactured from clay minerals formulated for various remediation applications), on two gram-negative organisms (Serratia marcescens and Burkholderia thailandensis) isolated from the Hg-contaminated EFPC bank soil. Pure cultures of S. marcescens or B. thailandensis were amended with 5% (w/v) and 25% (w/v) PM-199 and MRM, respectively, for 9 days. The samples were harvested, and bacterial cell viability was determined using a BacLight staining kit. Results showed that the growth of sorbent-amended S. marcescens was inhibited in contrast to that of unamended control. Furthermore, biochemical assays were used to analyze bacterial biofilm formation and integral biofilm components. Our results suggest that biofilm formation by sorbent-amended S. marcescens was negatively affected. In contrast, B. thailandensis amended with low concentrations of MRM showed enhanced growth and notable differences in biofilm morphology. These results suggest that the use of organoclay PM-199 and MRM at higher concentrations in field studies may hinder the growth of specific soil microorganisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Globally, mercury (Hg) contamination in the environment is a crucial issue due to its dangerous health impact on humans (Johs et al., 2019; Turner & Southworth, 1999). The Oak Ridge Reservation (ORR), situated in Oak Ridge, TN, USA, is one of the historical sites known for the use of Hg at industrial scales, which led to elevated levels of Hg in air, surface water, soil, and sediments (Brooks & Southworth, 2011). Contamination of the soils and sediments around East Fork Poplar Creek (EFPC) originated from the industrial use of Hg at the Y-12 National Security Complex within the ORR during the 1950s and 60s (Brooks & Southworth, 2011; Stewart et al., 2011). The release of Hg to EFPC is a significant concern in the region because of Hg widespread contamination of the downstream environment, the methylation of Hg to highly neurotoxic methylmercury (MeHg) by specific environmental microorganisms, and bioaccumulation and biomagnification of MeHg in fish (Brooks et al., 2017; Olsen et al., 2016; Parks et al., 2013). Studies have shown elevated levels of inorganic Hg in stream sediments, bank soils, and floodplains of EFPC (Dickson et al., 2019; Peterson et al., 2016; Watson et al., 2016) and MeHg levels exceeding the EPA’s regulatory limit of 0.3 mg/kg in fish tissues (EPA, 2001). As a result of environmental influences such as storms and erosion, a significant amount of Hg and MeHg is leached into the EFPC ecosystem annually (Brooks et al., 2017; Peterson et al., 2016). Remediation efforts, such as dechlorinating liquid waste and sewages introduced into EFPC and the purification of sump waters, have significantly reduced waterborne Hg concentrations (Southworth et al., 2000; Turner & Southworth, 1999). However, the levels of Hg and MeHg in fish are above the regulatory limits, leading to fish consumption advisories for EFPC (EPA, 2001; Peterson et al., 2016; Southworth et al., 2000).
One of the proposed cleanup strategies considered in EFPC is sorbent application. Sorbents are insoluble materials that immobilize contaminants by absorption or adsorption mechanisms (Chen et al., 1999; Ghosh et al., 2011). The effectiveness of a sorbent depends on its physical and chemical characteristics, which include surface area, pore size, and chemical interactions with the contaminant (He et al., 2015; Sarkar et al., 2010; Stathi et al., 2008). Among several sorbent materials proposed for Hg remediation in EFPC, biochar and activated carbon have been evaluated in laboratory studies with promising results (Goñez-Rodríguez et al., 2021; Johs et al., 2019). In situ studies involving activated carbon suggest effectiveness in Hg and MeHg absorption because of their competitive binding capacity in the presence of sediments (Gilmour et al., 2013, 2018). Related studies on the effectiveness of granular activated carbon (GAC) for the in-situ remediation of polychlorinated biphenyl (PCB) reduced contaminant levels while supporting biofilm formation through attachment to bacteria (Mercier et al., 2013). Other sorbents suggested for Hg cleanup are phyllosilicate clays modified with organic cations (Awad et al., 2019; Lee et al., 2004), such as Organoclay PM-199 and Organoclay MRM (CETCO, Hoffman Estates, IL, USA). Their chemical and physical properties have made them an option for remediating heavy metals such as Hg (Sarkar et al., 2010; Stathi et al., 2008). Previous studies suggest that Organoclays may be advantageous over activated carbon in Hg removal because of the positive charge on its surface that promotes hetero-aggregation with Hg species (Gai et al., 2019).
Organoclays PM-199 and MRM are modified small pore-sized clay sorbents with sorption characteristics. However, the sorption capability of sorbents may be inhibited due to blockage of their pores resulting from interactions with bacteria or natural organic matter (Fairey et al., 2010). Previous sediment studies suggested that Organoclay MRM may stimulate Hg methylation by sulfate-reducing bacteria (Gilmour et al., 2013; Paulson et al., 2018). Most recent studies in our laboratory investigating the microbial impact on sorbent’s effectiveness and efficiency suggest that PM-199 can immobilize Hg in the presence of two model microorganisms, Serratia marcescens and Burkholderia thailandensis (Egbo et al., 2021). These biofilm-forming, gram-negative microbes were shown to be relatively abundant in Hg-contaminated EFPC bank soil (Egbo et al., 2017). When incubated with these microbes, the sorption efficiency of PM-199 for Hg (II) can be attributed to the void volume fraction and accessible surface area (Egbo et al., 2021). Competitive binding studies aimed at elucidating Hg (II) absorption by PM-199 in the presence or absence of B. thailandensis showed no significant differences (Egbo et al., 2021). S. marcescens and B. thailandensis are facultative anaerobes intrinsic to soil and survive in harsh or contaminated environments by biofilm formation (Rice et al., 2005; Tseng et al., 2016; Vitale et al., 2020). Both microorganisms use quorum sensing to trigger biofilm formation of extracellular matrix, which serves as an essential protective layer for survival in a natural environment (Egbo et al., 2021; Fazli et al., 2014; Fekrirad et al., 2020; Tseng et al., 2016). S. marcescens grows on media in aerobic or anaerobic conditions, utilizing several compounds as a single carbon source, with optimum growth temperature ranging from 20 °C to 37 °C (Giri et al., 2004). B. thailandensis grows at optimum temperatures of 25 °C to 37 °C in aerobic and anaerobic (can use two or more compounds as a carbon source) (Wagley et al., 2014). The bacterium exhibits a competitive edge over other environmental microorganisms due to its genomic assembly (Ngamdee et al., 2015).
