Abstract
Water samples taken from the Çubuk Stream (Ankara, Turkey) were inoculated into nutrient broth media containing Setazol Navy Blue SBG (SNB), an organic pollutant, and heavy metal Cr(VI), an inorganic pollutant, to obtain a pollutant-resistant mixed microbial culture. Experiments were conducted with this culture to remove SNB and heavy metal. The optimum conditions, where the mixed bacterial culture removed the pollutants most effectively, were determined, showing that the highest capacity for removal took place at pH 8 with removal percentages 96.3% for Cr(VI) and 78.5% for SNB. In media with 50.4 mg/L SNB and 9.7 mg/L Cr(VI), the SNB removal was 87.3%, and the Cr(VI) removal was 96.6% at the end of the 7-day incubation period. The highest removal was observed with a biomass concentration of 8% (v/v) of mixed culture [50 mg/L SNB dye+25 mg/L Cr(VI)]. The removal was 100% for both Cr(VI) and the SNB dye. The bacteria with the highest removal were isolated and identified using 16S rDNA gene sequence analysis as Microbacterium oxydans and Leucobacter aridicollis. The role of various functional groups and the structures of the microorganisms that might be involved in the removal mechanisms were discussed using their FTIR spectra. This report is the first study that investigates a mixed bacterial culture and pure cultures (M. oxydans and L. aridicollis) isolated from that mixed culture, removing both SNB and Cr(VI) simultaneously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Today, the rapidly increasing population around the world, climate change, and industrial development are continuously impacting water quality significantly, resulting in environmental pollution, notably within the water itself. The effects of such pollution are severe and far-reaching, leading to ecosystem deterioration, loss of biodiversity, and increased health risks for humans and other living organisms. The rapid increase in wastewater containing toxic dyes and heavy metals from various industries poses a severe public health problem and a major challenge to conventional water treatment systems. (Haripriyan et al., 2023).
Water pollution varies depending on the type of pollutant, its amount in the water, and the environmental conditions of the water itself. Water pollutants are classified as inorganic toxic elements and organic chemicals; the former include various inorganic metallic elements such as mercury (Hg), cadmium (Cd), lead (Pb), chromium (Cr), and copper (Cu); whereas, the latter typically comprises organic dyes, pharmaceuticals, personal care products, pesticides, detergents, and common industrial organic wastes such as phenolics, halogens, and aromatics (Lu & Astruc, 2020).
Dyestuffs, another type of inorganic pollutants found in a wide variety of types and undesirable amounts in wastewater, cause color pollution, affect the photosynthetic activity of aquatic life, and are difficult to biodegrade. These dyes are used in various manufacturing sectors such as textile, leather, paint, plastic, pharmaceutical, etc. (Pavithra & Jaikumar, 2019; Rahayu et al., 2023; Sudarshan et al., 2023).
To preserve the environment and water resources, it is crucial to efficiently treat wastewater that contains dyes and heavy metals. To this end, there are three methods to remove pollutants, namely, chemical, physical, and biological. The physical and chemical methods are commonly used to eliminate dyes and heavy metals. Yet recently, biological-based treatments have gained popularity due to their cost-effective nature and superior results in wastewater treatment. This process involves bioremediation, which utilizes various microorganisms to remove pollutants. The use of microorganisms that are resistant to toxic substances is a method commonly employed in bioremediation. This method is considered highly effective as it is both cheap and environmentally friendly, making it an economical solution for treating wastewater that contains various pollutants (Zeng et al., 2022). In detail, biological treatment involves the use of organisms such as bacteria, fungi, yeasts, algae, and plants. The treatment process is carried out either by living cells, which use metabolic pathways, or by dead or inactive biomass, which is independent of metabolism.
The use of microorganisms is a highly effective solution for cleaning up pollutants. It has even been observed that utilizing a combination of these organisms yields better results than using only one type (Tufail et al., 2022). While individual strains are still being explored, one study has already demonstrated the remarkable capabilities of the salt-tolerant Bacillus circulans BWL1061 under anaerobic conditions for the efficient removal of the methyl orange dye and Cr(VI) from wastewater (Liu et al., 2017). In 2020, Louati and colleagues conducted a study on the ability of the P. aeruginosa Gb30 strain to remove both Reactive Black 5 (RB5) and Cd2+ from a heavy metal mixture, as well as on the mechanisms behind its heavy metal tolerance. Their findings showed that the strain was able to efficiently remove both dyes and heavy metals simultaneously and was highly resistant to the heavy metal mixture. In addition to Cd2+, it also removed zinc, copper, and chromium ions, and was able to detoxify the RB5 dye.
According to a study conducted by Plestenjak et al. in Plestenjak et al., 2022, it was found that enriched microbial groups were more effective than a single bacterial strain in removing Cr(VI) from industrial wastewater. These groups were also better suited to handling high concentrations of Cr(VI). In another study by Zabłocka-Godlewska and Przystaś in Zabłocka-Godlewska & Przystaś, 2020, the removal of dyes by pure and mixed bacterial cultures was examined. The study investigated the decolorization of a dye mixture (Evans blue and brilliant green) by selected bacterial strains, as well as the impact of changes in bacterial growth conditions on dye decolorization. The study found that a mixture of facultative Citrobacter sp. and obligate aerobic P. putida was able to remove the highest concentration of dye mixture (200 mg/L) in 288 hours, while other pure and mixed cultures required a longer time to remove the four dye mixtures (170 mg/L).
In this study, experiments were carried out with a mixed microbial culture obtained in water samples taken from the Çubuk Stream (Ankara, Turkey). Our aim was to determine the optimal conditions (including pH, pollutant concentration, and biomass concentration) under which the obtained mixed microbial culture exhibited the highest capacity for bioremediation of dyes and heavy metals. Additionally, pure cultures were also isolated from the mixed microbial culture, and their bioremediation capacities were assessed. In the end, Microbacterium oxydans and Leucobacter aridicollis were identified as the bacteria with the highest bioremediation capacities among the five pure cultures. The possible cell functional groups involved in the contaminant removal of pure bacterial cultures were determined by FTIR analysis. This is the first study that shows mixed bacterial culture and pure cultures (M. oxydans, L. aridicollis) isolated from the Çubuk Stream, removing both SNB and Cr(VI) simultaneously.
2 Materials and Methods
2.1 Chemicals
All the chemicals used were of analytical grade, and the solutions were prepared using deionized water. A pure form of Setazol Navy Blue SBG (SNB) was purchased from Setaş Kimya (Tekirdağ, Turkey). Potassium dichromate [K2Cr2O7] (Merck) was used for Cr(VI) stock solution at a final concentration of 10 g/L. A stock of SNB dye [2% (w/v)] was prepared, diluted with distilled water, and sterilized by autoclaving. Appropriate volumes of the stock solution were added to the media. The Cr(VI) complexing reagent, 1,5-diphenylcarbazide, was procured from Sigma-Aldrich (St. Louis, MO, USA).
