Abstract
The textile industry is expanding globally and is considered the backbone of the world’s largest source of foreign exchange. The development of the textile industry has caused environmental contamination due to its dye waste, which is complex and very difficult to resolve with chemical and physical treatments. Azo dye is one of the most widely used dyes in textile and other industries. It is one of the significantly toxic dyes, and when released in water bodies, it causes a serious threat to the environment. A bacterial strain having the potential to degrade a variety of azo dyes such as Congo red (CR), methylene blue (MB), Alizarin Red S (AR), and Remazol Brilliant Blue R (RBBR) was isolated from soil samples in the wood weathering area and further identified and characterized. Ligninolytic microorganisms produce laccase enzymes, lignin peroxidase, manganese peroxidase, and other enzymes that can decolorize dye waste from the textile industry. The research phase was qualitative and quantitative tests of ligninolytic bacteria in the decolorization process using several selected synthetic dyes, antagonism tests, and identification of potential bacteria based on 16S rDNA gene sequences. The L11 isolate showed high performance on CR dye of 82.79%, L1 isolate on dye AR of 40.51%, L7 isolate on dye MB of 38.69%, and L8 isolate on RBBR dye of 30.34%. The L11 isolate with the highest potency was identified as Bacillus paramycoides K7.2 with a similarity of 99.71%. After 7 days of incubation, the quantitative test findings are the same as the qualitative test results, with isolate L11 having the largest clear zone on CR, AR, and RBBR dyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The textile industry is one of the essential contributors to the world economy, but it is also involved in many industrial hazardous chemical effluents. Limited usage of natural dyes and growing textile demand have significantly increased the usage of synthetic dyes, which contributed to dye wastewater and has become a substantial source of severe environmental concerns in modern times. The synthetic dye waste generated is supposed to damage the environment because they seem to be insoluble in water and harmful to aquatic living organisms such as plants, animals, and microbes, and used in the batik textile industry, producing substantial long-term health impacts (Dewi et al., 2018). The dye waste in the water reduces the appeal, prevents sunshine from penetrating the water, disrupting aquatic organisms’ photosynthetic activity, decreasing oxygen availability in the water, and triggering anaerobic exercise, which generates undesirable odor compounds (Dafale et al., 2008). Concerning volume and effluent composition, textile industry wastewater is the most polluting of all industrial sectors because the textile industrial effluents have high chemical oxygen demand (COD), biochemical oxygen demand (BOD), color, pH, and metal ions, making it difficult to treat such effluents (Mezohegyi et al., 2009; Senan & Abraham, n.d.). As a result, textile industries produce millions of liters of untreated wastewater per day, discharged directly into chugging water resources like rivers and lakes. In addition, the changes in the pH raise the BOD and COD levels and produce intense coloration (Telke et al., 2010). Most of the artificial dyes used in the textile industry are azo-based dyes accounting for the most significant chunk. The discharge of these dyes into the environment is indeed a public health concern. Additionally, textile dyes reduce the aesthetic appeal of water bodies by raising the biochemical and chemical oxygen demand, which reduces photosynthesis, stunts plant development, enters the food chain, causes recalcitrance and bioaccumulation, and may even be poisonous, mutagenic, and carcinogenic (Mudhoo et al., 2020). The azo group accounts for approximately 60–70% of synthetic colors manufactured worldwide. As a result, the azo dye class is the most often utilized artificial dye class in the industrial sector. In addition, azo dyes are more commonly employed in staining because they are easier to get, offer a broader range of hues, and are less expensive than natural dyes. However, their presence in a water body is full of health risks due to their mutagenic and carcinogenic behavior, and they are challenging to handle chemically or photolytically (Sudiana et al., 2018). Diazotized amine, an amine/phenol, and one or more azo links are the main component of azo dyes. Approximately 300 distinct azo dyes are extensively used in the textile, paper, food, cosmetics, and pharmaceutical sectors. Previous research has looked at the impact of pH, temperature, the nature and amount of oxygen substrate, and oxygen consumption on the rate of biological reduction of a range of azo dyes (Wuhrmann et al., 1980).
Treatment of synthetic dye-containing wastewater has traditionally been carried out using appropriate conventional techniques (physical or chemical) such as flocculation, coagulation, adsorption, membrane filtration, precipitation, irradiation, ozonization, and Fenton oxidation. However, it may produce large quantities of chemical sludge, whose removal in a secure landfill increases process cost (Rajeswari, 2014; Lodha & Chaudhari, 2007; Kumar et al., 2006). As a result, novel and environmentally friendly wastewater treatment methods are desirable. Several published studies have described the decolorization of untreated wastewater by ligninolytic microorganisms such as Shewanella decoloration is MBTD16, Bacillus amyloliquefaciens, and Paenibacillus glucanolyticus SLM1 representing oxidative enzymes such as laccases and lignin peroxidases (Babu et al., 2013; Lončar et al., 2014; Mathews et al., 2016). The obtained data from these works confirmed the usage of ligninolytic bacteria is one of the most appealing remediation options.