While the sorption effectiveness of PM-199 and MRM in immobilizing Hg has been characterized (Egbo et al., 2021). Their toxicity on soil microorganisms is yet to be determined. Considering the significant role of microbes in nutrient cycling and conserving soil properties, it is paramount to introduce nontoxic sorbents into the environment. We hypothesize that the substances leaching from sorbents posses harmful effects on the indigenous soil microorganisms. This study’s premise is based on our preliminary study that evaluated the efficiency of organoclays in Hg immobilization. Due to the imminent direct application of these sorbents in the environment, it is crucial to investigate their eco-friendliness and understand their impact on soil microorganisms. Therefore, this present study aims to investigate the effect of Organoclay amendments on soil microorganisms in batch experiments. Specifically, we evaluated the effect of organoclay PM-199 and MRM on the viability of soil bacteria (S. marcescens and B. thailandensis). In addition, we assessed the role of the sorbents on the ability of bacteria to form biofilms, considering that the bacterial strains selected for this study can resist adverse conditions by forming biofilms. Further studies examined the molecular composition of biofilms and how sorbent amendments affect biofilm structure. We also considered the interaction between the sorbents and bacteria by evaluating the morphological characteristics of the sorbents post-amendment. This study endeavors to elucidate the eco-friendliness of the sorbents via the evaluation of microbial growth, survival, and biofilm composition.
2 Materials and Methods
2.1 Bacteria Culture
Serratia marcescens and Burkholderia thailandensis, two relatively abundant microorganisms in EFPC, were previously isolated and identified in our laboratory (Egbo et al., 2021). The bacteria were grown on Nutrient Agar No. 2 (Sigma-Aldrich, St. Louis, MO, USA) for 24 h at 25 °C (S. marcescens) or 30 °C (B. thailandensis). Single bacterial colonies were transferred to 500 mL of nutrient broth supplemented with a pancreatic digest of gelatin and beef extract (Carolina Biological Supply, Burlington, NC, USA), and incubated at 25 °C (S. marcescens) or 30 °C (B. thailandensis) with shaking (180 rpm). The bacterial cultures were grown to 0.5 (OD600) before amendment with the sorbents. For all biofilm assays, sorbent-amended S. marcescens and B. thailandensis cells were cultured in tryptic soy broth (TSB) and Luria–Bertani (LB) broth, respectively, and adjusted to 0.5 (OD600) before incubation.
2.2 Incubation with Organoclays PM-199 and MRM
To determine the impact of the sorbents on S. marcescens and B. thailandensis, in replicates of four, 25 mL of both bacteria cultures were amended with 1.25 g (5% w/v) and 6.25 g (25% w/v) of PM-199, and 1.25 g (5% w/v) and 6.25 g (25% w/v) of MRM (CETCO, Hoffman Estates, IL, USA), respectively. The samples were incubated at 25 °C (S. marcescens) or 30 °C (B. thailandensis) in an orbital shaker (180 rpm) for a 9-day period. Unamended bacteria cultures were used as a control. The OD600 of each sample was recorded every 24 h. At the endpoint, the samples were harvested, and the pH of all samples was recorded before further analysis. S. marcescens and B. thailandensis experiments were conducted independently.
2.3 Bacterial Purification, Preparation, and Staining for Fluorescence Microscopy
To remove all traces of the media components from the 9-day incubation samples, the bacterial cells were prepared according to the BacLight Bacterial Viability protocol (Invitrogen, 2004), with slight modifications. Briefly, bacterial pellets were obtained by centrifugation at 4200 rpm for 20 min, and the supernatants were discarded. The pellets were resuspended in 2 mL of 0.85% NaCl solution. Then, 1 mL of each suspension was added to a 50 mL polypropylene sterile conical tube filled with 20 mL of 0.85% NaCl solution. The suspensions were stored at room temperature for 1 h and mixed thoroughly every 15 min. The suspensions were centrifuged twice at 4200 rpm for 20 min. Finally, the resulting pellets were resuspended in 10 mL of 0.85% NaCl.
The live and dead cells staining was performed utilizing the LIVE/DEAD BacLight Bacterial Viability kit (ThermoFisher Scientific, Waltham, MA) with slight modification to the manufacturer's protocol. An equal volume of SYTO 9 and propidium iodide dyes was mixed in a 1.5 mL tube, and 3 mL of the mix was added to 1 mL of each resuspended pellet. The solutions were kept in the dark for 15 min at ambient temperature. Bacterial fixation was performed by centrifuging 900 µL of the solutions at 12,000 rpm for two minutes. The pellets were resuspended with 500 µL of 2% paraformaldehyde and centrifuged at 12,000 rpm for 2 min. The pellets were resuspended with 150 µL of nuclease-free water, and 5 µL was trapped between a slide and coverslip. The live and dead bacterial cell images were captured using a fluorescent microscope (Nikon Eclipse Ti fluorescence microscope, Nikon Instruments Inc., Melville, NY, USA). Cells stained green (intact cell membranes) and red (damaged cell membranes) were quantified using ImageJ, a product of the National Institutes of Health (NIH). The percent viable cells were calculated as previously described (Noshadi et al., 2017), using the following formula:
A comparison between the viable cells in unamended control and sorbent-amended samples was calculated by the following formula:
2.4 Bacteria Viability by Plate Count Method
Bacterial viability via plate count method was executed through a sequential series of eightfold dilutions as previously described (Reynolds, 2005). Briefly, 1 mL of each sample (after the 9-day incubation period) was aseptically transferred into sterile test tubes filled with 9 mL of 0.9% saline solution. Subsequently, 100 µL from each sterile tube was spread onto an agar plate and incubated for 24 h. The plates with countable colonies ranging from 25–250 were counted, recorded, and presented as. Colony-forming units per milliliter (CFU/mL) for each sample was calculated by the following formula:
2.5 Biofilm Formation Assay
The biofilm formation of bacteria was assessed using spectrophotometry analysis, as previously described (Fekrirad et al., 2020; Lemos et al., 2010; O’Toole, 2011; Okaro et al., 2021), with slight modification. Briefly, 1 mL of unamended control and sorbent-amended samples were transferred to sterile 15 mL polypropylene culture tubes (ThermoFisher Scientific, Waltham, MA, USA) containing 10 mL growth medium. TSB and LB broth were used for S. marcescens and B. thailandensis, respectively. The culture tubes were stored overnight in a shaker incubator at 37 °C, 200 rpm. Cultures were then diluted (1:100), and three replicates of 200 µL per sample were cultured in 96-well polystyrene plates (Corning Incorporated, Corning, NY, USA) for 3 days at 37 °C. The plates were gently dabbed on paper towels to discard spent media, and the wells were washed with 200 µL 1 × PBS twice to remove planktonic cells. Crystal violet (CV) staining assay was performed by treating the wells with 150µL of 0.1% CV solution for 15 min. Plates were rewashed with 1 × PBS and air-dried inside a biosafety cabinet. To quantify the biofilms, wells were further treated with 200 µL of 30% acetic acid, and the absorbance was measured at 550 nm using SpectraMax Abs Plus (Molecular Devices, San Jose, CA, USA).