2.2 Mixed Bacterial Culture and Culture Conditions
A mixed bacterial culture was obtained from water samples collected from the Çubuk Stream (Ankara, Turkey). The mixed culture was prepared by periodically subculturing the water samples in a Nutrient Broth (NB) medium (Neogen, USA) containing 10 mg/L Cr(VI) and 50 mg/L SNB. 1 mL of the water sample was inoculated into 25 mL of medium in 100 mL Erlenmeyer flasks. The flasks were incubated on a rotary shaker (NB 205V, Korea) at 100 rpm for 7 days at 30°C.
2.3 The Effect of Different Environmental Conditions on Bioremediation
In these trials, to determine the conditions under which the mixed microbial culture achieves the highest pollutant removal, the effect of different pH, initial pollutant concentration, and biomass concentrations on SNB and Cr(VI) removal were tested.
2.3.1 The Effect of pH on SNB and Cr(VI) Bioremediation
A range of values between 6 and 9 was tested to determine the effect of pH on binary pollutant removal by the mixed culture. The NB media were prepared at pH 6 (23.3 mg/L Cr(VI) + 52.0 mg/L SNB), pH 7 (24.3 mg/L Cr(VI) + 50.5 mg/L SNB), pH 8 (29.1 mg/L Cr(VI) + 55.8 mg/L SNB), and pH 9 (25.1 mg/L Cr(VI) + 50.6 mg/L SNB dye) as 100 mL in 250 mL flasks. These media were inoculated with 1% (v/v) of the mixed bacterial culture. The pH of the media, where the mixed culture removed the pollutants with the maximum capacity, was recorded.
2.3.2 The Effect of Different SNB Concentrations on SNB and Cr(VI) Bioremediation
The media with the determined optimum pH was prepared as 100 mL in 250 mL flasks with nearly constant Cr(VI) concentrations to investigate the effect of increasing the concentrations of SNB on the removal of both pollutants. For this purpose, three different media were used containing 24.7 mg/L SNB + 10.1 mg/L Cr(VI), 43.7 mg/L SNB + 10.4 mg/L Cr(VI), and 84.5 mg/L SNB + 12.1 mg/L Cr(VI). The activated mixed bacterial culture was inoculated into each medium at a concentration of 1% (v/v).
2.3.3 The Effect of Different Cr(VI) Concentrations on SNB and Cr(VI) Bioremediation
Three separate 100 mL media with the determined optimum pH were prepared in 250 mL flasks at a constant SNB concentration (50.4 mg/L). A variety of Cr(VI) concentrations were used to examine their effect on the treatment of both pollutants. These concentrations were 9.70 mg/L, 23.8 mg/L, and 55.0 mg/L. Inoculation was done with 1% (v/v) with the activated mixed bacterial culture.
2.3.4 The Effect of Different Biomass Concentration on SNB and Cr(VI) Bioremediation
Three separate 100 mL media were prepared in 250 mL flasks with constant SNB (50 mg/L) and Cr(VI) (25 mg/L) concentrations at optimum pH. Next, 1%, 4%, and 8% (v/v) of biomass concentration were added into each flask to determine their effect on the pollutant-removal.
2.4 Isolation and Selection of Pure Bacterial Cultures from Mixed Bacterial Culture
SNB- and Cr(VI)-removing mixed microbial cultures were obtained using the water samples taken from the Çubuk Stream (Ankara, Turkey). The samples were enriched by periodic subculturing in the NB using 25 mg/L Cr(VI) + 50 mg/L SNB. An aliquot of 0.1 ml of the growing, enriched mixed microbial culture sample was spread on a nutrient agar (1.5%, w/v) plate containing 25 mg/L Cr(VI) and 50 mg/L SNB (pH 8) and incubated at 30°C. Different colony morphotypes were monitored and isolated. The pure bacteria cultures were transferred to agar slants, kept at 4°C, and moved to fresh media every 3 months.
A medium of 100 mL at pH 8 was prepared in 250 mL flasks using 26.1 mg/L Cr(VI) + 46.5 mg/L SNB. The pure cultures of bacteria were separately inoculated [8% (v/v)] to the prepared medium in each flask. After incubation for 5 days, the residual pollutant concentrations were measured to find the bacterium with the highest capacity to remove both SNB and Cr(VI).
After selecting the pure cultures, their bioremediation capacity was evaluated. For this purpose, the bacteria were inoculated [8% v/v)] to the prepared media, all of which included a constant Cr(VI) concentration (14.4 mg/L), but varying concentrations of SNB, i.e., 26.2, 51.8, and 99.7 mg/L.
2.5 Scanning Electron Microscopy (SEM)
The analysis of the bacteria and their sizes was studied using a scanning electron microscope (SEM). For sample fixation, the bacteria were washed with a PBS buffer and placed overnight in a 2.5% glutaraldehyde solution prepared in a PBS buffer. Then, the samples were washed again with a PBS buffer and dehydrated upon exposure to EtOH solutions (30%-96%). At the end, the samples were coated with 5 nm Au for SEM imaging (ZEISS EVO 40, SEM). The bacteria were imaged in the media with and without pollutants.
2.6 Analytical Methods
During the incubation period, a 3 mL sample was taken daily from each flask. The aliquots of these samples were centrifuged at 6000 rpm (3468 x g) for 10 minutes to harvest the biomass (Hermle Z207A, Germany). The growth of the bacteria was measured by determining the dry weight of the washed biomass after the incubation period. The remaining concentration of the pollutant in the medium was determined spectrophotometrically (BioDrop, U.K.). The wavelength used for SNB was 602 nm. The chromium concentration in the supernatant was determined spectrophotometrically at 540 nm using a 1,5-diphenylcarbazide reagent in an acid solution as the complexing agent for Cr(VI) (Snell & Snell, 1959). The control flasks including media and pollutants but without inoculation were prepared to observe possible direct reactions between the media and each pollutant.
The pollutant removal was studied as a function of the pH, dye concentration, and biomasses, and the percentage was calculated by the following equation:
The pollutant removal can be measured based on the mass balance principle with the following equation:
The maximal specific pollutant removal (qm) shows the maximal amount of pollutant (mg) per unit of the dry weight of the bacterial cells (g). The maximal dried bacterial cell mass is Xm (g/L), Co is the initial, and Cf is the final dye concentration (mg/L).
2.7 PCR and Sequencing
Whole cells from an exponentially growing culture of each isolate were used for 16S rDNA gene amplification. Three microliters of cells were added directly to the Polymerase Chain Reaction (PCR) using the bacterial primer set to 27F and 1492R. The PCR was carried out by an initial denaturation step at 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 45 s, annealing at 57°C for 45 s, and elongation at 72°C for 60 s. The cycling was completed by a final elongation at 72°C for 5 min. ABI 3730XL Sanger sequencing instrument (Applied Biosystems, Foster City, CA) and BigDye Terminator v3.1 Cycle Sequencing Kit were used for Sanger Sequencing of the samples (Applied Biosystems, Foster City, CA). The phylogenetic analysis of the nearly complete data was performed using the BLAST. The phylogenetic analysis was also performed by the neighbor-joining way in the MEGA X software (Kumar et al., 2018).
