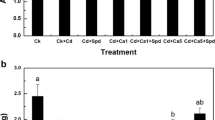
Abstract
Heavy metals are natural components that are formed as a result of biotic activities, accumulate in the ecosystem because they cannot be biodegraded, and thus cause environmental stress. In this study, the alleviation effect of the ectoine, which is the subject of many studies, on cadmium (Cd) toxicity was tested. Ectoine is produced by various bacteria in response to osmotic stress. Many different properties of this compound have been proven and studies on these compounds are constantly increasing. In the current study, we determined that ectoine has not any detrimental effect on plant (wheat) germination rate, antioxidant enzyme levels, H2O2 and malondialdehyde (MDA) contents. On the other hand, co-treatment of ectoine with Cd (110 ppm) significantly alleviated Cd toxicity [increased germination rate, antioxidant enzyme levels and genomic template stability (GTS) and decreased the content of H2O2 and MDA]. The findings mentioned above reports for the first time that ectoine has an amelioration effect on Cd toxicity. The alleviation effect of ectoine might arise from its reactive oxygen scavenging (singlet oxygen and hydroxyl radical) traits and macromolecule (DNA and protein) protection properties through which it decreases water molecules around macromolecules.
Novelty Statement
The findings of this study report for the first time that ectoine has an amelioration effect on Cd toxicity. The amelioration effect of ectoine against toxicity of cadmium was demonstrated by evaluating germination ratio, random amplified polymorphic DNA, content of H2O2 and MDA and antioxidant enzymes. The results of the study show that ectoine ameliorates the toxicity caused by cadmium in all the analysed parameters.









Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article.
References
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology., 105, 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Agar, G., Bozari, S., Adıgüzel, A., Barış, Ö., Güllüce, M., Şengül, M., & Şahin, F. (2009). Phenotypic and genetic variation among Astragalus species from Turkey. Romanian Biotechnology Letters, 14(2), 4267–4274.
Ahmad, P., Sarwat, M., Bhat, N. A., Wani, M. R., Kazi, A. G., & Tran, L.-S.P. (2015). Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS One, 10(1), e0114571. https://doi.org/10.1371/journal.pone.0114571
Ali, B., Deng, X., Hu, X., Gill, R., Ali, S., Wang, S., & Zhou, W. (2015). Deteriorative effects of cadmium stress on antioxidant system and cellular structure in germinating seeds of Brassica napus L. Journal of Agricultural Science and Technology, 17(1), 63–74.
Alizadeh, E., & Sanche, L. (2013). Role of humidity and oxygen level on damage to DNA induced by soft X-rays and low-energy electrons. Journal of Physical Chemistry C, 117(43), 22445–22453.
Anjum, N. A., Sofo, A., Scopa, A., Roychoudhury, A., Gill, S. S., Iqbal, M., Lukatkin, A. S., Pereira, E., Duarte, A. C., & Ahmad, I. (2015). Lipids and proteins—major targets of oxidative modifications in abiotic stressed plants. Environmental Science and Pollution Research, 22(6), 4099–4121. https://doi.org/10.1007/s11356-014-3917-1
Bandyopadhyay, A., & Mukherjee, A. (2011). Sensitivity of Allium and Nicotiana in cellular and acellular comet assays to assess differential genotoxicity of direct and indirect acting mutagens. Ecotoxicology and Environmental Safety, 74(4), 860–865. https://doi.org/10.1016/j.ecoenv.2010.12.002
Banerjee, A., & Roychoudhury, A. (2018). Abiotic stress, generation of reactive oxygen species, and their consequences: An overview. In S. Vijay Pratap, S. Samiksha, T. Durgesh Kumar, P. Sheo Mohan, & C. Devendra Kumar (Eds.), Reactive oxygen species in plants: Boon or bane? Revisiting the role of ROS (pp. 23–50). John Wiley & Sons Ltd.
Barceló, J., & Poschenrieder, C. (1990). Plant water relations as affected by heavy metal stress: A review. Journal of Plant Nutrition, 13(1), 1–37. https://doi.org/10.1080/01904169009364057
Baruah, N., Mondal, S. C., Farooq, M., & Gogoi, N. (2019). Influence of heavy metals on seed germination and seedling growth of wheat, pea, and tomato. Water, Air, and Soil Pollution, 230(12), 1–15. https://doi.org/10.1007/s11270-019-4329-0
Baycu, G., Tolunay, D., Özden, H., & Günebakan, S. (2006). Ecophysiological and seasonal variations in Cd, Pb, Zn, and Ni concentrations in the leaves of urban deciduous trees in Istanbul. Environmental Pollution, 143(3), 545–554. https://doi.org/10.1016/j.envpol.2005.10.050
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase improved assays and assay applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287.