2.6 Biofilm Growth and Extraction
The essential biofilm components of bacteria were analyzed as previously described (Okaro et al., 2021), with few modifications. Briefly, single colonies of unamended and sorbent-amended S. marcescens and B. thailandensis cells were cultured in 5 mL TSB and LB broth overnight, respectively. The overnight cultures were diluted 1:100 in TSB and LB broth, respectively, and grown to 0.5 (OD600). Samples were further diluted at 1:100 in fresh broth, and 1 mL of each sample was transferred to 24-well plates and incubated for 3 days at 37 °C with no agitation. After incubation, the spent media were discarded, and the wells were filled with 1 mL of 1 × PBS. Biofilms attached to wells were gently detached into the PBS and transferred to 1.5 mL Eppendorf tubes before centrifugation at 3700 rpm for 10 min. Biofilm components were extracted as previously described by Keithley and Kirisits (2018) and Okaro et al. (2021). Briefly, supernatants were gently discarded, and pellets were resuspended in 1.5 mL TE buffer (Integrated DNA Technologies) for 4 h in a rotating incubator at 35 °C, 200 rpm. The cells were pelleted at 4 °C for 10 min at 13,000 rpm, and the extractants were filtered through a 0.45 µm sterile Acrodisc (ThermoFisher Scientific, Waltham, MA). The extractants were used for subsequent analysis.
2.7 Protein Assay
Protein concentrations of the extracted biofilms were evaluated using the Pierce Bicinchoninic Acid (BCA) protein assay (ThermoFisher Scientific, Waltham, MA, USA). BCA reagent A and reagent B were mixed in a 50:1 ratio to form a solution. In accordance with the manufacturer's protocol, 25 µL of each extractant was combined with the BCA mixture (200 µL) in a 96-well plate and incubated for 30 min at 37 °C. The absorbance was measured at 562 nm using SpectraMax Abs Plus (Molecular Devices, San Jose, CA, USA). All samples were analyzed in triplicate.
2.8 Extracellular Polysaccharide Concentrations
The widely used phenol–sulfuric method was adopted to quantify extracellular polysaccharides in extracted biofilms, as previously described by Keithley and Kirisits (2018). Briefly, each extractant (150 µL) and glucose standards (0/0.2/0.4/0.6/0.8 mg/mL) were added to a glass tube containing a mixture of sulfuric acid (450 µL) and 5% phenol (90 µL). After a gentle swirling, the tubes were incubated in a 90 °C water bath for 10 min and then allowed to cool down. In replicates of three, 200 µL of solution from each test tube was transferred to a 96-well plate, and absorbance was measured at 490 nm using SpectraMax Abs Plus (Molecular Devices, San Jose, CA, USA).
2.9 Extracellular DNA (eDNA) Concentration
The eDNA in the biofilms were quantified using the propidium iodide (PI) method previously described (Okaro et al., 2021). Briefly, in replicates of three, 175 µL of 0.1% PI solution (prepared in ultra-distilled water) was mixed with 25 µL of extractant in a 96-well plate. Two hundred microliters of PI was used as a blank. The plates were immediately incubated in the dark room for 10 min, with gentle shaking every 2 min. The plates were read using a BioTek Cytation 3 image reader (Agilent Technologies, Santa Clara, CA, USA) with an excitation at 535 nm, and the fluorescence emission recorded at 620 nm.
2.10 Biofilm Morphology
Biofilm morphology of untreated and sorbent-treated S. marcescens and B. thailandensis samples were analyzed by growing biofilms in 24-well plates as previously described (Fekrirad et al., 2020). The biofilms were gently detached and transferred to microscope slides, then treated with 2.5% glutaraldehyde for 2 h and washed with distilled water. The biofilms were further dehydrated with 50% ethanol and then air-dried. The dried biofilms were placed on a metal stud and gold-coated before inspection under a scanning electron microscope (Phenom XL G2 Desktop SEM, ThermoFisher Scientific).
2.11 Structural Evaluation of Organoclay PM-199 and MRM Post-Amendment
Structural analysis of sorbent was conducted as previously described (Egbo et al., 2021).
Briefly, after the 9-day incubation period, 0.1 g of PM-199 and MRM were retrieved from all sorbent-amended samples. The control was 5% of each sorbent (PM-199 and MRM) in 25 mL nutrient broth incubated during the 9-day period. Retrieved sorbents were transferred to 2 mL Eppendorf tubes and washed three times with 1 × PBS by centrifugation (3500 rpm, 30 s). The sorbents were transferred to a microscopic slide, then treated with 2% paraformaldehyde, and air-dried. The prepared sorbents were gold coated on a metal stud and examined under a scanning electron microscope (Phenom XL G2 Desktop SEM, ThermoFisher Scientific).
2.12 Statistical Analysis
All the obtained values were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test using GraphPad Prism Software version 10.1 (GraphPad Software, San Diego, CA, USA). Statistical significance was considered at p < 0.05.
3 Results
3.1 Growth of S. marcescens and B. thailandensis
We monitored the growth of unamended and sorbent-amended S. marcescens and B. thailandensis samples by measuring the absorbance at OD600 every 24 h during the 9-day incubation period. The growth curve of S. marcescens shows that there is no difference between the growth pattern of 5% PM-199 (P5) and 5% MRM (M5) treated S. marcescens compared with that of untreated samples (Fig. 1a). But when S. marcescens was amended with 25% PM-199 (P25) and 25% MRM (M25), the growth curve showed a decreased trend (Fig. 1a). The growth curve of B. thailandensis indicated a similar growth trajectory among the 5% PM-199, 5%, and 25% MRM (P5, P25 and M25) amended samples when compared with the nontreated samples (Fig. 1b). The growth pattern of 5% MRM (M5) amended samples showed a difference in growth trajectory compared to the other amended samples (P5, P25 and M25 (Fig. 1b).
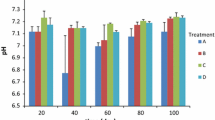
We examined the pH of S. marcescens and B. thailandensis post-treatment to establish the role of pH in bacterial growth. The unamended S. marcescens samples (Control) had an average pH of 9.4. In contrast, sorbent-treated samples (P5, P25, M5, and M25) indicated an average pH of 9.1, 8.9, 8.2, and 7.4, respectively (Fig. 2a). Compared with the control, we noticed a decrease in pH with M5 and M25 by 1.2 and 2.0, respectively. The pH of B. thailandensis samples was also observed post-treatment. The unamended B. thailandensis samples showed an average pH of 9.0. In contrast, the sorbent-treated samples (P5, P25, M5, and M25) displayed average pH values of 8.9, 8.6, 7.3, and 6.4, respectively (Fig. 2b). In comparison with the unamended control, we observed a decrease in pH with M5 and M25 by 1.7 and 2.6 respectively (Fig. 2b).