2.8 Fourier Transform Infrared Spectroscopy (ATR-FTIR)
The bacterial biomass grown in the presence and absence of pollutants was collected by centrifuging and washed twice with double-distilled water (d2H2O). The Pellet was vortexed with 100 microliters of d2H2O until a homogeneous solution was obtained. Five microliters of this solution were pipetted onto the diamond and dried with a gentle stream of N2 gas for 15 min to remove excess water. The infrared spectra were obtained using a Thermo-Scientific Nicolet 6700 FTIR spectrometer equipped with a diamond attenuated total reflection (ATR, ConcentratIR2, Harrick) accessory. A clean diamond surface was used for the background interferogram. The absorbance spectra were recorded by collecting and averaging 128 scans at 4 1/cm resolution using the instrument software OMNIC (v. 8.2.388). The average of the repeated experiments was used for the visuals presented in this study. All the spectra were first area-normalized to eliminate the concentration differences due to the differences in the bacterial population. The peak area calculations were performed by integrating the peak from the normalized absorbance spectra. A second derivative calculation was performed using a 15-point Savitsky-Golay derivative with the polynomial order set to 3.
The Shapiro-Wilk test was applied to the peak area calculation results to analyze the normality of distribution among the repeated measurements of the same sample. The data were found to be normally distributed. One-way ANOVA was used to determine the statistical significance of the difference between the control and sample groups.
3 Results and Discussion
3.1 SNB and Cr(VI) Bioremediation in Media with Varying pH
The data obtained from these trials are summarized in Table 1. At the end of the incubation period, the microorganisms removed Cr(VI) with approximately similar removal percentages ranging from 96.3% to 97.5% at all the tested pH levels. However, the SNB removal reached the highest value at pH 8 (78.5%). Thus, further experiments were performed with the media at pH 8.
Lyu et al. (2021) demonstrated that the optimum pH of a bacterial community Cr(VI) removal was between 7 and 8. They emphasized that pH is a critical factor that can influence the efficiency of microbial remediation processes due to its impact on the solubility, speciation, and metabolic activities of the pollutants. In 2021, Kholisa and colleagues conducted a study on reducing Cr(VI) using a mixed culture of microbes from municipal wastewater treatment sludge. Their findings revealed that the Cr(VI) reduction increased as the initial pH increased, reaching its optimum level at pH 7. However, further increases in pH resulted in reduced capacity in removing Cr(VI). In another study, Tan et al. (2020) showed that the Bacillus sp. CRB-B1 bacteria can effectively reduce Cr(VI) in a pH range of 6-8. In this pH range, microbial cell surfaces facilitate the binding of negatively charged chromate and provide protons for Cr(VI) reduction.
According to Mishra (Mishra & Maiti, 2019), the enzyme-mediated decolorization of dyes takes place through degradation or biotransformation mechanisms under optimum environmental conditions, where pH plays a significant role. Bera and Tank (2021) showed that Pseudomonas stutzeri exhibited a maximum decolorization of the Procion Red H-3B dye at pH values between 6 and 8, the optimum being at pH 7.5. On the other hand, Alaya et al. (2021) reported that S. maltophilia had the highest malachite green removal at pH 8.
In the present study, the Cr(VI) bioremediation capacity of the mixed bacterial culture was not adversely affected by pH changes. Cr(VI) was removed with similar removal percentages at all the tested pH values; however, the SNB dye was removed with the highest capacity at pH 8.0. Our data showed that the tested bacterial mixed culture was suitable for effective bioremediation of alkaline wastewaters, such as those from textile industries, containing reactive dyes and Cr(VI).
3.2 SNB and Cr(VI) Bioremediation in Media with Varying SNB Concentrations
The experiments were conducted in media containing approximately 10 to 12 mg/L Cr(VI) and increasing SNB (25-85 mg/L) concentrations to investigate the pollutant bioremediation by the mixed bacterial culture (Fig. 1a).
In these experiments, after incubation for 2 days, all the applied Cr(VI) was removed by the bacterial culture in the media containing varying SNB concentrations. On the other hand, after incubation for 1 day, the SNB removal was 79.6% in the media with 24.7 mg/L SNB and 10.1 mg/L Cr(VI). Under the same conditions, the removal of SNB was 87.4% after incubation for 7 days. In the media containing 43.7 mg/L SNB and 10.4 mg/L Cr(VI), the SNB removal was 62.3% after incubation for 1 day and reached 86.9% at the end of the seventh day of the incubation. At the highest SNB concentration (84.5 mg/L), its removal was 84.8% at the end of the incubation period.
3.3 SNB and Cr(VI) Bioremediation in Media with Varying Cr(VI) Concentrations
In a medium with 50.4 mg/L SNB and 9.7 mg/L Cr(VI), the removal was 68.5% and 88.5%, respectively, at the end of the first day of incubation. Under the same conditions, the SNB removal was 87.3%, and the Cr(VI) removal was 96.6% at the end of the incubation period (Fig. 1b). When the Cr(VI) concentration was increased to 23.8 mg/L, the removal was 93.6% for Cr(VI) and 58.9% for SNB after incubation for 2 days. The removals were 85.1% (SNB) and 96% [Cr(VI)] in the same medium at the end of the incubation period. After incubation for 7 days, the highest SNB removal was 52.9%, while that of Cr(VI) was 97.7% in the medium with 50.4 mg/L SNB and 55.0 mg/L Cr(VI). Therefore, it can be stated that, with an increase in heavy metal concentration, the removal pertaining to both pollutants decreased owing to the effect of their toxicity on the mixed bacterial culture.
During the assessment of removal percentages at constant Cr(VI) with increasing SNB concentrations and constant SNB with increasing Cr(VI) concentrations, it was observed that Cr(VI) was consistently removed from the environment under all the tested conditions, while SNB exhibited greater resistance to removal under the tested conditions. This result showed that the tested mixed bacterial culture was more resistant to Cr(VI) but more sensitive to dye.
Our results agree well with previous studies regarding the effect of pollutant concentration on bioremediation. In their study, Chaudhari et al. (2013) reported that increasing the dye concentration reduced its decolorization rate while enhancing Cr(VI) removal. The Lysinibacillus sp. KMK-A decolorized 58.0% of the Orange M2R dye and removed Cr(VI) by 100% within 48 h. The inverse relationship of the Cr(VI) concentration with the dye decolorization rate might be due to the toxicity effect of Cr(VI) that causes loss in cellular growth and microbial activity (Rahman, 2020).