Bilstein, A., Heinrich, A., Rybachuk, A., & Mösges, R. (2021). Ectoine in the treatment of irritations and inflammations of the eye surface. BioMed Research International, 2021, 16. https://doi.org/10.1155/2021/8885032
Borgohain, P., Saha, B., Agrahari, R., Chowardhara, B., Sahoo, S., van der Vyver, C., & Panda, S. K. (2019). Sl NAC2 overexpression in Arabidopsis results in enhanced abiotic stress tolerance with alteration in glutathione metabolism. Protoplasma, 256(4), 1065–1077. https://doi.org/10.1007/s00709-019-01368-0
Boroujeni, M. B., & Nayeri, H. (2018). Stabilization of bovine lactoperoxidase in the presence of ectoine. Food Chemistry, 265, 208–215.
Bownik, A., Zofia, S., & Tadeusz, S. (2014). Protective effects of ectoine on heat-stressed Daphnia magna. Journal of Comparative Physiology B, 184(8), 961–976.
Brands, S., Schein, P., Castro-Ochoa, K. F., & Galinski, E. A. (2019). Hydroxyl radical scavenging of the compatible solute ectoine generates two N-acetimides. Archives of Biochemistry and Biophysics, 674, 108097.
Buenger, J., & Driller, H. (2004). Ectoin: An effective natural substance to prevent UVA-induced premature photoaging. Skin Pharmacology and Physiology, 17(5), 232–237.
Buommino, E., Schiraldi, C., Baroni, A., Paoletti, I., Lamberti, M., De Rosa, M., & Tufano, M. A. (2005). Ectoine from halophilic microorganisms induces the expression of hsp70 and hsp70B′ in human keratinocytes modulating the proinflammatory response. Cell Stress and Chaperones, 10(3), 197.
Casalino, E., Sblano, C., Calzaretti, G., & Landriscina, C. (2006). Acute cadmium intoxication induces alpha-class glutathione S-transferase protein synthesis and enzyme activity in rat liver. Toxicology, 217(2–3), 240–245. https://doi.org/10.1016/j.tox.2005.09.020
Catalá, A. (2006). An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. International Journal of Biochemistry & Cell Biology, 38(9), 1482–1495. https://doi.org/10.1016/j.biocel.2006.02.010
Chowardhara, B., Borgohain, P., Saha, B., Awasthi, J. P., & Panda, S. K. (2020). Differential oxidative stress responses in Brassica juncea (L.) Czern and Coss cultivars induced by cadmium at germination and early seedling stage. Acta Physiologiae Plantarum, 42, 1–12. https://doi.org/10.1007/s11738-020-03094-0
Chrestensen, C. A., Starke, D. W., & Mieyal, J. J. (2000). Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. Journal of Biological Chemistry, 275(34), 26556–26565. https://doi.org/10.1074/jbc.M004097200
Clemens, S. (2006). Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie, 88(11), 1707–1719. https://doi.org/10.1016/j.biochi.2006.07.003
Cobbett, C., & Goldsbrough, P. (2002). Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology, 53(1), 159–182. https://doi.org/10.1146/annurev.arplant.53.100301.135154
Dai, T., Wang, Y., & Yang, G. (2022). Visualization of DNA damage and protection by atomic force microscopy in liquid. International Journal of Molecular Sciences, 23(8), 4388.
Demidchik, V. (2015). Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environmental and Experimental Botany, 109, 212–228. https://doi.org/10.1016/j.envexpbot.2014.06.021
Demir, E., Qin, T., Li, Y., Zhang, Y., Guo, X., Ingle, T., Yan, J., Orza, A. I., Biris, A. S., & Ghorai, S. (2020). Cytotoxicity and genotoxicity of cadmium oxide nanoparticles evaluated using in vitro assays. Mutation Research, Genetic Toxicology and Environmental Mutagenesis, 850, 503149.