Bacteria secrete enzymes that can degrade lignin and organic compounds, such as lignin peroxidase, lignin manganese, and laccase enzymes, and certain microorganisms, such as ligninolytic bacteria, act as bioremediation agents. The ligninolytic bacteria as bioconversion agents are applied in various issues. As a result, this novel bacterium is applied in this research to determine its potential in waste management, specifically azo-dye decolorization, a competent organism to resolve these textile waste problems. The primary goal is to find high-potency ligninolytic bacteria strains that can approach azo-dye treated wastewater and act as bioremediation agents. In this regard, the ligninolytic bacteria as functional bioremediation agents are isolated and characterized for remediation application. In the experiment, the activity of color-degrading enzymes in isolated bacteria is evaluated spectrophotometrically by spectrophotometry to assess the movement of color-degradation enzymes in isolated bacteria. In addition, the rate of color removal efficiency is used to determine the capability of bacterial lignin peroxidases in decolorizing azo-dye-containing wastewater. This study provides a milestone for low-cost and environmentally friendly solutions for the textile industry and essential information on the ability of ligninolytic bacteria to be used as bioremediation agents in dye biodegradation for high efficiency.
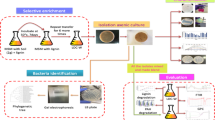
2 Materials and Methods
2.1 Screening Test of Ligninolytic Bacteria
Measuring the clear zone index created on the lignin agar medium may provide a semi-quantitative assessment of bacteria’s ability to break down lignin. This experiment was carried out on the surface of a lignin agar plate utilizing blank disk diffusion. A ligninolytic bacterial isolate was inoculated into a culture vial containing 5 mL of liquid lignin medium, and then incubated for 5 days at 30 °C. A spectrophotometer with a wavelength of 600 nm was used to determine the absorbance value of each isolate’s starting culture. Each starting culture was equalized using a liquid lignin medium and its cell density value. A sterile blank disk was filled with 30 mL of ligninolytic bacterial culture. The blank disk was placed on the surface of the lignin agar medium and incubated for 5 days at 30 °C after it had been entirely absorbed. The formation of a clear zone around the colony shows the potential for bacteria. A 10-min immersion aided in assessing the clear zone in the 0.1% Congo red dye medium. Solution of 0.1 M NaCl rinsed the medium discarding the residual Congo red.
In this study, to calculate the ligninolytic activity, the ligninolytic index used the ratio of the diameter of the clear zone to the colony (Rahayu et al. 2010). By breaking or degrading 1,4 glycosidic connections in lignin and releasing Congo red, ligninolytic bacteria can hydrolyze the lignin medium, allowing the hydrolyzed medium to bind Congo red and produce a clear zone. As a result, the semi-quantitative tests were repeated three times. Following that, the isolate with the largest clear zone has a good chance of being used in the next test.
2.2 Potential Bacterial Isolates for Synthetic Dye Decolorization
Inoculating ligninolytic bacteria on an agar dye medium with 100 mg/L dye concentrations for methylene blue (MB), Congo red (CR), Alizarin Red S (AR), and Remazol Brilliant Blue R (RBBR) were used for screening. For 7 days, the bacterial isolates were cultured at 30 °C. Synthetic dyes decolorize the colony according to a clear zone around it, and these experiments were repeated three times.
2.2.1 Dye Decolorization Test
This bacterial isolate was chosen using a medium containing (in grams per liter): 2.5 NaNO3, 1 KH2PO4, 0.01 CaCl2•2H2O, 0.3 MgSO4•7H2O, 0.1 NaCl, 0.01 FeCl3•6H2O, one yeast extract, 15 bacteriological agar, and synthetic colors with graded concentrations (multiples of 100 mg/L). The isolates were then firmly streaked into four-quadrant serving as duplicates and cultured at 30 °C for 7 days. Next, bacterial isolates with a clear zone were streaked again onto a synthetic dye medium carrying a greater concentration of synthetic dye (Bandounas et al., 2011). The bacteria would be chosen for the next test when creating the largest clear zone with a high dye concentration in the medium.
The decolorization test on the liquid medium used the modified Alalewi & Jiang (2012) method with three replications. The test used four synthetic dyes: Congo red (CR), methylene blue (MB), Alizarin Red S (AR), and Remazol Brilliant Blue R in Spec 160% (RBBR). The bacterial isolates were injected in 50 mL of 25 mg/L liquid dye medium and cultured on a rotary shaker at 150 rpm and 30 °C until they reached the exponential phase. After equalizing the cell density to 108 CFU/ml, 10 mL of culture was inoculated into 100 mL of simple liquid mineral with 1 g/L yeast extract. It had the most significant quantity of synthetic dye from the previous test results, which bacteria could never decolorize. As a control, a medium without inoculum was employed. The medium was incubated on a rotary shaker at 30 °C, 150 rpm for 7 days, then took 5 mL on 0, 1, 3, and 7 days. A spectrophotometer with a wavelength of 600 nm was used to quantify cell density in the samples. The bacterial cells were separated by centrifugation at 10,000 rpm for 10 min at 20 °C. A UV–Vis spectrophotometer with wavelengths of MB (λ 520 nm), AR (λ 427 nm), CR (λ 470 nm), and RBBR (λ 595 nm) was used to investigate dye decolorization (595 nm). The proportion of decolorization was calculated using an algorithm devised by López et al. (2006).