3.4 SNB and Cr(VI) Bioremediation in Media with Varying Biomass Concentrations
The comparison of removals in samples with different biomass concentrations is shown in Fig. 2. In our experiments, the removal percentages were 1.3%, 38.2%, and 62.9% for SNB in samples with 1%, 4%, and 8% (v/v) of biomass concentration, respectively, after 1 day. It is clearly observed that with an increasing biomass concentration, the SNB removal also increased. Furthermore, at the end of the incubation period, the removal was 85.1% for the 1% (v/v) biomass sample, while it was 100% for both the 4% and 8% (v/v) biomass samples.
The effect of different biomass concentrations on the SNB and Cr(VI) removal (Removal %) by mixed bacterial culture in NB media containing 50 mg/L SNB dye and 25 mg/L Cr(VI) concentrations. Biomass concentrations tested include 1%, 4%, and 8% (v/v), with a 7-day incubation period; (pH: 8; T: 30 ± 1 °C)
When the Cr(VI) removal by 1%, 4%, and 8% (v/v) biomass concentrations after 1- and 7-day incubation periods was compared; it was observed that the Cr(VI) was cleared mostly within 24 h. The removal was 69.7% in the medium with 1% (v/v) of biomass. However, the removals were rather close as 92.8% and 93.1% in samples with 4% and 8% (v/v) of biomass, respectively. After incubation for 7 days, nearly all the heavy metal content was removed (96%) in the medium with 1% (v/v) of biomass. The removal percentages reached 100% in samples with 4% and 8% (v/v) of biomass within 5 days. As a result, it can be said that increased biomass concentrations shortened the duration of the removal process; in other words, the time required for the completed bioremediation was reduced from 7 to 5 days.
Similar to our findings, Emadi et al. (2022) reported that the dye removal increased when the inoculum size was increased from 2% to 5%. However, in the same study, researchers emphasized that increasing the biomass concentration by more than 5% did not provide further removal. Afrin et al. (2021) also reported a significant increase in pollutant removal with an increase in biomass concentration from 2% to 5% and to 10%; however, after 10% biomass concentration, the dye removal declined.
3.5 Isolation, Selection, and Identification of SNB- and Cr(VI)-removing Bacteria from Mixed Microbial Culture
Five different colony morphotypes (Isolate A, B, C, D, and E) were observed on nutrient agar plates incubated with samples of the mixed microbial culture that were able to remove SNB and Cr(VI). These colonies were purified and analyzed under the microscope after Gram staining. Among them, three isolates were Gram-positive and rod-shaped; one was coccoid-shaped and Gram-positive; the last was rod-shaped and Gram-negative.
The SNB and Cr(VI) removal of pure bacterial cultures are shown in Fig. 3. All the isolates were able to remove the applied pollutants, with removal percentages of at least 94.6% for Cr(VI) and 86.5% for SNB. Of these, pure cultures B and C were selected for their higher binary pollutant removal. The percentages of removal for B were 96.9% (Cr(VI)) and 89.9% (SNB), while those were 97.5% (Cr(VI)) and 91.0% (SNB) for C at the end of the 5-day incubation period.
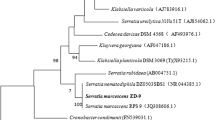
These two isolates (B and C) were identified by amplifying and sequencing their 16S rDNA gene. BLAST search was performed to find the phylogenetic analysis of the nearly complete sequence data. Alignment and further analysis revealed a 100% similarity to Microbacterium oxydans for isolate B and a 100% similarity to Leucobacter aridicollis for isolate C. The evolutionary history was inferred by using the Maximum Likelihood method and Tamura-Nei model (Tamura & Nei, 1993). The tree with the highest log likelihood (-3399.88) was shown in Fig. 4. The percentage of trees, in which the associated taxa clustered together, was shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree was drawn to scale, with branch lengths measured in the number of substitutions per site. This analysis involved 10 nucleotide sequences. There were a total of 1380 positions in the final dataset. The bacteria were submitted to NCBI GenBank with accession numbers as Microbacterium oxydans OR127276 and Leucobacter aridicollis OR127277.
3.6 SNB and Cr(VI) Bioremediation by M. oxydans and L. aridicollis
M. oxydans and L. aridicollis were tested separately for their removal in media containing an initial constant concentration of 14.4 mg/L Cr(VI) and increasing dye concentrations of 26.2, 51.8, 99.7 mg/L SNB (Fig. 5a-f). M. oxydans removed Cr(VI) within 24 h with a removal of 95.7% and removed SNB with a removal of 30% within the same incubation period (Fig. 5a, b, c) in the media with 14.4 mg/L Cr(VI ) + 26.2 mg/L SNB. Subsequently, Cr(VI) was entirely removed on the fourth day and SNB was removed by 79.2% by the end of the seventh day. When the SNB concentration was doubled (51.8 mg/L), the Cr(VI) removal by M. oxydans was not adversely affected. The bacterium removed 94.0% of the heavy metal and 62.1% of SNB by the end of the incubation period. In the media with the highest SNB concentration (99.7 mg/L), the Cr(VI) removal dropped to 82.8% after incubation for 7 days. In contrast, the SNB removal slightly improved, reaching 63.0% under the same conditions.
The removal (Removal %) of SNB and Cr(VI) by M. oxydans (a, b, c) and L. aridicollis (d, e, f) in media containing 14.4 mg/L Cr(VI) and increasing concentrations of SNB (incubation period: 7 days; pH: 8; biomass concentration: 8% (v/v); T: 30 ± 1 °C; measurement errors are shown with error clouds in all plots)
Figure 5d, e, f summarize Cr(VI) and SNB bioremediation by L. aridicollis. In the media with 14.4 mg/L Cr(VI) and 26.2 mg/L SNB, the removal percentages were 97.4% and 25.0%, respectively, within 24 h. The Cr(VI) was removed by 100% within 2 days, and by the end of the incubation period, the removal of SNB was 83.0% under the same conditions. When the initial SNB concentration was increased to 51.8 mg/L, L. aridicollis removed the applied 14.4 mg/L Cr(VI) with a removal of 93.2%, while it removed the dye with a removal of 47.2% at the end of the incubation period. For an initial SNB concentration of 99.7 mg/L, the bacterium was affected negatively by this increment of the dye; consequently, Cr(VI) and SNB removal dropped to 79.6% and 18.3%, respectively.
These results suggest that the isolates could efficiently remove Cr(VI) at various concentrations in the presence of both pollutants. The Cr(VI) removal notably dropped when the SNB concentration was increased from 51.8 mg/L to 99.7 mg/L. However, the SNB removal was significantly compromised upon an increase of its initial concentration. Analysis by ANOVA showed that (Table 2) the capacity of different bacterial species with respect to pollutant removal under the same conditions were significantly different.
Table 3 shows the maximum specific heavy metal removal of M. oxydans and L. aridicollis in the media with constant Cr(VI) (14.4 mg/L) and increasing SNB (26.2-99.7 mg/L) concentrations.