El-Amier, Y., Elhindi, K., El-Hendawy, S., Al-Rashed, S., & Abd-ElGawad, A. (2019). Antioxidant system and biomolecules alteration in Pisum sativum under heavy metal stress and possible alleviation by 5-aminolevulinic acid. Molecules, 24(22), 4194. https://doi.org/10.3390/molecules24224194
Esterbauer, H., & Cheeseman, K. H. (1990). Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods in Enzymology, 186, 407–421. https://doi.org/10.1016/0076-6879(90)86134-H
Faller, P., Kienzler, K., & Krieger-Liszkay, A. (2005). Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+ site. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1706(1–2), 158–164. https://doi.org/10.1016/j.bbabio.2004.10.005
Farmer, E. E., & Mueller, M. J. (2013). ROS-mediated lipid peroxidation and RES-activated signaling. Annual Review of Plant Biology, 64, 429–450. https://doi.org/10.1146/annurev-arplant-050312-120132
Galinski, E. A. (1993). Compatible solutes of halophilic eubacteria: Molecular principles, water-solute interaction, stress protection. Experientia, 49(6), 487–496.
Galinski, E. A., Stein, M., Amendt, B., & Kinder, M. (1997). The kosmotropic (structure-forming) effect of compensatory solutes. Comparative Biochemistry and Physiology, 117(3), 357–365.
Garnier, L., Simon Plas, F., Thuleau, P., Agnel, J.-P., Blein, J.-P., Ranjeva, R., & Montillet, J.-l. (2006). Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant, Cell and Environment, 29(10), 1956–1969. https://doi.org/10.1111/j.1365-3040.2006.01571.x
Graf, R., Anzali, S., Buenger, J., Pfluecker, F., & Driller, H. (2008). The multifunctional role of ectoine as a natural cell protectant. Clinics in Dermatology, 26(4), 326–333. https://doi.org/10.1016/j.clindermatol.2008.01.002
Grether-Beck, S., Timmer, A., Felsner, I., Brenden, H., Brammertz, D., & Krutmann, J. (2005). Ultraviolet A-induced signaling involves a ceramide-mediated autocrine loop leading to ceramide de novo synthesis. The Journal of Investigative Dermatology, 125(3), 545–553. https://doi.org/10.1111/j.0022-202X.2005.23782.x
Hahn, M. B., Meyer, S., Schröter, M.-A., Kunte, H.-J., Solomun, T., & Sturm, H. (2017). DNA protection by ectoine from ionizing radiation: Molecular mechanisms. Physical Chemistry Chemical Physics, 19(37), 25717–25722. https://doi.org/10.1039/C7CP02860A
Hashem, A., Abd_Allah, E. F., Alqarawi, A. A., Malik, J. A., Wirth, S., & Egamberdieva, D. (2019). Role of calcium in AMF-mediated alleviation of the adverse impacts of cadmium stress in Bassia indica [Wight] AJ Scott. Saudi Journal of Biological Sciences, 26(4), 828–838. https://doi.org/10.1016/j.sjbs.2016.11.003
He, J., Ren, Y., Chen, X., & Chen, H. (2014). Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L.) under cadmium stress. Ecotoxicology and Environmental Safety, 108, 114–119. https://doi.org/10.1016/j.ecoenv.2014.05.021
Idrees, S., Shabir, S., Ilyas, N., Batool, N., & Kanwal, S. (2015). Assessment of cadmium on wheat (Triticum aestivum L.) in hydroponics medium. Agrociencia, 49(8), 917–929.