Noted:
2.3 Identification of the Decolorization Bacteria Potency by Using 16S rDNA Sequences
The Quick-DNATM Fungal/Bacterial Miniprep Kit was used to extract genomic DNA molecules from bacterial cultures potentially. The 16S rDNA gene was amplified using forward primer 27F (5′-AGAGTTTGATCCTGGCTCAG-3') and reverse primer 1492R (5′-GGTTACCTTACCTTGTTACGACTT-3′) that were introduced into a PCR master mix that included 2 L template DNA, 25 L GoTaq®Green Master Mix, 2 L of forwarding primer and 2 L of reverse primer, and 19 L of sterile distilled water.
The PCR process was conducted at pre-denatured settings of 95 °C for 300 s for one cycle, then denatured at 95 °C for 30 s, annealing at 52 °C for 45 s, and extension at 72 °C for 90 s for 35 cycles each, followed by an extension period of 300 s at 72 °C. Electrophoresis was used to confirm the 16S rDNA sequence amplicon, and the quantity of DNA recovered was quantified using a Nano-Drop Spectrophotometer. The agarose concentration was 1.5%, and running electrophoresis was performed for 30 min at 100 V. The buffer solution used is Tris–borate EDTA (TBE) 1X. The DNA bands were recorded with Gel Doc, and the PCR product was purified with the PCR Purification Kit. Samples are sent to First Base Pte. Malaysia for sequencing (Rupaedah et al., 2019).
With the Bioedit application, the 16S rDNA sequence was contiguous. The contig findings were evaluated by online Blast and matched on GenBank for nucleotide sequences. The neighbor-joining tree technique is used in the MEGA11 software to generate a phylogenetic tree using Bootstrap 1000.
2.4 Data Analysis
Each test was carried out in 3 replications, and the mean value and standard deviation were shown (Karim, 2018). The data obtained were analyzed statistically using one-way analysis of variance (ANOVA) with p 0.05, which has previously been tested for normality of the data; if the results were significantly different, further tests were performed. Data analysis was calculated by SPSS 16.0 for Windows software.
3 Result
3.1 The Potency of the Isolate in Degrading Lignin
The blank disk diffusion technique was used on a specific agar medium to examine bacteria’s capacity to break down lignin. After 7 days of incubation, colonies on lignin agar media for ligninolytic bacteria reached an apparent zone size, with typically equal growth rates amongst isolates. This study performed on 11 distinct ligninolytic bacteria isolates (L1, L2, L3, L4, L5, L6, L7, L8, L9, L10, and L11). The presence of lignin breakdown by isolates is represented by producing a clear zone surrounding the colony.
The diameter of the clear zone differed significantly (α 0.05) across the tested isolates; three isolates demonstrated the capacity to break down lignin, including isolates L5, L6, and L8, which had clear zones of 1.65 mm, 2.11 mm, and 1.96 mm, respectively (Fig. 1). The cleared zone index is comparable to Seesatat et al. (2021) work using bacteria isolated from soil and a cleared zone index of 2 to 3 mm. The ability of bacteria to degrade lignin varies depending on the type of enzyme present in each bacterium.
The ability of isolates to break down lignin was regulated by incubation time, lignin concentration, and pH in the medium. At this point, ligninolytic bacterial isolates were chosen based on the diameter index of the generated clear zone. The cleared zone index of the isolates was higher than 1.5 mm, with an L6 isolate exhibited the highest clear zone index.
3.2 Screening of Maximum Tolerance Concentration (MTC) on Varieties Concentration of Azo Dye
In this study, azo dyes such as Congo red (CR) and methylene blue (MB) were utilized, as well as anthraquinone dyes such as Alizarin Red S (AR) and Remazol Brilliant Blue R Spec 160% (RBBR) were also subjected to bacteria. The intensity of its application in the textile sector and the structural resemblance to lignin influence the color selection.
The tight scratch method was used to select prospective bacterial isolates by analyzing the growth rate and capacity to generate a clear zone after seven days of incubation. After 24 h of incubation at 30 °C, almost all bacterial isolates were able to grow on media with RBBR, AR, CR, and MB dyes at the concentration of 100 mg/L, which shows the presence of the clear zone around the colony, suggesting its ability to decolorize the dye. Bacterial isolates L4 and L5 were unable to grow on all mediums with all colors and were subsequently not used.