3.2 Viability of S. marcescens and B. thailandensis in the Presence of PM-199 and MRM
S. marcescens and B. thailandensis were ideal for this study because of their relative abundance among microbes isolated from the EFPC soil. S. marcescens amended with 5% PM-199 (P5) and MRM (M5) showed no difference in the number of live cells when compared with the control (Fig. 3a; and supplemental material Fig. S1 for a complete panel showing live, dead, and merged images). However, visual analysis of the fluorescent micrographs indicated a reduction in the population of live cells at 25% PM-199 (P25) and 25% MRM (M25) compared to the unamended control (Fig. 3a). Using ImageJ, the live and dead cells (green and red) were counted with a threshold setting of 7- infinity (this is to accurately count the precise number of green and red dots across all samples). The viable cells among the control and sorbent-amended samples were compared. We did not notice a significant difference between the control, P5, and M5 (Fig. 3b). However, P25 and M25 exhibited 53% and 46% lower cell viability compared to the unamended control (p < 0.001) (Fig. 3b). Also, P25 and M25 indicated a significant decrease in percentage viability compared to P5 and M5 (p < 0.01) (Fig. 3b).
Live/dead staining analyzed with fluorescence microscopy (a) S. marcescens merged (live/dead) cells (b) percentage viability of S. marcescens. (c) B. thailandensis merged (live/dead) cells (d) percentage viability of B. thailandensis. Scale bars: 100 µm. Each bar represents the mean ± standard deviation of four replicate samples. Significance between groups was established at **p < 0.01, ***p < 0.001, and ****p < 0.0001
The fluorescence micrograph of B. thailandensis treated with 5% PM-199 (P5), 25% PM-199 (P25), and 25% MRM (M25) showed no difference between viable cells in comparison with the unamended control. However, 5% MRM (M5) indicated more viable cells compared to the unamended control (Fig. 3c; and supplemental material Fig. S2 for a complete panel showing live, dead, and merged images). The percentage of viable cells in the unamended control was compared with sorbent-amended samples. P5, P25, and M25 showed no significant difference in comparison to the unamended control (Fig. 3d). However, M5 showed a highly significant increase in live cells compared to the control (p < 0.0001) (Fig. 3d). It is important to note that there were fewer dead cells (red) among the sorbent-amended samples when compared to the control. This indicates that the higher concentrations of sorbents did not induce bacterial cell death but caused growth inhibition, especially in S. marcescens.
3.3 Viability of Bacteria by Colony Count Method
We conducted a series of eightfold dilutions at the 9-day endpoint to corroborate our viability results. For S. marcescens samples, the control, 5% PM-199 and 5% MRM plates, were countable at 106, 25% PM-199 and MRM were countable at 105 (Fig. S3 supplemental material). The data sets displayed on a chart indicate that there is no significant difference between the control, P5, and M5 (Fig. 4a; and Table S1 supplemental material). However, the control plate had 15 and 5 times more colonies in contrast to P25 and M25, respectively (p < 0.000) (Fig. 4a).
For our B. thailandensis samples, the control plate, 5% PM-199, 25% PM-199, and 25% MRM were countable at 105, except 5% MRM at 10–6 (Fig. S4 supplemental material). The data sets on the chart indicate that there is no significant difference between the control, P5, P25, and M25 (Fig. 4b; and Table S2 supplemental material). Whereas M5 had seven times more countable colonies than any other sample, including the control (p < 0.0001), (Fig. 4b).
3.4 Biofilm Formation
S. marcescens and B. thailandensis are soil microorganisms known to thrive in harsh conditions through biofilm formation (Fazli et al., 2014; Tseng et al., 2016). Hence, we assessed the impact of Organoclay PM-199, and MRM on the ability of the microbes to form biofilms (see Fig. S5 and S6 in the supplemental material showing CV-stained biofilms). As shown in Fig. 5a, the biofilms of sorbent-amended S. marcescens (P5, P25, M5, M25) are significantly decreased (p < 0.05) compared to the unamended control. B. thailandensis amended with PM-199 and MRM did not inhibit the biofilm formation of the bacterium (Fig. 5b). We observed that each species formed a surface adherent biofilm irrespective of sorbent amendments.
3.5 Protein Quantification
Proteins involved in biofilm formation were analyzed in unamended and sorbent-amended bacteria. To quantify the total protein content of biofilms, we utilized BCA assays. As shown in Fig. 6a, 5% PM-199 (P5) and MRM (M5) amended S. marcescens and unamended bacterium (control) have similar quantities of proteins. However, protein concentration was slightly higher in P25 and M25 compared to unamended control (p < 0.05, p < 0.01) (Fig. 6a). As exhibited in Fig. 6b, there was no significant difference between protein concentrations of sorbent-amended B. thailandensis (P5, P25, M5, and M25) and untreated B. thailandensis (control).
Protein quantification by BCA (a) S. marcescens protein concentration (b) B. thailandensis protein concentration. Experiments were repeated at least three times. Each bar represents the mean ± standard deviation of four replicate samples. Significance between groups was established at *p < 0.05 and **p < 0.01
3.6 Extracellular Polysaccharide (EPS) Concentrations
EPS concentrations in S. marcescens and B. thailandensis were quantified using the phenol–sulfuric method. As shown in Fig. 7a, there is a significant difference between sorbent-treated S. marcescens (P5, P25, M5, and M25) compared to untreated S. marcescens (control). The sorbent-amended samples did not produce enough EPS for biofilm formation. Sorbent-treated B. thailandensis samples (P5, P25, M5, and M25) did not show significant differences in EPS concentration compared to the control (Fig. 7b). However, we observed slightly high levels of EPS in 5% MRM treated B. thailandensis (M5).
3.7 Extracellular DNA (eDNA) Concentration
We quantified the biofilm eDNA expressed by treated and untreated bacteria. Results indicate that biofilms formed by sorbent-amended and unamended S. marcescens samples expressed similar concentrations of eDNA (Fig. 8a). Likewise, biofilms formed by sorbent-amended and unamended B. thailandensis samples showed no significant difference in eDNA expression (Fig. 8b).
Figure 8 eDNA concentrations quantified after a 3-day incubation period (a) S. marcescens (b) B. thailandensis. Experiments were repeated at least three times. Each bar represents the mean ± standard deviation of four replicate samples.
3.8 Biofilm Morphology by Scanning Electron Microscopy (SEM)
The structural arrangement of the biofilm formed by sorbent-treated and untreated bacteria was observed under SEM. As shown in Fig. 9, biofilms of S. marcescens treated with PM-199 at 5% and 25% (9b and 9c) appear disorganized on the surface and are slightly porous compared to biofilms formed by untreated bacteria (9a). The architectural appearance of the biofilm matrix formed by MRM-amended S. marcescens (9d and 9e) displayed irregularities on their surface compared to the untreated control (Fig. 9).
Biofilms formed by B. thailandensis treated with PM-199 at 5% (10b) appear to have similar surface characteristics, but those treated with 25% of PM-199 (10c) display slightly porous matrix assemblage on the surface compared to the untreated bacterium (10a). However, the surface of biofilms formed by samples treated with MRM at 5% and 25% (10d and 10e) appears compact but displays roughness on the surfaces (Fig. 10).