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60. https://doi.org/10.2478/intox-2014-0009
Jozefczak, M., Keunen, E., Schat, H., Bliek, M., Hernández, L. E., Carleer, R., Remans, T., Bohler, S., Vangronsveld, J., & Cuypers, A. (2014). Differential response of Arabidopsis leaves and roots to cadmium: Glutathione-related chelating capacity vs antioxidant capacity. Plant Physiology and Biochemistry, 83, 1–9. https://doi.org/10.1016/j.plaphy.2014.07.001
Jung, H.-i, Lee, B.-R., Chae, M.-J., Lee, E.-J., Lee, T.-G., Jung, G.-B., Kim, M.-S., & Lee, J. (2020). Ascorbate-mediated modulation of cadmium stress responses: Reactive oxygen species and redox status in Brassica napus. Frontiers in Plant Science, 11, 1823. https://doi.org/10.3389/fpls.2020.586547
Kanapathipillai, M., Ku, S. H., Girigoswami, K., & Park, C. B. (2008). Small stress molecules inhibit aggregation and neurotoxicity of prion peptide 106–126. Biochemical and Biophysical Research Communications, 365(4), 808–813. https://doi.org/10.1016/j.bbrc.2007.11.074
Kapoor, D., Kaur, S., & Bhardwaj, R. (2014). Physiological and biochemical changes in Brassica juncea plants under Cd-induced stress. BioMed Research International, 2014, 13. https://doi.org/10.1155/2014/726070
Kaya, C., Ashraf, M., Alyemeni, M. N., & Ahmad, P. (2020). Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiologia Plantarum, 168(2), 345–360. https://doi.org/10.1111/ppl.13012
Khan, A., Khan, S., Khan, M. A., Qamar, Z., & Waqas, M. (2015). The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environmental Science and Pollution Research, 22(18), 13772–13799. https://doi.org/10.1007/s11356-015-4881-0
Li, S., Yang, W., Yang, T., Chen, Y., & Ni, W. (2015). Effects of cadmium stress on leaf chlorophyll fluorescence and photosynthesis of Elsholtzia argyi—a cadmium accumulating plant. International Journal of Phytoremediation, 17(1), 85–92. https://doi.org/10.1080/15226514.2013.828020
Lippert, K., & Galinski, E. A. (1992). Enzyme stabilization be ectoine-type compatible solutes: Protection against heating, freezing and drying. Applied Microbiology and Biotechnology, 37(1), 61–65.
Liu, W., Li, P., Qi, X., Zhou, Q., Zheng, L., Sun, T., & Yang, Y. (2005). DNA changes in barley (Hordeum vulgare) seedlings induced by cadmium pollution using RAPD analysis. Chemosphere, 61(2), 158–167. https://doi.org/10.1016/j.chemosphere.2005.02.078
Maheshwari, R., & Dubey, R. (2009). Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regulation, 59(1), 37–49. https://doi.org/10.1007/s10725-009-9386-8
Manquián-Cerda, K., Escudey, M., Zúñiga, G., Arancibia-Miranda, N., Molina, M., & Cruces, E. (2016). Effect of cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets grown in vitro. Ecotoxicology and Environmental Safety, 133, 316–326. https://doi.org/10.1016/j.ecoenv.2016.07.029
Martins, L. L., Mourato, M. P., Cardoso, A. I., Pinto, A. P., Mota, A. M., Maria de Lurdes, S. G., & de Varennes, A. (2011). Oxidative stress induced by cadmium in Nicotiana tabacum L.: effects on growth parameters, oxidative damage and antioxidant responses in different plant parts. Acta Physiologiae Plantarum, 33(4), 1375–1383. https://doi.org/10.1007/s11738-010-0671-y
Mishra, S., Jha, A., & Dubey, R. (2011). Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma, 248(3), 565–577. https://doi.org/10.1007/s00709-010-0210-0
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7(9), 405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Muradoglu, F., Gundogdu, M., Ercisli, S., Encu, T., Balta, F., Jaafar, H. Z., & Zia-Ul-Haq, M. (2015). Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biological Research, 48, 1–7. https://doi.org/10.1186/S40659-015-0001-3
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22(5), 867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nikolić, N., Zorić, L., Cvetković, I., Pajević, S., Borišev, M., Orlović, S., & Pilipović, A. (2017). Assessment of cadmium tolerance and phytoextraction ability in young Populus deltoides L. and Populus× euramericana plants through morpho-anatomical and physiological responses to growth in cadmium enriched soil. Forest-Biogeosciences and Forestry., 10(3), 635. https://doi.org/10.3832/ifor2165-010
Nouairi, I., Ammar, W. B., Youssef, N. B., Miled, D. D. B., Ghorbal, M. H., & Zarrouk, M. (2009). Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiologiae Plantarum, 31(2), 237–247. https://doi.org/10.1007/s11738-008-0224-9
Olmos, E., Martínez-Solano, J. R., Piqueras, A., & Hellín, E. (2003). Early steps in the oxidative burst induced by cadmium in cultured tobacco cells (BY-2 line). Journal of Experimental Botany, 54(381), 291–301. https://doi.org/10.1093/jxb/erg028
Oprzeska-Zingrebe, E. A., Meyer, S., Roloff, A., Kunte, H.-J., & Smiatek, J. (2018). Influence of compatible solute ectoine on distinct DNA structures: Thermodynamic insights into molecular binding mechanisms and destabilization effects. Physical Chemistry Chemical Physics, 20(40), 25861–25874. https://doi.org/10.1039/C8CP03543A
Ozfidan-Konakci, C., Elbasan, F., Arikan, B., Alp, F. N., Yildiztugay, E., Keles, R., & Kucukoduk, M. (2022). Ex-foliar applied extremolyte ectoine improves water management, photosystem, antioxidant system and redox homeostasis in Zea mays under cadmium toxicity. South+A1443 African Journal of Botany, 147, 130–141.