After obtaining pure isolates, screening is carried out to determine potential bacteria used for further tests. The manufacture of the dye medium in the screening test was carried out by following the method of Tian et al. (2016). First, except for the synthetic dyes used, all medium components were sterilized using an autoclave. Then, the synthetic dye was added aseptically to the medium after being fixed and conditioned at 80 °C for 1 h with occasional shaking; the concentration of the synthetic dyes used in this screening process was 100 mg/L. The screening was performed using the streak plate method with four replications and incubated for 7 days at 30 °C. The required isolates for further testing were chosen based on their ability to develop in the dyes medium and decolorize the additional synthetic dyes; a clear zone showed the ability to degrade around the colony. Eleven bacterial isolates were used to degrade four dyes (MB, CR, AR, and RBBR).
The cleared zone formation indicated that the test isolates could decolorize the synthetic dyes added to the agar medium. The bacterial colonies can consume the supplied dyes as nutrition for their growth (Bandounas et al., 2011). The ability of bacteria to degrade synthetic dyes is presented in Table 1. Almost of bacteria can grow in media with synthetic dyes after 24 h of incubation at 30 °C. In contrast, the isolates L4 and L5 may not grow up in each media with synthetic dyes (Table 1).
The MTC test was performed using a dense streak approach to one quadrant and incubated for 7 days at 30 °C with varying doses to test all ligninolytic bacteria, as indicated in Table 1. The clear zone created by the test isolates in each color at 200 mg/L was not significantly different from the 100 mg/L concentration. Based on the results of this test, the L1, L6, L7, L8, and L11 bacterial isolates have significant potential for dye degradation. Table 1 shows that increasing the concentration of synthetic dyes tested can reduce the percentage of decolorization efficiency and reduce the growth of almost all the tested bacteria due to the dye’s toxic effect, which blocks the active site of the azo-reductase enzyme with dyes molecules of different structures. Furthermore, a decline in decolorization efficiency might be due to a low ratio of cells in the medium to break down all colors transported across the cell membrane (Tony et al., 2009).
Based on this qualitative test, it was discovered that isolate L1 could decolorize AR dye, isolate L6 had potency in decolorizing CR dye, isolate L7 had a single potential as bacteria to degrade MB dye, isolate L8 could decolorize MB and RBRR dyes, and isolate L11 had triple potency in decolorizing AR, CR, and RBRR dyes (Table 1). The creation of the clear zone indicated that the test isolate could decolorize the synthetic dyes introduced to the medium. The bacterial colonies could use the supplied dyes as nutrition for their growth. No additional experiments were performed on several other bacterial isolates since the bacteria did not thrive at low doses, implying that these bacteria could not decolorize colors.
Furthermore, bacteria with the most significant capacity for degrading dyes to the highest concentrations are evaluated in a liquid medium. The liquid medium decolorization test was performed statically to simulate the original environmental conditions of industrial textile waste. The dyes degradation test yielded a variety of results. As seen in Table 2, each bacterial isolate has a unique capacity to break down azo dyes.
The statistical analysis of the decolorization test results for several azo dyes, namely MB, CR, AR, and RBBR, demonstrates a homogeneity in their results. Furthermore, there was a significant difference in sampling times. Nonetheless, there was no significant difference between the isolates used and no interaction between the isolates and the sampling time. On the seventh day of incubation, the maximum decolorization percentage in a medium containing synthetic dyes MB, CR, AR, and RBBR was 38.96%, 82.79%, 40.51%, and 30.34% on isolate L7, L8, L1, and L11, respectively. The L11 was the most promising isolate in the decolorization process, with the highest percentage of decolorization in CR dye of 82.79%. It also showed a significant response to the degradation of other dyes, such as AR and RBBR. After 7 days of incubation, the quantitative test findings are the same as the qualitative test results, with isolate L11 having the largest clear zone on CR, AR, and RBBR dyes and isolate L7 having the most extensive clear zone on MB dye at a concentration of 500 mg/L.
According to Bandounas et al. (2011), the percentage of MB dye decolorization by Bacillus sp. after 25 h, MB showed excellent performance for the degradation of 53% at a concentration of 25 mg/L lower than the dose applied in this study, which was 100 mg/L. Sarim et al. (2019) conducted research for the CR decolorization procedure, achieving a decolorization percentage of 84.5% using a bacterial isolate of Bacillus subtilis strain HAU-KK01. The incubation temperature employed in this investigation was 30 °C, while 35 °C was recommended temperature for the incubation of CR dye decolorization. Another study found that after 72 h of incubation with a dye concentration of 10 mg/100 mL, the bacteria Lysinibacillus sphaericus decolorized the synthetic dye RBBR by 58% (Chantarasiri & Boontanom, 2017). The temperature of incubation in this study was 30 °C. In contrast, according to Illakkiam et al. (2016), the optimal incubation temperature for the AR decolorization process was 37 °C, with the percentage decolorization reaching 67% using Pseudomonas sp. after 48 h of incubation. That is why the rate of decolorization in this study was low. He also stated that pH affects the Alizarin Red (AR) dye decolorization process, and the effective pH used is pH 7.0. According to the results of this study, the isolates L1 and L11 generated the highest percentage of decolorization on the seventh day of sampling, with a pH of the medium near pH 7, precisely 6.84 and 6.85, respectively. The change in pH seen in this study was most likely caused by the dye’s high number of azo linkages, which degraded to generate aromatic amine metabolites, which are more alkaline than the original azo dyes (Hanis et al., 2020).