Concerning M. oxydans, the qm was 3.7 mg/g, 6.2 mg/g, and 13.4 mg/g in the media with 26.2, 51.8, and 99.7 mg/L SNB concentrations, respectively. The Cr(VI) removal per one gram of M. oxydans was similar and ranged from 2.5 to 2.6 mg, consistent with the constant initial concentration. It is seen that Cr(VI) can be removed at high percentage rates in different media containing M. oxydans. Apart from this, the qm values of SNB increased parallel to increasing SNB concentrations in these media. Therefore, it appears that M. oxydans was only slightly affected by the tested pollutant concentrations, allowing it to perform bioremediation highly efficiently. It can be said that the reasons for the difference in the qm values of the samples with increasing pollutant concentrations were the biomass produced and the removal percentage of the pollutant at these conditions. In the media with increasing dye concentration and constant heavy metal, the biomass produced by the bacteria was slightly less. More specifically, the dye concentration removal increased by 1 gram of the bacteria.
With respect to L. aridicollis, the qm values reflect a more concentration-dependent bioremediation performance. The maximum specific SNB removals were 3.09, 4.70, and 3.85 mg/g, and the Cr(VI) removals were 2.05, 2.6, and 2.4 mg/g in media with 26.2, 51.8, and 99.7 mg/g of SNB concentrations, respectively. It was seen that L. aridicollis could tolerate Cr(VI) but was affected negatively by added SNB concentrations. Thus, the SNB removal per 1 gram of L. aridicollis dropped in the medium with the highest SNB concentration, while the lowest value was 3.09 mg/g; it increased in the medium with constant Cr(VI) concentration. In the L. aridicollis samples with 14.4 mg/L Cr(VI)+ 99.7 mg/L SNB according to the low dye removal percentage, the qm for dye and Cr(VI) declined.
Our results show similarities with those reported by Dang Son et al. (2016), in that the efficiency of dye removal diminishes with higher initial dye concentrations due to the saturation of adsorption sites on the adsorbent surface. Conversely, an increase in the initial dye concentration leads to a higher adsorption capacity, attributed to the elevated driving force of mass transfer at higher concentrations.
Ghanbari et al. (2022) reported that the M. oxydans strain M7 can be used to treat Acid Orange 7. In another study, two strains of M. oxydans, CM3 and CM7, were tested for their Pb(II) bioremediation capacities (Ashrafi et al., 2022). The studies concerning L. aridicollis are scant. The bacterium was isolated using samples taken from chromium-contaminated areas. The strain is known to be resistant to Cr(VI) and capable of removing chromium from the environment (Morais et al., 2004). In the current study, the bacteria removed both pollutants; therefore, our findings agree with previous reports.
3.7 Fourier Transform Infrared Spectroscopy (ATR-FTIR) Analysis
The FTIR spectrum of a bacterium can be divided into regions based on its underlying molecular structure. The broad band centered at ~3300 1/cm corresponds to the O–H and N–H stretching vibrations. The 3000–2800 1/cm region is due to the C–H stretching vibrations in fatty acids. Various proteins, peptides, and enzymes produce the amide I and II bands in the 1700–1500 1/cm region. The 1500–1200 1/cm region is more complex due to several contributors, such as the protein amide III band, fatty acids, and phosphate groups. The RNA/DNA functional groups show up in the 1200–900 1/cm region together with polysaccharides in the cell wall (Kardas et al., 2014; Talari et al., 2017).
The analysis of M. oxydans showed all the principal peaks listed above (Fig. 6a). The same bacterium grown in the presence of pollutants showed absorbance variations at positions 3500, 1640, 1530, 1055, 972, 932, and 915 1/cm relative to the one grown in the absence of pollutants. The second derivative profiles of these two spectra showed additional differences in the protein amide I and amide II bands, at 1724, 1400, 1382, 1342, 1300, 1220, 1146, 1013, 970, and 914 1/cm (Fig. 6c, d). These changes agree well with the findings of a previous study that investigated the effects of acute and gradual Cd acclimation of M. oxydans (Kepenek et al., 2019).
The area-normalized FTIR absorbance spectra of M.oxydans (a) grown in the absence (black) and presence of 14.4 mg/L Cr(VI )+ 26.2 mg/L SNB (red). The calculated second derivative profiles are shown for the lipid region (b); for the 1770-1490 1/cm window showing lipid C=O and the protein amide I and II peaks (c); and for the fingerprint region (d)
The FTIR spectra of L. aridicollis grown with or without the same pollutants are shown in Fig. 7a. The bacterium grown with the pollutants showed changes in the high-frequency lipid region (3000-2800 1/cm), in the protein amide I and II regions (1700-1500 1/cm), at 3500 1/cm assigned to the O-H stretching modes, at 1740 1/cm assigned to the lipid C=O vibration, and in the fingerprint region at 1335, 1161, 1083, 967, 935 and 860 1/cm. The second derivative profile also confirmed these variations (Fig. 7b,d).
The area-normalized FTIR absorbance spectra of L. aridicollis (a) grown in the absence (black) and presence of pollutants (14.4 mg/L Cr(VI) + 26.2 mg/L SNB) (red). The calculated second derivative profiles are shown for the lipid region (b); for the 1770-1490 1/cm window showing lipid C=O and the protein amide I and II peaks (c); and for the fingerprint region (d)
To quantify the observed changes, the areas of peaks corresponding to lipids, protein, and nucleic acid content were calculated by integrating the peaks from the normalized absorbance spectra. The results of these calculations are tabulated in Table 4.
The lipid content of M. oxydans significantly decreased when grown in the presence of pollutants; however, the lipid structure was not changed, as shown by the second derivative profile of the 3000-2800 1/cm region (Fig. 6b). The shrunk lipid area might indicate the synthesis of shorter chain lipids in the membrane (Kardas et al., 2014). However, the pollutants had the opposite effect on L. aridicollis; the lipid area calculation results showed that the lipid content had significantly increased compared to the control (Table 4). While the large peaks corresponding to the CH2 and CH3 stretching modes remained unchanged in their positions, the other C-H stretching modes showed differences, such as 3001, 2987, and 2900 1/cm shifted down to 2996, 2980, and 2896 1/cm, respectively. These positions are tentatively assigned to the C-H modes originating from cell structures other than the lipid acyl chains (Movasaghi et al., 2008; Vaynberg & Ng, 2005). Generally, a change in the lipid content is followed by membrane fluidity differences, which can be observed and measured as changes in bandwidth and which become more ordered and less fluid with increasing acyl chain length. However, this was not the case for either bacterium in this study. Such outcomes have been suggested to be due to changes in the number and/or type of molecules and proteins harbored by the membrane (Kardas et al., 2014).