Parwata, I. P., Wahyuningrum, D., Suhandono, S., & Hertadi, R. (2021). Ability of ectoine to stabilize lipase against elevated temperatures and methanol concentrations. Indonesian Journal of Chemistry, 21(2), 494–501.
Porebski, S., Bailey, L. G., & Baum, B. R. (1997). Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Molecular Biology Reporter, 15(1), 8–15.
Razinger, J., Dermastia, M., Koce, J. D., & Zrimec, A. (2008). Oxidative stress in duckweed (Lemna minor L.) caused by short-term cadmium exposure. Environmental Pollution, 153(3), 687–694. https://doi.org/10.1016/j.envpol.2007.08.018
Rombel-Bryzek, A., Rajfur, M., Żuk, O., & Zając, P. (2018). The effect of cadmium on oxidative stress in Beta vulgaris. Ecological Chemistry and Engineering, 25(3), 457–467. https://doi.org/10.1515/eces-2018-0031
Romero-Puertas, M., Rodríguez-Serrano, M., Corpas, F., Gomez, M. D., Del Rio, L., & Sandalio, L. (2004). Cadmium-induced subcellular accumulation of O2·− and H2O2 in pea leaves. Plant, Cell and Environment, 27(9), 1122–1134. https://doi.org/10.1111/j.1365-3040.2004.01217.x
Sajjad, W., Qadir, S., Ahmad, M., Rafiq, M., Hasan, F., Tehan, R., McPhail, K., & Shah, A. (2018). Ectoine: a compatible solute in radio-halophilic Stenotrophomonas sp. WMA-LM 19 strain to prevent ultraviolet-induced protein damage. Journal of Applied Microbiology, 125(2), 457–467. https://doi.org/10.1111/jam.13903
Salmannejad, F., & Nafissi-Varcheh, N. (2017). Ectoine and hydroxyectoine inhibit thermal-induced aggregation and increase thermostability of recombinant human interferon Alfa2b. European Journal of Pharmaceutical Sciences, 97, 200–207.
Salvador, M., Argandoña, M., Naranjo, E., Piubeli, F., Nieto, J. J., Csonka, L. N., & Vargas, C. (2018). Quantitative RNA-seq analysis unveils osmotic and thermal adaptation mechanisms relevant for ectoine production in Chromohalobacter salexigens. Frontiers in Microbiology., 9, 1845. https://doi.org/10.3389/fmicb.2018.01845
Schröter, M.-A., Meyer, S., Hahn, M. B., Solomun, T., Sturm, H., & Kunte, H.-J. (2017). Ectoine protects DNA from damage by ionizing radiation. Science and Reports, 7(1), 1–7. https://doi.org/10.1038/s41598-017-15512-4
Shafiq, M., & Iqbal, M. Z. (2006). The toxicity effects of heavy metals on germination and seedling growth of Cassia siamea Lamk. Journal of New Seeds, 7(4), 95–105. https://doi.org/10.1300/J153v07n04_07
Shah, K., Kumar, R. G., Verma, S., & Dubey, R. (2001). Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Science, 161(6), 1135–1144. https://doi.org/10.1016/S0168-9452(01)00517-9
Shamsi, I., Wei, K., Zhang, G., Jilani, G., & Hassan, M. (2008). Interactive effects of cadmium and aluminum on growth and antioxidative enzymes in soybean. Biologia Plantarum, 52(1), 165–169. https://doi.org/10.1007/s10535-008-0036-1
Sharma, P., & Dubey, R. S. (2005). Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regulation, 46(3), 209–221. https://doi.org/10.1007/s10725-005-0002-2
Sharma, V. K., & Rokita, S. E. (2012). Oxidation of amino acids, peptides, and proteins: Kinetics and mechanism, (Vol. 9). John Wiley & Sons.