3.9 Effect of Microbial Interactions on the Morphology of PM-199 and MRM
The morphology of PM-199 and MRM post-incubation with S. marcescens and B. thailandensis was examined to understand bacteria-sorbent interaction. SEM micrograph indicates that PM-199 incubated with S. marcescens (11b and 11c) shows dissociation compared to the control (11a) (Fig. 11). Surface of 25% PM-199 (11c) indicates the presence of external attachment from the bacterium establishing sorbent-bacteria interaction (Fig. 11). MRM (11e and 11f) displays dissociation and irregular surface in comparison to control (11d) in the absence of bacteria (Fig. 11).
SEM micrograph of sorbents after 9 days of incubation (a) 5% PM-199 incubated with media (control) (b) 5% PM-199 incubated with S. marcescens (c) 25% PM-199 incubated with S. marcescens (d) 5% MRM incubated with media (control) (e) 5% MRM incubated with S. marcescens (f) 25% MRM incubated with S. marcescens. Scale bar: 10 µm
PM-199 incubated with B. thailandensis (12b and 12c) showed notable dissociation and external attachments by the bacteria compared to the control (12a) (Fig. 12). Whereas MRM incubated with the bacterium (12e and 12f) displayed particle dissociation and uneven surface in comparison to the control (12d) (Fig. 12).
SEM micrograph of sorbents after 9 days of incubation (a) 5% PM-199 incubated with media (control) (b) 5% PM-199 incubated with B. thailandensis (c) 25% PM-199 incubated with B. thailandensis (d) 5% MRM incubated with media (control) (e) 5% MRM incubated with B. thailandensis (f) 25% MRM incubated with B. thailandensis. Scale bar: 10 µm
4 Discussion
This study aimed to assess the potential effect of sorbents, organoclay PM-199, and MRM on selected soil microorganisms isolated from Hg-contaminated soil. Engineered sorbents are proposed as a component of Hg remediation strategies for contaminated soils. Deploying effective, efficient, and eco-friendly sorbents in field applications is paramount. Hence, there is a need to assess sorbent toxicity before introducing such amendments into the environment. In situ studies have shown that activated carbon and biochar have a low toxic effect on Lumbriculus variegatus, an aquatic organism (Gilmour et al., 2013). However, the toxicity of PM-199 and MRM on soil microorganisms has not been critically evaluated. PM-199 and MRM are organocation-modified small-grain phyllosilicate-based sorbent media with tunable sorption properties (Chen et al., 1999; Egbo et al., 2021; Johs et al., 2019). Studies suggest that the absorption properties of sorbents may be compromised as their interaction with bacteria or natural organic matter (NOM) may result in sorbent pore blockage (Fairey et al., 2010). Sulfur-containing sorbents are suspected to affect sulfate-reducing bacteria promoting Hg methylation (Gilmour et al., 2013; Johs et al., 2019; Paulson et al., 2018). More recent studies in our laboratory investigating the microbial impact on sorbent’s effectiveness and efficiency suggest that PM-199 can immobilize Hg in the presence of S. marcescens and B. thailandensis (Egbo et al., 2021). These biofilm-forming Gram-negative microbes are among the relatively abundant species isolated from Hg-contaminated EFPC bank soils.
For this study, the viability of S. marcescens and B. thailandensis in the presence of PM-199 and MRM was examined by laboratory incubations. Fluorescence microscopy images showed no significant difference in viable cells among 5% PM-199 and MRM-amended S. marcescens, compared to unamended control. We observed that sorbent incubation at lower concentrations has no impact on bacteria growth. However, we noticed a reduction in the number of viable cells among S. marcescens samples amended with 25% PM-199 and 25% MRM, respectively (Fig. 3b). Analysis of the micrograph images indicates that amendment of the strain with 25% PM-199, and 25% MRM decreased the viable cells by 53% and 46%, respectively. We suspect that higher concentrations of sorbents provide minerals above the tolerance level for the bacteria in the solution, leading to growth inhibition. B. thailandensis interaction with PM-199 and MRM amendments showed that the growth of the bacterium was sustained. MRM enhanced the bacterium growth, but we observed a decrease in the growth when the concentration of the sorbent is increased to 25% (Fig. 3d). These results suggest that B. thailandensis may harness the minerals such as sulfur and iron in MRM as a nutrient. These results are consistent with the findings of Gilmour et al. (2013). Amendment with higher concentrations of MRM may suggest microbial growth inhibition as we see a decrease in viable cells when MRM is increased from 5 to 25% (Fig. 3d). Likewise, we observed a drop in pH from 7.3 to 6.4 among B. thailandensis samples incubated with 5% and 25% MRM.
In addition, the plate count method was used to further examine the viability of S. marcescens in the presence of PM-199 and MRM. The unamended control plates (containing 1.1 × 109 CFU/mL) had 15 times more colonies than 25% PM-199 (containing 7.4 × 107 CFU/mL) and five times more colonies than 25% MRM (containing 2.0 × 108 CFU/mL) (Fig. 4a; and Table S1 supplemental material). As the concentrations of the sorbents were increased, we noticed a reduction in the number of viable cells. A higher concentration of MRM decreased the pH of amended samples. However, pH may not have influenced the growth of S. marcescens because we observed that the average pH of the control and 5% MRM was 9.3 and 8.2, respectively. However, there was no considerable difference in viable cell counts when compared to each other. Likewise, 25% PM-199 had an average pH of 9.0, but there was a significant difference in viability compared to the control (Fig. 4a).
The B. thailandensis viability assessment by plate count method indicated that the control, 5% PM-199, 25% PM-199, and 25% MRM samples contained relatively the same number of viable cells (Fig. 4b) Nevertheless, we observed that sorbent-amended samples had more countable colonies in comparison to the control (Table S2 supplemental material), suggesting that sorbent amendments did not impede bacteria growth. Compared to the control, 5% MRM samples (4.5 × 108 CFU/mL) had seven times more countable colonies. These results suggest that the sorbents, especially MRM, are being utilized as growth nutrients by B. thailandensis. We observed that 5% MRM amendments adjusted the pH to an average value of 7.1, which was more favorable for bacteria growth, but at a higher concentration of MRM (25%), we saw a decline in pH (6.4), which ultimately resulted to a decrease in countable colonies (Fig. 4b). B. thailandensis likely harnessed the minerals present in MRM (Gilmour et al., 2013) as nutrients for growth in moderate proportion, but higher concentrations led to a decline in viable cells. Gilmour et al. (2013) reported that organoclay MRM, specifically infused with sulfur, demonstrated low toxicity in aquatic organisms. Similarly, we observed that MRM amendment at 5 and 25% displayed no toxicity toward B. thailandensis, but a significant decrease in the number of viable cells was observed at higher concentrations. The decrease in cell viability observed among 25% sorbent amended samples could be attributed to the toxicity level of the sorbents at an increased concentration. The difference in viability between S. marcesens and B. thailandensis could also be attributed to their cellular composition and the ability of the latter to withstand the toxicity level of the sorbents.