The area of the 1740 1/cm band shrank slightly in the presence of pollutants for M. oxydans. The second derivative of the same region showed that the 1742 1/cm component shifts down to 1740 1/cm when grown with pollutants (Fig. 6c). Additionally, there was a change in the contribution of the 1723 1/cm component. It can be assigned to the H-bonded ester C=O groups. Therefore, it can be concluded that some of the C=O groups form H-bonds, which positions them at a lower wavenumber, hence leading to a shrinkage in the overall peak area at 1740 1/cm. On the other hand, the amplitude of the 1740 1/cm band significantly spiked for L. aridicollis. The second derivative profile of the same region for L. aridicollis showed components at 1744, 1730, and 1715 1/cm (Fig. 7c). For the same bacterium grown with the pollutants, these components relocated to 1740, 1725, and 1712 1/cm. The relative proportion of each component was also different compared to the control group. The shifting components and elevated peak areas indicate an increased number of C=O groups that interact more with the environment or with the membrane components. These findings also agree well with the observed changes in the C-H stretching modes (3000-2830 1/cm).
The pollutant-induced changes in the protein content and structure were followed from the amide I band (Table 4). For M. oxydans, it was positioned at 1644 1/cm when measured from the-center-of-mass. This position indicates a mixture of alpha helical- and beta sheet-structured protein content. The second derivative analysis showed four components constituting the amide I band at 1693, 1680, 1655, and 1637 1/cm (Fig. 6c). When grown with the pollutants, the 1637-component was replaced by a 1630-component with comparable size and by a second, but smaller 1639-component. The amide I peak position shifted down to 1640 1/cm, and the bandwidth expanded asymmetrically on the low wavenumber side as a result of this change in the structure. Both positions, 1637 and 1630 1/cm, can be assigned to the beta sheet secondary structure of proteins. Factors such as stronger H-bonding and tight packing shift the beta sheet signal to lower wavenumbers (Korkmaz et al., 2012). However, it remains uncertain whether the component at 1630 1/cm represents new protein structures or represents the existing beta sheet-structured proteins being affected by the changing environment. The fact that the amide I peak area also significantly expanded in the presence of pollutants suggests that both of the previously mentioned scenarios could have occured. Taken together, the data suggest altered protein structure and content for M. oxydans to adapt to the new conditions. For L. aridicollis, the amide I peak was at 1639 1/cm for the control group and, shifted to 1643 1/cm in the presence of pollutants (Fig. 7a). This shift was due to the increased contribution of the 1652 1/cm component, as shown by the second derivative profile (Fig. 7c). The amide I peak area also significantly increased. It can be suggested that the overall protein content of L. aridicollis increased due to elevated alpha helical- and/or random-structured proteins in the presence of pollutants. As for the amide II peak position, its shift from 1543 1/cm down to 1538 1/cm, and the declining intensity at 1514 1/cm also confirm alterations in the protein/enzyme population within the cell. In some studies, the increase in protein concentration has been suggested to serve as a coping mechanism when exposed to a toxic environment (Gupta et al., 1992; Gupta et al., 1993; Jarosławiecka & Piotrowska-Seget, 2014; Kepenek et al., 2019; Naik & Dubey, 2013; Nithya et al., 2011). Promoted production of metal sequestering proteins, metal efflux proteins, and metal and toxic molecule binding transmembrane proteins have been proposed as the survival mechanism in such environments.
The nucleic acid content was studied by measuring the asymmetric and symmetric stretching mode of the phosphate groups originating mainly from RNA and DNA with a lesser contribution from phospholipid headgroups. In both bacteria, M. oxydans and L. aridicollis, the amount of nucleic acid declined significantly, suggesting adaptation at the DNA/RNA level to environmental pollutants. The second derivative profiles also showed changes at 970, 960, 930, and 914 1/cm, confirming changes at the nucleic acid level (Fig. 6d-Fig. 7d).
3.8 SEM Analysis
When the bacteria were exposed to 14.4 mg/L Cr(VI) + 26.2 mg/L SNB, M. oxydans kept its cell size (Fig. 8a, b) while the cell size of L. aridicollis became smaller (Fig. 9a, b). In a pollutant-free media, the average cell size of M. oxydans was measured as 1 μm ± 0.2, while it was 0.9 μm ± 0.2 in the media with pollutants. On the other hand, the shrinking of L. aridicollis was more noteworthy than that of M. oxydans and was measured to be nearly 30% in media with pollutants compared to the size in pollutant-free media. Unfavorable conditions, such as exposure to organic and inorganic pollutants, induce several stress response mechanisms to withstand the heavy toxicity of pollutants. Among these mechanisms are the morphological changes in bacterial size and shape (Mathivanan et al., 2021). Louati et al. (2020) reported small changes in the bacterial cell size upon exposure to Cd(II) (70 mg/L). In line with our findings, Giovanella et al. (2017) and Naik and Dubey (2011) reported that the cell size of Pseudomonas spp. decreased when the bacterium was exposed to heavy metals.
4 Conclusions
Lately, there has been a surge in the use of mixed cultures for pollutant removal processes. This is partly due to the rise in the availability of molecular tools that aid the study of the composition and interactions within microbial communities. Nevertheless, mixed cultures can pose a challenge in their study and interpretation as the results may not always be consistent. In contrast, pure cultures enable more reliable data and a deeper insight into biodegradation mechanisms (Mohanty et al., 2023).
The current research investigates the treatment of water polluted with SNB dye and Cr(VI) using a mixed bacterial culture. The optimum conditions were determined as for the highest capacity demonstrated by the mixed bacterial culture to remove pollutants. Accordingly, the highest capacity occurred at pH 8 with a biomass concentration of 8% (v/v) of mixed culture (50 mg/L SNB dye and 25 mg/L Cr(VI)). Under these conditions, the removal percentages were 100% for both Cr(VI) and the SNB dye. The bacterial strains showing the highest bioremediation capacity were isolated and identified as M. oxydans and L. aridicollis. When the morphological changes of pure bacterial cultures were compared via SEM upon exposure to the pollutant, it was observed that the size of M. oxydans remained the same, while L. aridicollis decreased in size.
The presence of various functional groups and structures of the microorganisms that are involved in the removal mechanisms were confirmed by FTIR analysis, showing that both bacteria adapt structural alterations to cope with the new environment. Still these mechanisms seem to be different for each; while M. oxydans conformed by forming less lipids and more protein content, L. aridicollis did so by generating more lipids and protein content compared to their controls. The analysis of the lipid acyl chain and the ester C=O group characteristics suggest differences in the membrane-harbored components in both bacteria. The nucleic acid characteristics are also affected, implying structural modifications of cell components for adaptation.
To sum up, both the mixed cultures and the pure bacterial strains of M. oxydans and L. aridicollis have demonstrated promising potentials as microbial agents for the removal of both dye and heavy metal content from wastewaters.
Data Availability
The data that support the findings of this study are not available on the public domain, and can be provided by the corresponding author upon reasonable request.