Under anaerobic conditions, the decolorization of azo dyes (MB and CR) can begin by reducing the -N = N- link to produce colorless aromatic amines, which can be degraded aerobically or anaerobically (Sh Alabdraba et al., 2014). According to Saratale et al. (2011), the azoreductase enzyme transfers four electrons (reducing equivalents) throughout two transfer phases, with two electrons transferred to the azo dyes functioning as the final electron acceptor form a colorless or decolorizing solution. The anaerobic decolorization of azo dyes is generally regarded as a straightforward and non-specific procedure, as seen in the CR dye decolorization process, which has a more significant percentage of decolorization in anaerobic circumstances than other dyes decolorization procedures. Meanwhile, because it is challenging to validate the breakdown route, the decolorization of anthraquinone dyes has not precisely clarified the decolorization mechanism under anaerobic circumstances (Routoula & Patwardhan, 2020). According to Li et al. (2019), bacteria’s degeradation of an anthraquinone dye is a complicated process combining adsorption, degradation, and enzyme catalysis. Adsorption happens when the dyes adhere to the surface of the bacterial cell and causes the bacterial cell to become more concentrated or change color depending on the absorbed color before further degradation occurs.
The elements that contributed to the low decolorization percentage were incubation temperature, medium pH, oxygen and agitation, initial concentration of added dyes, dye structure, incubation period, the density of inoculation cells, and bacteria species employed. The activity medium in this study is a simple mineral medium with 0.1% yeast extract added, which works as a co-substrate in the co-metabolism of the synthetic dye tested to regenerate NADH, which functions as an electron donor in the reduction process by microorganisms, allowing the effectiveness of decolorization to be detected (Chang et al., 2000; Chantarasiri & Boontanom, 2017; Illakkiam et al., 2016; Saratale et al., 2011; Sarim et al., 2019). The bacterial isolates used in this study were ligninolytic bacteria, which might also generate enzymes such as lignin peroxidase (LiP), and dye-decolorizing peroxidase (DyP), manganese peroxidase (MnP), catalase, and laccase, all of which are used to boost the efficacy of decolorization. According to Tian et al. (2016), the decolorization of RBBR and MB cannot be accomplished by low redox potential oxides like manganese peroxidase (MnPs) and laccase. However, it can be accomplished by high redox potential agents like lignin peroxidase (LiP).
3.3 The Identification of Decolorization Bacteria by Using 16S rDNA Sequences
The bacteria that will be detected molecularly have the most significant capacity for lowering the concentration of the synthetic dyes tested, which is defined as a high percentage of decolorization in this study. In this investigation, the isolate with the highest potential was L11. The isolate exhibited a 99.71% resemblance to the species Bacillus paramycoides strain K7.2 as determined by BLAST-N using the 16S rDNA sequencing. The phylogenetic tree was built by comparing isolates L11 with the genus Bacillus sp. as in groups, specifically Bacillus paramycoides and E. coli species as outgroups (Fig. 2). Bacterial isolates with a resemblance greater than 99% are considered as one strain. However, isolates with lower than 95% similarity are said to represent a new genus (novel genera) (Schlaberg et al., 2012). Identification based on molecular characterization was reported and revealed the existence of a strain belonging to the genera of Bacillus sp. was isolated from soil samples in the wood weathering area. In another research, the genus Bacillus is commonly used to degrade azo dye (Avci et al., 2023; Srivastava et al., 2022; Wu et al., 2022).
According to Liu et al. (2017), Bacillus paramycoides is a facultative anaerobic bacterium isolate and a form of a rod cell with a length of 1.8–2.2 m and a width of 0.8–1.2 m. These gram-positive bacteria are nonmotile, can generate endospores, and thrive at temperatures ranging from 15 to 39 °C (optimum at 30 °C), pH ranges from 5 to 9, and a salt concentration of 0.5%. Bacterial colonies showed a waxy appearance, were circular, non-permeable to light, and 2–3 mm in diameter after 48 h at 32 °C, which was consistent with the findings of this investigation. In addition, Bacillus paramycoides performed well in catalase and oxidase tests and were able to hydrolyze starch, skim milk, and casein. Based on the results of the API 20E test, Bacillus paramycoides can produce acetoin (Voges-Proskauer), gelatinase, and acid, while based on the results of the API 50CHB test, the bacteria can produce acid from D-ribose, D-galactose, D-glucose, D-fructose, D-mannose, N-acetylglucosamine, amygdalin, arbutin, aesculin ferric citrate, cellobiose, maltose, trehalose, and starch.