S. marcescens and B. thailandensis form biofilm to thrive in adverse conditions such as Hg-contaminated environments (Harimawan et al., 2017; Rice et al., 2005; Tseng et al., 2016). These Gram-negative bacteria form surface adherent biofilms during stress or nutrient-limiting conditions (Fekrirad et al., 2020; Okaro et al., 2021). Bacterial biofilms consist of proteins, eDNA, and extracellular polysaccharides necessary for biofilm structure (Karygianni et al., 2020; Mirzaei & Ranjbar, 2022; Tang et al., 2013). Therefore, we examined the impact of organoclay PM-199 and MRM on the ability of these bacteria to form biofilms. We observed that the capability of S. marcescens to form biofilms when incubated with higher concentrations of PM-199 and MRM is significantly decreased (Fig. 5a). However, sorbents did not entirely inhibit biofilm formation. The quantification of biofilm components (proteins, eDNA, and EPS) by molecular assays showed that 25% PM-199 and 25% MRM incubated samples indicated higher protein concentrations than the control (Fig. 6a). Slightly higher protein concentrations in sorbent-amended samples may indicate robust biofilm formation when other biofilm components, such as EPS, are reduced under these conditions (Okaro et al., 2021).
eDNA concentrations were similar across all samples. However, EPS concentrations were significantly lower in sorbent-treated samples (Fig. 7a). Differences in biofilm structure can be attributed to low EPS concentrations because of their role in biofilm formation (Karygianni et al., 2020; Mirzaei & Ranjbar, 2022). Due to low EPS concentrations, the biofilm structure of PM-199-treated samples appeared different from the control. Irregularity and disorganization of the biofilm surface indicated a stress response (Fig. 9). Similarly, the structure of biofilm formed by MRM-treated samples seemed rough and clustered, suggesting that low expression of EPS induced morphological changes in biofilms.
The capability of B. thailandensis to form biofilms when incubated with sorbents was examined. Both the control and sorbent-amended samples displayed similar quantities of biofilm (Fig. 5b), suggesting that biofilm formation by B. thailandensis was not inhibited by sorbent amendments. Furthermore, biofilm components quantified by molecular assays showed that proteins, eDNA, and EPS concentrations were all similar across all samples. Morphological assessment of biofilm by SEM showed that biofilms formed by PM-199-incubated B. thailandensis displayed similarities to the control (10a, 10b, and 10c). However, biofilms formed by 5% MRM-treated samples appeared more compact, and those from 25% MRM-treated samples displayed roughness on the surface (10d and 10e). The compactness may be due to slightly higher levels of EPS in 5% MRM samples compared to any other sample (Fig. 7b). Increased EPS levels may be due to the bacteria responding to stress induced by MRM. Hence, structural changes in a biofilm were observed. These results align with that of Tseng et al., 2016 who suggested that overproduction of EPS can lead to structural changes in biofilm.
The morphology of the sorbents post-incubation was examined to understand the interaction of the bacteria with the sorbents. Our results indicate that the interaction of S. marcescens with PM-199 and MRM led to particle dissociation from the sorbent compared to media-amended sorbents (Fig. 11). The bacteria may have initiated the dissociation of sorbent particles, thereby acquiring nutrients, but in the presence of 25% sorbents, excessive nutrients suppress bacterial growth. Similarly, the interaction of B. thailandensis with PM-199 and MRM observed through SEM analysis showed that the sorbents appeared dissociated and irregular near the surface compared to media-incubated sorbents (Fig. 12). PM-199 incubated with bacteria appeared to have external attachments resulting from sorbent-bacteria interaction. These results indicate that B. thailandensis may promote the dissociation of mineral particles from the sorbent surface to harness the minerals present in PM-199 and MRM as nutrients for growth.
5 Conclusion
Organoclay PM-199 and MRM are sorbents that can potentially be used in field applications for Hg remediation. However, sorbents for remediation applications in soil are expected to be effective and non-detrimental to human and soil health. Considering the crucial role of microbes in soil health and nutrient cycling it is paramount to assess the toxicity and eco-friendliness of the sorbents toward soil microorganisms. In this study, we investigated the viability of soil microbes, S. marcescens and B. thailandensis, in the presence of Organoclay PM-199 and MRM, respectively. Sorbent amendments at increased concentrations inhibited the growth of S. marcescens. In addition, a decreased biofilm formation was observed, including induced structural changes in bacterial biofilms.
Unlike S. marcescens, B. thailandensis displayed enhanced growth when amended with sorbents, especially Organoclay MRM. In this study, we have been able to show that specific soil microorganisms can thrive in the presence of Organoclay PM-199 and MRM in reduced concentrations; however, in higher concentrations, bacteria growth may be hindered. Our results further indicate that several soil microbes may respond to sorbent amendment differently; therefore, long-term batch experiments involving the total microbial community need to be conducted to establish the toxicity and eco-friendliness of PM-199 and MRM. Also, such experiments will address the long-term effectiveness of the sorbents in absorbing Hg in a field setting because of the potential of bacteria dissociating the sorbents during bacteria-sorbent interactions.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Awad, A., Shaikh, S., Jalab, R., Gulied, M., Nasser, M., Benamor, A., & Adham, S. (2019). Adsorption of Organic Pollutants by Natural and Modified Clays: A Comprehensive Review. Separation and Purification Technology, 228, 115719. https://doi.org/10.1016/j.seppur.2019.115719
Brooks, S. C., & Southworth, G. R. (2011). History of mercury use and environmental contamination at the Oak Ridge Y-12 Plant. Environmental Pollution, 159(1), 219–228. https://doi.org/10.1016/j.envpol.2010.09.009
Brooks, S., Eller, V., Dickson, J., Earles, J., Lowe, K., Mehlhorn, T., Olsen, T., Derolph, C., Watson, D., Phillips, D., & Peterson, M. (2017). Mercury Content of Sediments in East Fork Poplar Creek: Current Assessment and Past Trends. Retrieved December 8, 2023, from https://www.osti.gov/scitech/
Chen, X., Feng, X., Liu, J., Fryxell, G. E., & Gong, M. (1999). Mercury separation and immobilization using self-assembled monolayers on mesoporous supports (SAMMS). Separation Science and Technology, 34(6–7), 1121–1132. https://doi.org/10.1080/01496399908951084
Dickson, J. O., Mayes, M. A., Brooks, S. C., Mehlhorn, T. L., Lowe, K. A., Earles, J. K., Goñez-Rodriguez, L., Watson, D. B., & Peterson, M. J. (2019). Source relationships between streambank soils and streambed sediments in a mercury-contaminated stream. Journal of Soils and Sediments, 19(4), 2007–2019. https://doi.org/10.1007/s11368-018-2183-0
Egbo, T. E., Dickson, J. O., Miller, C., Johs, A., Sanders, C. A., & Robertson, B. K. (2017). Characterization, Identification and Seasonal Evaluation of Microbes in Mercury Contaminated Soils. In Frontiers Science Technology. Engineering Math, 1(1), 15–26.