References
Afrin, S., Shuvo, H. R., Sultana, B., Islam, F., Rus'd, A. A., Begum, S., & Hossain, M. N. (2021). The degradation of textile industry dyes using the effective bacterial consortium. Heliyon, 7. https://doi.org/10.1016/j.heliyon.2021.e08102
Alaya, V., Kodi, R. K., Ninganna, E., Gowda, M., & Shivanna, M. B. (2021). Decolorization of Malachite green dye by Stenotrophomonas maltophilia a compost bacterium. Abstract Bulletin of the National Research Centre, 45(81), 1–13. https://doi.org/10.1186/s42269-021-00518-w
Ashrafi, Z., Heidari, P., & Mojerlou, S. (2022). Does the long-term contamination of lead (PbII) affect the bioremediation mechanisms of Microbacterium oxydans strain CM3 and CM7? Soil and Sediment Contamination: An International Journal, 31, 959–973. https://doi.org/10.1080/15320383.2022.2028719
Bera, S. P., & Tank, S. K. (2021). Screening and identification of newly isolated Pseudomonas sp. for biodegrading the textile azo dye CI Procion Red H-3B. Journal of Applied Microbiology, 130(6), 1949–1959. https://doi.org/10.1111/jam.14920
Chaudhari, A. U., Tapase, S. R., Markad, V. L., & Kodam, K. M. (2013). Simultaneous decolorization of reactive Orange M2R dye and reduction of chromate by Lysinibacillus sp. KMK-A. Journal of Hazardous Materials, 262, 580–588. https://doi.org/10.1016/j.jhazmat.2013.09.006
Dang Son, B. H., Quang Mai, V., Xuan Du, D., Hai Phong, N., & Quang Khieu, D. (2016). A study on astrazon black AFDL dye adsorption onto Vietnamese diatomite. Journal of Chemistry, 2016. https://doi.org/10.1155/2016/8685437
Emadi, Z., Sadeghi, M., Forouzandeh, S., Sadeghi, R., Sadeghi, R., & Mohammadi-Moghadam, F. (2022). Simultaneous decolorization/degradation of AB-113 and chromium (VI) removal by a salt-tolerant Klebsiella sp. AB-PR and detoxification of biotransformed-metabolites. International journal of Environmental Science and Technology, 19, 2007–2024. https://doi.org/10.1007/s13762-021-03360-9
Ghanbari, M., Dargahi, A., Ahmadi, M., Ghafari, S., Jaafarzadeh, N., & Jorfi, S. (2022). Biodegradation of Acid Orange 7 dye using consortium of novel bacterial strains isolated from Persian Gulf water and soil contaminated with petroleum compounds. Biomass Conversion and Biorefinery, 1-12. https://doi.org/10.1007/s13399-022-03692-7
Giovanella, P., Cabral, L., Costa, A. P., de Oliveira Camargo, F. A., Gianello, C., & Bento, F. M. (2017). Metal resistance mechanisms in Gram-negative bacteria and their potential to remove Hg in the presence of other metals. Ecotoxicology and Environmental Safety, 140, 162–169. https://doi.org/10.1016/j.ecoenv.2017.02.010
Gupta, A., Morby, A. P., Turner, J. S., Whitton, B. A., & Robinson, N. J. (1993). Deletion within the metallothionein locus of cadmium-tolerant Synechococcus PCC 6301 involving a highly iterated palindrome (HIP1). Molecular Microbiology, 7(2), 189–195. https://doi.org/10.1111/j.1365-2958.1993.tb01110.x
Gupta, A., Whitton, B. A., Morby, A. P., Huckle, J. W., & Robinson, N. J. (1992). Amplification and rearrangement of a prokaryotic metallothionein locus smt in Synechococcus PCC 6301 selected for tolerance to cadmium. Proceedings of the Royal Society of London. Series B: Biological Sciences, 248, 273–281. https://doi.org/10.1098/rspb.1992.0072
Haripriyan, U., Arun, J., Gopinath, K. P., Mythili, R., Kim, W., & Govarthanan, M. (2023). A mini-review on innovative strategies for simultaneous microbial bioremediation of toxic heavy metals and dyes from wastewater. Archives of Microbiology, 205, 29. https://doi.org/10.1007/s00203-022-03367-x
Jarosławiecka, A., & Piotrowska-Seget, Z. (2014). Lead resistance in micro-organisms. Microbiology, 160, 12–25. https://doi.org/10.1099/mic.0.070284-0
Kardas, M., Gozen, A. G., & Severcan, F. (2014). FTIR spectroscopy offers hints towards widespread molecular changes in cobalt-acclimated freshwater bacteria. Aquatic Toxicology, 155, 15–23. https://doi.org/10.1016/j.aquatox.2014.05.027
Kepenek, E. S., Gozen, A. G., & Severcan, F. (2019). Molecular characterization of acutely and gradually heavy metal acclimated aquatic bacteria by FTIR spectroscopy. Journal of Biophotonics, 12, e201800301. https://doi.org/10.1002/jbio.201800301
Kholisa, B., Matsena, M., & Chirwa, E. M. (2021). Evaluation of Cr (VI) reduction using indigenous bacterial consortium isolated from a municipal wastewater sludge: Batch and kinetic studies. Catalysts, 11, 1100. https://doi.org/10.3390/catal11091100
Korkmaz, F., Köster, S., Yildiz, Ö., & Mäntele, W. (2012). In situ opening/closing of OmpG from E. coli and the splitting of β-sheet signals in ATR–FTIR spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 91, 395–401. https://doi.org/10.1016/j.saa.2012.01.025
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
Liu, W., Liu, C., Liu, L., You, Y., Jiang, J., Zhou, Z., & Dong, Z. (2017). Simultaneous decolorization of sulfonated azo dyes and reduction of hexavalent chromium under high salt condition by a newly isolated salt-tolerant strain Bacillus circulans BWL1061. Ecotoxicology and Environmental Safety, 141, 9–16. https://doi.org/10.1016/j.ecoenv.2017.03.005
Louati, I., Elloumi-Mseddi, J., Cheikhrouhou, W., Hadrich, B., Nasri, M., Aifa, S., et al. (2020). Simultaneous cleanup of Reactive Black 5 and cadmium by a desert soil bacterium. Ecotoxicology and Environmental Safety, 190, 110103. https://doi.org/10.1016/j.ecoenv.2019.110103
Lu, F., & Astruc, D. (2020). Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coordination Chemistry Reviews, 408, 213180. https://doi.org/10.1016/j.ccr.2020.213180
Lyu, Y., Yang, T., Liu, H., Qi, Z., Li, P., Shi, Z., et al. (2021). Enrichment and characterization of an effective hexavalent chromium-reducing microbial community YEM001. Environmental Science and Pollution Research, 28, 19866–19877. https://doi.org/10.1007/s11356-020-11863-0
Mathivanan, K., Chandirika, J. U., Vinothkanna, A., Yin, H., Liu, X., & Meng, D. (2021). Bacterial adaptive strategies to cope with metal toxicity in the contaminated environment–A review. Ecotoxicology and Environmental Safety, 226, 112863. https://doi.org/10.1016/j.ecoenv.2021.112863
Mishra, S., & Maiti, A. (2019). Applicability of enzymes produced from different biotic species for biodegradation of textile dyes. Clean Technologies and Environmental Policy, 21, 763–781. https://doi.org/10.1007/s10098-019-01681-5
Mohanty, S., Dash, S., Pradhan, N., & Maji, S. K. (2023). Bio-remediation of organic dyes from wastewater by microbial colony—a short review. Nano-engineered Materials for Textile Waste Remediation, 61-104. https://doi.org/10.1007/978-981-19-7978-1_4
Morais, P. V., Francisco, R., Branco, R., Chung, A. P., & Da Costa, M. S. (2004). Leucobacter chromiireducens sp. nov, and Leucobacter aridicollis sp. nov., two new species isolated from a chromium contaminated environment. Systematic and Applied Microbiology, 27(6), 646–652. https://doi.org/10.1078/0723202042369983
Movasaghi, Z., Rehman, S., & ur Rehman, D. I. (2008). Fourier transform infrared (FTIR) spectroscopy of biological tissues. Applied Spectroscopy Reviews, 43, 134–179. https://doi.org/10.1080/05704920701829043
Naik, M. M., & Dubey, S. K. (2011). Lead-enhanced siderophore production and alteration in cell morphology in a Pb-resistant Pseudomonas aeruginosa strain 4EA. Current Microbiology, 62, 409–414.