Siedlecka, A., & BaszyńAski, T. (1993). Inhibition of electron flow around photosystem I in chloroplasts of Cd-treated maize plants is due to Cd-induced iron deficiency. Physiologia Plantarum, 87(2), 199–202. https://doi.org/10.1111/j.1399-3054.1993.tb00142.x
Silva, J., Fernandes, A., Junior, M. S., Santos, C., & Lobato, A. (2018). Tolerance mechanisms in Cassia alata exposed to cadmium toxicity–potential use for phytoremediation. Photosynthetica, 56(2), 495–504. https://doi.org/10.1007/s11099-017-0698-z
Srivastava, S., & Dubey, R. (2011). Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regulation, 64(1), 1–16. https://doi.org/10.1007/s10725-010-9526-1
Stadtman, E. R. (1992). Protein oxidation and aging. Science, 257(5074), 1220–1224. https://doi.org/10.1126/science.1355616
Street, R., Kulkarni, M., Stirk, W., Southway, C., & Van Staden, J. (2010). Effect of cadmium on growth and micronutrient distribution in wild garlic (Tulbaghia violacea). South+A1443 African Journal of Botany, 76(2), 332–336. https://doi.org/10.1016/j.sajb.2009.12.006
Triantaphylides, C., Krischke, M., Hoeberichts, F. A., Ksas, B., Gresser, G., Havaux, M., Van Breusegem, F., & Mueller, M. J. (2008). Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiology, 148(2), 960–968. https://doi.org/10.1104/pp.108.125690
Ulusu, Y., Öztürk, L., & Elmastaş, M. (2017). Antioxidant capacity and cadmium accumulation in parsley seedlings exposed to cadmium stress. Russian Journal of Plant Physiology, 64(6), 883–888. https://doi.org/10.1134/S1021443717060139
Ünyayar, S., Celik, A., Çekiç, F. Ö., & Gözel, A. (2006). Cadmium-induced genotoxicity, cytotoxicity and lipid peroxidation in Allium sativum and Vicia faba. Mutagenesis, 21(1), 77–81.
Van-Thuoc, D., Hashim, S. O., Hatti-Kaul, R., & Mamo, G. (2013). Ectoine-mediated protection of enzyme from the effect of pH and temperature stress: A study using Bacillus halodurans xylanase as a model. Applied Microbiology and Biotechnology, 97(14), 6271–6278.
Velikova, V., Yordanov, I., & Edreva, A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Science, 151(1), 59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Verbruggen, N., Hermans, C., & Schat, H. (2009). Mechanisms to cope with arsenic or cadmium excess in plants. Current Opinion in Plant Biology, 12(3), 364–372. https://doi.org/10.1016/j.pbi.2009.05.001
Verma, S., & Dubey, R. (2003). Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Science, 164(4), 645–655. https://doi.org/10.1016/S0168-9452(03)00022-0
Vogeli-Lange, R., & Wagner, G. J. (1990). Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves: Implication of a transport function for cadmium-binding peptides. Plant Physiology, 92(4), 1086–1093. https://doi.org/10.1104/pp.92.4.1086
Wang, C.-R., Nguyen, J., & Lu, Q.-B. (2009). Bond breaks of nucleotides by dissociative electron transfer of nonequilibrium prehydrated electrons: A new molecular mechanism for reductive DNA damage. Journal of the American Chemical Society, 131(32), 11320–11322.
Wardman, P., & Candeias, L. P. (1996). Fenton chemistry: An introduction. Radiation Research, 145(5), 523–531. https://doi.org/10.2307/3579270
Yang, J., Li, K., Zheng, W., Zhang, H., Cao, X., Lan, Y., Yang, C., & Li, C. (2015). Characterization of early transcriptional responses to cadmium in the root and leaf of Cd-resistant Salix matsudana Koidz. BMC Genomics, 16(1), 1–15. https://doi.org/10.1186/s12864-015-1923-4
Zhang, M., Deng, X., Yin, L., Qi, L., Wang, X., Wang, S., & Li, H. (2016). Regulation of galactolipid biosynthesis by overexpression of the rice MGD gene contributes to enhanced aluminum tolerance in tobacco. Frontiers in Plant Science, 7, 337. https://doi.org/10.3389/fpls.2016.00337
Author information
Authors and Affiliations
Contributions
F.O. conducted the experimentation and each author (F.O., K.U.P., S.B. and D.T.) wrote the part of the manuscript related to their area of expertise. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
Authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Orhan, F., Parlak, K.U., Tabay, D. et al. Alleviation of the Cadmium Toxicity by Application of a Microbial Derived Compound, Ectoine. Water Air Soil Pollut 234, 534 (2023). https://doi.org/10.1007/s11270-023-06562-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06562-8