Bacillus paramycoides may also generate enzymes such as oxidase, catalase, amylase, cellulase, urease, proteases, and caseinase, which can be employed in the industry. In addition, Bacillus paramycoides is a ligninolytic bacterium capable of degrading lignin and is hypothesized to be capable of producing enzymes required in the bio-delignification process, such as Li-P (lignin peroxidase) and Mn-P (manganese peroxidase) (Çağlayan, 2021; Rupaedah, 2019). Bacillus paramycoides were employed as immobilized bacteria in nanofibers in the decolorization process for processing methylene blue (MB) colored trash.
4 Conclusion
Four isolates of ligninolytic bacteria have a specific ability to decrease BOD5 in leachate water and decolorize azo dyes. L1, L7, L8, and L11 are efficient candidates of biodegradation agents because of their capability to break down and decolorize azo dyes in the textile dyeing industry’s final effluent. Bacterial isolate L8 was considered the most potential to reduce BOD5 values up to 95.87% on day ten, and the maximum decolorization percentage in a medium containing synthetic dyes MB, CR, AR, and RBBR was 38.96%, 82.79%, 40.51%, and 30.34% on isolates L7, L8, L1, and L11, respectively. Isolate L11 exhibited a 99.71% resemblance to the species Bacillus paramycoides strain K7.2 as determined by BLAST-N using the 16S rDNA sequencing was the most promising isolate in the decolorization process, with the highest percentage of decolorization in CR dye of 82.79%, and it was also capable of decolorizing other dyes (AR and RBBR). The isolate L7 has the most extensive clear zone on MB dye at a concentration of 500 mg/L. The obtained results are comparatively high and good. Thus, it can be used as a candidate for the degradation of pollutants (dyes) to reduce water pollution.
Data Availability
Not applicable.
References
Alalewi, A., & Jiang, C. (2012). Bacterial influence on textile wastewater decolorization. Journal of Environmental Protection, 03(08), 889–903. https://doi.org/10.4236/jep.2012.328104
Avcı, A., Yıldırım, A., Cerit, I., Keskin, S. Y., Hamk, M., Keskin, C. S., & Demirkol, O. (2023). Influence of culture age and environmental conditions on the decolorization and biodegradation of Reactive Orange 13 by Bacillus sp. SBT8. Biomass Conversion and Biorefinery, 13, 4353–4363. https://doi.org/10.1007/s13399-022-03157-x
Babu, S. S., Mohandass, C., Raj, A. S. V., Rajasabapathy, R., & Dhale, M. A. (2013). Multiple approaches towards decolorization and reuse of a textile dye (VB-B) by a marine bacterium shewanella decolorationis. Water, Air, and Soil Pollution, 224(4), 1500. https://doi.org/10.1007/s11270-013-1500-x
Bandounas, L., Wierckx, N. J., de Winde, J. H., & Ruijssenaars, H. J. (2011). Isolation and characterization of novel bacterial strains exhibiting ligninolytic potential. BMC Biotechnology, 11, 94. https://doi.org/10.1186/1472-6750-11-94
Çağlayan, P. (2021). Determination of important enzymes and antimicrobial resistances of gram-positive haloalkaliphilic bacteria isolated from Salda Lake. Ege Journal of Fisheries and Aquatic Sciences, 38(3), 375–382. https://doi.org/10.12714/egejfas.38.3.14
Chang, J.-S., Kuo, T.-S., Chao, Y.-P., Ho, J.-Y., & Lin, P.-J. (2000). Azo dye decolorization with a mutant Escherichia coli strain. Biotechnology Letters, 22, 807–812. https://doi.org/10.1023/a:1005624707777
Chantarasiri, A., & Boontanom, P. (2017). Decolorization of synthetic dyes by ligninolytic lysinibacillus sphaericus JD1103 isolated from Thai wetland ecosystems. AACL Bioflux, 10(4), 814–819. https://www.researchgate.net/publication/319270611
Dafale, N., Wate, S., Meshram, S., & Nandy, T. (2008). Kinetic study approach of remazol black-B use for the development of two-stage anoxic-oxic reactor for decolorization/biodegradation of azo dyes by activated bacterial consortium. Journal of Hazardous Materials, 159(2–3), 319–328. https://doi.org/10.1016/j.jhazmat.2008.02.058
Dewi, R. S., Kasiamdari, R. S., Martani, E., & Purwestri, Y. A. (2018). Decolorization and detoxification of batik dye effluent containing Indigosol Blue-04B using fungi isolated from contaminated dye effluent. Indonesian Journal of Biotechnology, 23(2), 54–60. https://doi.org/10.22146/ijbiotech.32332
Hanis, K. K. A., Muhammad Nasri, A. R., Wan Farahiyah, W. K., & Mohd Rabani, M. Y. (2020). Bacterial degradation of azo dye Congo red by Bacillus sp. Journal of Physics: Conference Series, 1529(2), 022048. https://doi.org/10.1088/1742-6596/1529/2/022048
Illakkiam, D., S, D., A, V., & G, N. (2016). Decolorization of alizarin red s dye by bacterial strains isolated from industrial effluents. International Journal of Plant, Animal and Environmental Sciences, 6(1), 268–275.