Naik, M. M., & Dubey, S. K. (2013). Lead resistant bacteria: lead resistance mechanisms, their applications in lead bioremediation and biomonitoring. Ecotoxicology and Environmental Safety, 98, 1–7. https://doi.org/10.1016/j.ecoenv.2013.09.039
Nithya, C., Gnanalakshmi, B., & Pandian, S. K. (2011). Assessment and characterization of heavy metal resistance in Palk Bay sediment bacteria. Marine Environmental Research, 71, 283–294. https://doi.org/10.1016/j.marenvres.2011.02.003
Pavithra, K. G., & Jaikumar, V. J. J. O. I. (2019). Removal of colorants from wastewater: A review on sources and treatment strategies. Journal of Industrial and Engineering Chemistry, 75, 1–19. https://doi.org/10.1016/j.jiec.2019.02.011
Plestenjak, E., Kraigher, B., Leskovec, S., Mandic Mulec, I., Marković, S., Ščančar, J., & Milačič, R. (2022). Reduction of hexavalent chromium using bacterial isolates and a microbial community enriched from tannery effluent. Scientific Reports, 12, 20197. https://doi.org/10.1038/s41598-022-24797-z
Rahayu, F., Mustafa, I., Marjani, R. F., Qazi, R. A., Zeb, K., & Ullah, N. (2023). Newly Isolated Ligninolytic Bacteria and Its Applications for Multiple Dye Degradation. Water, Air, & Soil Pollution, 234, 359. https://doi.org/10.1007/s11270-023-06377-7
Rahman, Z. (2020). An overview on heavy metal resistant microorganisms for simultaneous treatment of multiple chemical pollutants at co-contaminated sites, and their multipurpose application. Journal of Hazardous Materials, 396, 122682. https://doi.org/10.1016/j.jhazmat.2020.122682
Snell, F. D., & Snell, C. T. (1959). Colorimetric methods of analysis. D. van Nostrand.
Sudarshan, S., Harikrishnan, S., RathiBhuvaneswari, G., Alamelu, V., Aanand, S., Rajasekar, A., & Govarthanan, M. (2023). Impact of textile dyes on human health and bioremediation of textile industry effluent using microorganisms: current status and future prospects. Journal of Applied Microbiology, 134, lxac064. https://doi.org/10.1093/jambio/lxac064
Talari, A. C. S., Martinez, M. A. G., Movasaghi, Z., Rehman, S., & Rehman, I. U. (2017). Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Applied Spectroscopy Reviews, 52, 456–506. https://doi.org/10.1080/05704928.2016.1230863
Tamura, K., & Nei, M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10, 512–526. https://doi.org/10.1093/oxfordjournals.molbev.a040023
Tan, H., Wang, C., Zeng, G., Luo, Y., Li, H., & Xu, H. (2020). Bioreduction and biosorption of Cr (VI) by a novel Bacillus sp. CRB-B1 strain. Journal of Hazardous Materials, 386, 121628. https://doi.org/10.1016/j.jhazmat.2019.121628
Tufail, M. A., Iltaf, J., Zaheer, T., Tariq, L., Amir, M. B., Fatima, R., Asbat, A., Kabeer, T., Fahad, M., Naeem, H., Shoukat, U., Noor, H., Awais, M., Umar, W., & Ayyub, M. (2022). Recent advances in bioremediation of heavy metals and persistent organic pollutants: A review. Science of the Total Environment, 850, 157961. https://doi.org/10.1016/j.scitotenv.2022.157961
Vaynberg, J., & Ng, L. M. (2005). Surface chemistry of fluoroethanols II. a FTIR study of the reaction of 2, 2, 2-trifluoroethanol on Al2O3 surface. Surface Science, 577, 188–199. https://doi.org/10.1016/j.susc.2004.12.032
Zabłocka-Godlewska, E., & Przystaś, W. (2020). Fed-batch decolourization of mixture of Brilliant Green and Evans Blue by bacteria species applied as pure and mixed cultures: influence of growth conditions. Water, Air, & Soil Pollution, 231, 1–23. https://doi.org/10.1007/s11270-020-4441-1
Zeng, G., He, Y., Liang, D., Wang, F., Luo, Y., Yang, H., Wang, Q., Wang, J., Pei Gao, P., Wen, X., Chunyi Yu, C., & Sun, D. (2022). Adsorption of heavy metal ions copper cadmium and nickel by Microcystis aeruginosa. International Journal of Environmental Research and Public Health, 19(21), 13867. https://doi.org/10.3390/ijerph192113867
Acknowledgments
We are grateful for the financial support provided by the Ankara University Research Fund under Project No. FYL-2023-2873.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
A. İrem Gündüz (Formal analysis, Investigation, Project administration, Software, Writing – original draft), Esra Erkoç (Formal analysis, Investigation, Software, Writing – original draft), Filiz Korkmaz (Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing), Nur Koçberber Kılıç (Data curation, Investigation, Project administration, Supervision, Validation, Writing – review & editing).
Corresponding author
Ethics declarations
Conflict of Interest
No potential conflict of interest is reported by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gündüz, A.İ., Erkoç, E., Korkmaz, F. et al. Simultaneous Removal of Setazol Navy Blue and Cr(VI) By Mixed Microbial Culture Isolated from the Çubuk Stream. Water Air Soil Pollut 235, 345 (2024). https://doi.org/10.1007/s11270-024-07173-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07173-7