Egbo, T. E., Johs, A., Sahu, R., Abdelmageed, Y., Ogbudu, J., & Robertson, B. K. (2021). Interaction of Soil Microbes with Organoclays and their Impact on the Immobilization of Hg under Aerobic Conditions. Water, Air, and Soil Pollution, 232(4). https://doi.org/10.1007/s11270-021-05093-4
Environmental Protection Agency. (2001). Water quality criteria: Notice of availability of water quality criterion for the protection of human health: methylmercury. Federal register. Retrieved December 20, 2023, from https://www.federalregister.gov/d/01-217
Fairey, J. L., Wahman, D. G., & Lowry, G. V. (2010). Effects of Natural Organic Matter on PCB-Activated Carbon Sorption Kinetics: Implications for Sediment Capping Applications. Journal of Environmental Quality, 39(4), 1359–1368. https://doi.org/10.2134/jeq2009.0505
Fazli, M., Almblad, H., Rybtke, M. L., Givskov, M., Eberl, L., & Tolker-Nielsen, T. (2014). Regulation of biofilm formation in Pseudomonas and Burkholderia species. In Environmental Microbiology 16 (7), 1961–1981. Blackwell Publishing Ltd. https://doi.org/10.1111/1462-2920.12448
Fekrirad, Z., Gattali, B., & Kashef, N. (2020). Quorum sensing-regulated functions of Serratia marcescens are reduced by eugenol. Iranian Journal of Microbiology, 12(5), 451–459. https://doi.org/10.18502/ijm.v12i5.4607
Gai, K., Avellan, A., Hoelen, T. P., Lopez-Linares, F., Hatakeyama, E. S., & Lowry, G. V. (2019). Impact of mercury speciation on its removal from water by activated carbon and organoclay. Water Research, 157, 600–609. https://doi.org/10.1016/j.watres.2019.04.006
Ghosh, U., Luthy, R. G., Cornelissen, G., Werner, D., & Menzie, C. A. (2011). In-situ sorbent amendments: A new direction in contaminated sediment management. In Environmental Science and Technology, 45(4), 1163–1168. https://doi.org/10.1021/es102694h
Gilmour, C. C., Riedel, G. S., Riedel, G., Kwon, S., Landis, R., Brown, S. S., Menzie, C. A., & Ghosh, U. (2013). Activated carbon mitigates mercury and methylmercury bioavailability in contaminated sediments. Environmental Science and Technology, 47(22), 13001–13010. https://doi.org/10.1021/es4021074
Gilmour, C., Bell, T., Soren, A., Riedel, G., Riedel, G., Kopec, D., Bodaly, D., & Ghosh, U. (2018). Activated carbon thin-layer placement as an in-situ mercury remediation tool in a Penobscot River salt marsh. Science of the Total Environment, 621, 839–848. https://doi.org/10.1016/j.scitotenv.2017.11.050
Giri, A. V., Anandkumar, N., Muthukumaran, G., & Pennathur, G. (2004). A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiology, 4, 11. https://doi.org/10.1186/1471-2180-4-11
Goñez-Rodríguez, L., Johs, A., Lowe, K. A., Carter, K. E., Löffler, F. E., & Mayes, M. A. (2021). Evaluation of engineered sorbents for the sorption of mercury from contaminated bank soils: A column study. Environmental Science and Pollution Research International, 28(18), 22651–22663. https://doi.org/10.1007/s11356-020-12073-4
Harimawan, A., Devianto, H., Kurniawan, I. C., & Utomo, J. C. (2017). Influence of Solution Initial pH on Biofilm Formation and Corrosion of Carbon Steel by Serratia marcescens. 17(2), 89–95. https://doi.org/10.14710/reaktor.17.2.89-96
He, F., Gao, J., Pierce, E., Strong, P. J., Wang, H., & Liang, L. (2015). In situ remediation technologies for mercury-contaminated soil. Environmental Science and Pollution Research, 22(11), 8124–8147. https://doi.org/10.1007/s11356-015-4316-y
Invitrogen. (2004). LIVE / DEAD® BacLight Bacterial Viability Kits. Retrieved March 10, 2022, from https://assets.thermofisher.com/TFS-Assets/LSG/manuals/mp07007.pdf
Johs, A., Eller, V. A., Mehlhorn, T. L., Brooks, S. C., Harper, D. P., Mayes, M. A., Pierce, E. M., & Peterson, M. J. (2019). Dissolved organic matter reduces the effectiveness of sorbents for mercury removal. Science of the Total Environment, 690, 410–416. https://doi.org/10.1016/j.scitotenv.2019.07.001
Karygianni, L., Ren, Z., Koo, H., & Thurnheer, T. (2020). Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. In Trends in Microbiology 28(8), 668–681. Elsevier Ltd. https://doi.org/10.1016/j.tim.2020.03.016
Keithley, S. E., & Kirisits, M. J. (2018). An improved protocol for extracting extracellular polymeric substances from granular filter media. Water Research, 129, 419–427. https://doi.org/10.1016/J.WATRES.2017.11.020
Lee, S. Y., Kim, S. J., Chung, S. Y., & Jeong, C. H. (2004). Sorption of hydrophobic organic compounds onto organoclays. Chemosphere, 55(5), 781–785. https://doi.org/10.1016/j.chemosphere.2003.11.007
Lemos, J. A., Abranches, J., Koo, H., Marquis, R. E., & Burne, R. A. (2010). Protocols to study the physiology of oral biofilms. Methods in Molecular Biology (clifton, N.J.), 666, 87–102. https://doi.org/10.1007/978-1-60761-820-1_7
Mercier, A., Wille, G., Michel, C., Harris-Hellal, J., Amalric, L., Morlay, C., & Battaglia-Brunet, F. (2013). Biofilm formation vs. PCB adsorption on granular activated carbon in PCB-contaminated aquatic sediment. Journal of Soils and Sediments, 13(4), 793–800. https://doi.org/10.1007/s11368-012-0647-1
Mirzaei, R., & Ranjbar, R. (2022). Hijacking host components for bacterial biofilm formation: An advanced mechanism. International Immunopharmacology, 103, 108471. https://doi.org/10.1016/j.intimp.2021.108471
Ngamdee, W., Tandhavanant, S., Wikraiphat, C., Reamtong, O., Wuthiekanun, V., Salje, J., Low, D. A., Peacock, S. J., & Chantratita, N. (2015). Competition between Burkholderia pseudomallei and B thailandensis Ecological and evolutionary microbiology. BMC Microbiology, 15, 56. https://doi.org/10.1186/s12866-015-0395-7
Noshadi, I., Walker, B. W., Portillo-Lara, R., Shirzaei Sani, E., Gomes, N., Aziziyan, M. R., & Annabi, N. (2017). Engineering Biodegradable and Biocompatible Bio-ionic Liquid Conjugated Hydrogels with Tunable Conductivity and Mechanical Properties. Scientific Reports, 7(1), 4345. https://doi.org/10.1038/s41598-017-04280-w
O’Toole, G. A. (2011). Microtiter dish Biofilm formation assay. Journal of Visualized Experiments., 47, 2437. https://doi.org/10.3791/2437