Karim, D. H. (2018). Decolorization of textile reactive dyes by bacterial monoculture and consortium screened from textile dyeing effluent. Journal of Genetic Engineering and Biotechnology, 16, 375–380. https://doi.org/10.1016/j.jgeb.2018.02.005
Kumar, K., Saravana Devi, S., Krishnamurthi, K., Gampawar, S., Mishra, N., Pandya, G. H., & Chakrabarti, T. (2006). Decolorisation, biodegradation and detoxification of benzidine based azo dye. Bioresource Technology, 97(3), 407–413. https://doi.org/10.1016/j.biortech.2005.03.031
Li, Hh., Wang, Yt., Wang, Y., Wang, Hx., Sun, Kk., & Lu, Zm. (2019). Bacterial degradation of anthraquinone dyes. In Journal of Zhejiang University: Science B, 20(6), 528–540. https://doi.org/10.1631/jzus.B1900165. Zhejiang University Press.
Liu, Y., Du, J., Lai, Q., Zeng, R., Ye, D., Xu, J., & Shao, Z. (2017). Proposal of nine novel species of the bacillus cereus group. International Journal of Systematic and Evolutionary Microbiology, 67(8), 2499–2508. https://doi.org/10.1099/ijsem.0.001821
Lodha, B., & Chaudhari, S. (2007). Optimization of Fenton-biological treatment scheme for the treatment of aqueous dye solutions. Journal of Hazardous Materials, 148(1–2), 459–466. https://doi.org/10.1016/j.jhazmat.2007.02.061
Lončar, N., Gligorijević, N., Božić, N., & Vujčić, Z. (2014). Congo red degrading laccases from Bacillus amyloliquefaciens strains isolated from salt spring in Serbia. International Biodeterioration and Biodegradation, 91, 18–23. https://doi.org/10.1016/j.ibiod.2014.03.008
López, M. J., Guisado, G., Vargas-García, M. C., Suárez-Estrella, F., & Moreno, J. (2006). Decolorization of industrial dyes by ligninolytic microorganisms isolated from composting environment. Enzyme and Microbial Technology, 40(1), 42–45. https://doi.org/10.1016/j.enzmictec.2005.10.035
Mathews, S. L., Grunden, A. M., & Pawlak, J. (2016). Degradation of lignocellulose and lignin by Paenibacillus glucanolyticus. International Biodeterioration and Biodegradation, 110, 79–86. https://doi.org/10.1016/j.ibiod.2016.02.012
Mezohegyi, G., Fabregat, A., Font, J., Bengoa, C., Stuber, F., & Fortuny, A. (2009). Advanced bioreduction of commercially important azo dyes: Modeling and correlation with electrochemical characteristics. Industrial and Engineering Chemistry Research, 48(15), 7054–7059. https://doi.org/10.1021/ie9003893
Mudhoo, A., Ramasamy, D. L., Bhatnagar, A., Usman, M., & Sillanpää, M. (2020). An analysis of the versatility and effectiveness of composts for sequestering heavy metal ions, dyes and xenobiotics from soils and aqueous milieus. Ecotoxicology and Environmental Safety, 197, 110587. https://doi.org/10.1016/j.ecoenv.2020.110587
Rahayu, F., Untung Setyo Budi Balai Penelitian Tanaman Tembakau dan Serat, dan, Jl Raya Karangploso, M., & Pos, K. (2010). Seleksi dan Pengujian Potensi Bakteri Indigenous Air Rendaman Kenaf (Hibiscus cannabinus L.) Sebagai Bakteri Selulolitik, Pektinolitik, dan Lignolitik. Buletin Tanaman Tembakau, Serat & Minyak Industri, 2(2), 81–87.
Rajeswari, K. (2014). Degradation of textile dyes by isolated Lysinibacillus Sphaericus Strain RSV-1 and Stenotrophomonas maltophilia strain RSV-2 and toxicity assessment of degraded produc. Journal of Environmental & Analytical Toxicology, 4(4), 1000222. https://doi.org/10.4172/2161-0525.1000222
Routoula, E., & Patwardhan, S. V. (2020). Degradation of anthraquinone dyes from effluents: A review focusing on enzymatic dye degradation with industrial potential. In Environmental Science and Technology, 54(2), 647–664. https://doi.org/10.1021/acs.est.9b03737. American Chemical Society.