Okaro, U., Mou, S., & Deshazer, D. (2021). Production and molecular composition of Burkholderia pseudomallei and Burkholderia thailandensis biofilms. Authorea https://doi.org/10.22541/au.162135371.13380628/v1
Olsen, T. A., Brandt, C. C., & Brooks, S. C. (2016). Periphyton Biofilms Influence Net Methylmercury Production in an Industrially Contaminated System. Environmental Science & Technology, 50(20), 10843–10850. https://doi.org/10.1021/acs.est.6b01538
Parks, J. M., Johs, A., Podar, M., Bridou, R., Hurt, R. A., Smith, S. D., Tomanicek, S. J., Qian, Y., Brown, S. D., Brandt, C. C., Palumbo, A. V., Smith, J. C., Wall, J. D., Elias, D. A., & Liang, L. (2013). The genetic basis for bacterial mercury methylation. Science (new York, n.y.), 339(6125), 1332–1335. https://doi.org/10.1126/science.1230667
Paulson, K. M. A., Ptacek, C. J., Blowes, D. W., Gould, W. D., Ma, J., Landis, R. C., & Dyer, J. A. (2018). Role of Organic Carbon Sources and Sulfate in Controlling Net Methylmercury Production in Riverbank Sediments of the South River, VA (USA). Geomicrobiology Journal, 35(1), 1–14. https://doi.org/10.1080/01490451.2016.1247483
Peterson, M. J., Brooks, S. C., Mathews, T. J., Mayes, M., Johs, A., Watson, D. B., Poteat, M. D., Smith, J. G., Mehlhorn, T., Lester, B., Morris, J., Lowe, K., Dickson, J. O., Eller, V., & Derolph, C. R. (2016). Mercury remediation technology development for lower east fork poplar Creek-FY 2015 progress report. Retrieved February 28, 2024, from https://www.osti.gov/scitech/
Reynolds, J. (2005). Serial Dilution Protocols. Retrieved November 10, 2023, from https://asm.org/protocols/serial-dilution-protocols
Rice, S. A., Koh, K. S., Queck, S. Y., Labbate, M., Lam, K. W., & Kjelleberg, S. (2005). Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. Journal of Bacteriology, 187(10), 3477–3485. https://doi.org/10.1128/JB.187.10.3477-3485.2005
Sarkar, B., Xi, Y., Mallavarapu, M., Krishnamurti, G., Dharmarajan, R., & Naidu, R. (2010). Remediation of hexavalent chromium through adsorption by bentonite based Arquad (R) 2HT-75 organoclays. Journal of Hazardous Materials, 183, 87–97. https://doi.org/10.1016/j.jhazmat.2010.06.110
Southworth, G., Turner, R., Peterson, M., Bogle, M., & Ryon, M. (2000). Response of Mercury Contamination in Fish to Decreased Aqueous Concentrations and Loading of Inorganic Mercury in a Small Stream. Environmental Monitoring and Assessment, 63, 481–494. https://doi.org/10.1023/A:1006237610383
Stathi, P., Litina, K., Gournis, D., Giannopoulos, T., & Deligiannakis, Y. (2008). Physicochemical study of novel organoclays as heavy metal ion adsorbents for environmental remediation. Journal of Colloid and Interface Science, 316, 298–309. https://doi.org/10.1016/j.jcis.2007.07.078
Stewart, A. J., Smith, J. G., & Loar, J. M. (2011). Long-Term Water-Quality Changes in East Fork Poplar Creek, Tennessee: Background, Trends, and Potential Biological Consequences. Environmental Management, 47(6), 1021–1032. https://doi.org/10.1007/s00267-011-9630-7
Tang, L., Schramm, A., Neu, T. R., Revsbech, N. P., & Meyer, R. L. (2013). Extracellular DNA in adhesion and biofilm formation of four environmental isolates: A quantitative study. FEMS Microbiology Ecology, 86(3), 394–403. https://doi.org/10.1111/1574-6941.12168
Tseng, B. S., Majerczyk, C. D., da Silva, D. P., Chandler, J. R., Greenberg, E. P., & Parsek, M. R. (2016). Quorum sensing influences Burkholderia thailandensis biofilm development and matrix production. Journal of Bacteriology, 198(19), 2643–2650. https://doi.org/10.1128/JB.00047-16
Turner, R. R., & Southworth, G. R. (1999). Mercury-contaminated industrial and mining sites in North America: an overview with selected case studies. In Mercury contaminated sites: Characterization, risk assessment and remediation (pp. 89–112). Berlin, Heidelberg: Springer Berlin Heidelberg.https://doi.org/10.1007/978-3-662-03754-6_4
Vitale, A., Paszti, S., Takahashi, K., Toyofuku, M., Pessi, G., & Eberl, L. (2020). Mapping of the denitrification pathway in Burkholderia thailandensis by genome-wide mutant profiling. Journal of Bacteriology, 202(23). https://doi.org/10.1128/JB.00304-20
Wagley, S., Hemsley, C., Thomas, R., Moule, M. G., Vanaporn, M., Andreae, C., Robinson, M., Goldman, S., Wren, B. W., Butler, C. S., & Titball, R. W. (2014). The twin arginine translocation system is essential for aerobic growth and full virulence of Burkholderia thailandensis Journal of Bacteriology, 196(2), 407–416. https://doi.org/10.1128/JB.01046-13
Watson, D., Brooks, S., Mathews, T., Bevelhimer, M., Derolph, C., Brandt, C., Peterson, M., & Ketelle, R. (2016). Evaluation of lower east fork poplar creek mercury sources. Oak Ridge National Laboratory. Retrieved January 15, 2024, from https://www.osti.gov/scitech/
Acknowledgements
The authors are very grateful to Dr. Derrick Dean for the sorbent SEM analysis.
Funding
This work was supported by grants from Savannah River Nuclear Solution (SRNS) under subcontract No. 0000217390 and the U.S. Department of Energy’s (DOE) Oak Ridge Office of Environmental Management (ORO-EM) and United Cleanup Oak Ridge LLC (UCOR) and under ORNL’s Mercury Remediation Technology Development Program. ORNL is managed by UT-Battelle, LLC, for the U.S. DOE under Contract No. DE-AC05-00OR22725.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of the manuscript. All authors have approved the manuscript in its final form.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Notice: This manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the manuscript for publication, acknowledges that the US government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript or allow others to do so for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ogbudu, J., Egbo, T.E., Johs, A. et al. The Impact of Sorbent Amendments for Mercury Remediation on the Viability of Soil Microorganisms. Water Air Soil Pollut 235, 448 (2024). https://doi.org/10.1007/s11270-024-07219-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07219-w