Rupaedah, B., Purwoko, D., Safarrida, A., Tajuddin, T., Wahid, A., Sugianto, M., Sudjai, I., & Suyono, A. (2019). Skrining dan identifikasi mikroba ligninolitik pada pengomposan alami tandan kosong kelapa sawit. Jurnal Bioteknologi & Biosains Indonesia, 6(1), 139–148. https://doi.org/10.29122/jbbi.v6i1.3237
Saratale, R. G., Saratale, G. D., Chang, J. S., & Govindwar, S. P. (2011). Bacterial decolorization and degradation of azo dyes: A review. In Journal of the Taiwan Institute of Chemical Engineers, 42, 138–157. https://doi.org/10.1016/j.jtice.2010.06.006
Sarim, K. M., Kukreja, K., Shah, I., & Choudhary, C. K. (2019). Biosorption of direct textile dye Congo red by Bacillus subtilis HAU-KK01. Bioremediation Journal, 23(3), 185–195. https://doi.org/10.1080/10889868.2019.1641466
Schlaberg, R., Simmon, K. E., & Fisher, M. A. (2012). A systematic approach for discovering novel, clinically relevant bacteria. In Emerging Infectious Diseases, 18(3), 422–430. https://doi.org/10.3201/eid1803.111481
Seesatat, A., Rattanasuk, S., Bunnakit, K., Maneechot, P., Sriprapakhan, P., & Artkla, R. (2021). Biological degradation of rice straw with thermophilic lignocellulolytic bacterial isolates and biogas production from total broth by rumen microorganisms. Journal of Environmental Chemical Engineering, 9(1), 104499. https://doi.org/10.1016/j.jece.2020.104499
Senan, R. C., & Abraham, T. E. (2004). Bioremediation of textile azo dyes by aerobic bacterial consortium Aerobic degradation of selected azo dyes by bacterial consortium. Biodegradation, 15(4), 275–280. https://doi.org/10.1023/b:biod.0000043000.18427.0a
Sh Alabdraba, W. M., Bayati, M., Mohammed Sheet Alabdraba, W., & Burhan Ali Albayati, M. (2014). Biodegradation of Azo Dyes a Review Industrial wastewater treatment by advanced oxidation processes-A Review View project EM technology View project Biodegradation of Azo Dyes-A Review. International Journal of Environmental Engineering and Natural Resources, 1(4), 179–189. https://www.researchgate.net/publication/262564975
Srivastava, A., Dangi, L. K., Kumar, S., & Rani, R. (2022). Microbial decolorization of Reactive Black 5 dye by Bacillus albus DD1 isolated from textile water effluent: kinetic, thermodynamics & decolorization mechanism. Heliyon, 7, e08834. https://doi.org/10.1016/j.heliyon.2022.e08834
Sudiana, I. K., Sastrawidana, I. D. K., & Sukarta, I. N. (2018). Decolorization study of remazol black B textile dye using local fungi of Ganoderma sp. and their ligninolytic enzymes. Journal of Environmental Science and Technology, 11(1), 16–22. https://doi.org/10.3923/jest.2018.16.22
Telke, A. A., Joshi, S. M., Jadhav, S. U., Tamboli, D. P., & Govindwar, S. P. (2010). Decolorization and detoxification of Congo red and textile industry effluent by an isolated bacterium Pseudomonas sp. SU-EBT. Biodegradation, 21(2), 283–296. https://doi.org/10.1007/s10532-009-9300-0
Tian, J. H., Pourcher, A. M., & Peu, P. (2016). Isolation of bacterial strains able to metabolize lignin and lignin-related compounds. Letters in Applied Microbiology, 63(1), 30–37. https://doi.org/10.1111/lam.12581
Tony, B. D., Goyal, D., & Khanna, S. (2009). Decolorization of textile azo dyes by aerobic bacterial consortium. International Biodeterioration and Biodegradation, 63(4), 462–469. https://doi.org/10.1016/j.ibiod.2009.01.003
Wu, K., Shi, M., Pan, X., Zhang, J., Zhang, X., Shen, T., & Tian, Z. (2022). Decolourization and biodegradation of methylene blue dye by a ligninolytic enzyme-producing Bacillus thuringiensis: degradation products and pathway. Enzyme and Microbial Technology, 156, 109999. https://doi.org/10.1016/j.enzmictec.2022.109999
Wuhrmann, K., Mechsner, K., & Kappeler, T. (1980). Investigation on rate — determining factors in the microbial reduction of azo dyes. European Journal of Applied Microbiology and Biotechnology, 9, 325–338. https://doi.org/10.1007/BF00508109
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahayu, F., Mustafa, I., Marjani et al. Newly Isolated Ligninolytic Bacteria and Its Applications for Multiple Dye Degradation. Water Air Soil Pollut 234, 359 (2023). https://doi.org/10.1007/s11270-023-06377-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06377-7