Abstract
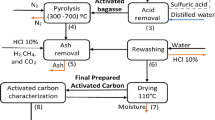
Textile wastewaters (TW) contain contaminants such as organic matter, dyes, sulfate, and many salts. Many methods have been used to remove these pollutants and to reduce the environmental impact associated with their release into water bodies. In the present study, physically activated carbon made of sugar cane bagasse was used as an adsorbent to remove color and chemical oxygen demand (COD) from textile effluent. The experiments were carried out with a real effluent from a textile jeans laundry. The effect of the variables, such as adsorbent amount, pH, contact time, temperature, and particle size of the adsorbent, was tested. The most efficient results were obtained for the adsorbent mass of 0.25 g, pH 6.0, contact time of 60 min, and adsorbent size smaller than 0.42 mm. The adsorbent performed well for color removal of textile effluent, removing up to 93% and 55% of color and COD, respectively. The Brunauer–Emmett–Teller (BET) surface area and total pore volume were 188.2 m2 g−1 and 0.13 cm3 g−1, respectively, and average sizes of pore diameter of 2.7 nm and 3.6 nm. This indicates the ability of agro-industrial waste for application in the process of textile wastewater treatment.
Graphical abstract







Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmed, M. J., & Theydan, S. K. (2012). Equilibrium isotherms, kinetics and thermodynamics studies of phenolic compounds adsorption on palm-tree fruit stones. Ecotoxicology and Environmental Safety, 84, 39–45. https://doi.org/10.1016/j.ecoenv.2012.06.019 [online].
Amaral, F. M., Florêncio, L., Kato, M. T., Santa-Cruz, P. A., & Gavazza, S. (2017). Hydraulic retention time influence on azo dye and sulfate removal during the sequential anaerobic-aerobic treatment of real textile wastewater. Water Science and Technology, 76(12), 3319–3327.
APHA (2005). Standard methods for the examination of water and wastewater. In A. D. Eaton, L. S. Clesceri, E. W. Rice, & A. E. Greenberg (Eds.), American Water Works Association/ Water Environment Federation (20th ed). Washington DC, USA
Arikan, E. B., Isik, Z., Bouras, H. D., & Dizge, N. (2019). Investigation of immobilized filamentous fungi for treatment of real textile industry wastewater using up flow packed bed bioreactor. Bioresource Technology Reports, 7, 100197.
Attia, A. A., Girgis, B. S., & Fathy, N. A. (2008). Removal of methylene blue by carbons derived from peach stones by H3PO4 activation: Batch and column studies. Dyes and Pigments, 76(1), 282–289.
Bahadur, N., & Bhargava, N. (2019). Novel pilot scale photocatalytic treatment of textile & dyeing industry wastewater to achieve process water quality and enabling zero liquid discharge. Journal of Water Process Engineering, 32, 100934.
Bilinska, L., Blus, K., Gmurek, M., & Ledakowicz, S. (2019). Coupling of electrocoagulation and ozone treatment for textile wastewater reuse. Chemical Engineering Journal, 358, 992–1001.
Contreras, E., Sepúlveda, L., & Palma, C. (2012). Valorization of agroindustrial wastes as biosorbent for the removal of textile dyes from aqueous solutions. International Journal of Chemical Engineering, 2012. https://doi.org/10.1155/2012/679352.
Demiral, H., & Gündüzoǧlu, G. (2010). Removal of nitrate from aqueous solutions by activated carbon prepared from sugar beet bagasse. Bioresource Technology, 101(6), 1675–1680.
El Naga, A. O. A., El Saied, M., Shaban, S. A., & El Kady, F. Y. (2019). Fast removal of diclofenac sodium from aqueous solution using sugar cane bagasse-derived activated carbon. Journal of Molecular Liquids, 285, 9–19.
Foo, K. Y., & Hameed, B. H. (2012). An overview of dye removal via activated carbon adsorption process. Desalination and Water Treatment, 19(1–3), 255–274.
Foo, K. Y., Lee, L. K., & Hameed, B. H. (2013). Preparation of tamarind fruit seed activated carbon by microwave heating for the adsorptive treatment of landfill leachate: A laboratory column evaluation. Bioresource Technology, 133, 599–605.
Foroutan, R., Mohammadi, R., Razeghi, J., & Ramavandi, B. (2019). Performance of algal activated carbon/Fe3O4 magnetic composite for cationic dyes removal from aqueous solutions. Algal Research, 40, 101509.
Giraldo-Gutiérrez, L., & Moreno-Piraján, J. C. (2008). Pb(II) and Cr(VI) adsorption from aqueous solution on activated carbons obtained from sugar cane husk and sawdust. Journal of Analytical and Applied Pyrolysis, 81(2), 278–284.
Giusto, L. A. R., Pissetti, F. L., Castro, T. S., & Magalhães, F. (2017). Preparation of activated carbon from sugarcane bagasse soot and methylene blue adsorption. Water, Air, and Soil Pollution, 228(7), 249.
Guo, Y., Tan, C., Sun, J., Li, W., Zhang, J., & Zhao, C. (2020). Porous activated carbons derived from waste sugarcane bagasse for CO2 adsorption. Chemical Engineering Journal, 381, 122736.
Jaguaribe, E. F., Medeiros, L. L., Barreto, M. C. S., & Araujo, L. P. (2005). The performance of activated carbons from sugarcane bagasse, babassu, and coconut shells in removing residual chlorine. Brazilian Journal of Chemical Engineering, 22(1), 41–47.
Marsh, H., & Rodríguez-Reinoso, F. (2006a). Chapter 4- Characterization of activated carbon. In H. Marsh, & F. Rodríguez-Reinoso (Eds.), Activated carbon (pp.143–242). Oxford Elsevier Science Ltd.
Marsh, H., & Rodríguez-Reinoso, F. (2006b). Chapter 5 - Activation processes (thermal or physical). In H. Marsh, & F. Rodríguez-Reinoso (Eds.), Activated carbon (pp. 243–321). Oxford Elsevier Science Ltd.
Marsh, H., & Rodríguez-Reinoso, F. (2006c). Charpter 8 - Applicability of activated carbon. In H. Marsh, & F. Rodríguez-Reinoso (Eds.), Activated carbon (pp. 383–453). Oxford Elsevier Science Ltd.
McCabe, W. L., Smith, J. C., & Harriott, P. (2001). Unit operations of chemical engineering. McGraw-Hill Science/Engineering/Math.
Mohammad-pajooh, E., Turcios, A. E., Cuff, G., Weichgrebe, D., Rosenwinkel, K. H., Vedenyapina, M. D., & Sharifullina, L. R. (2018). Removal of inert COD and trace metals from stabilized landfill leachate by granular activated carbon (GAC) adsorption. Journal of Environmental Management, 228, 189–196.
Nam, H., Choi, W., Genuino, D. A., & Capareda, S. C. (2018). Development of rice straw activated carbon and its utilizations. Journal of Environmental Chemical Engineering, 6(4), 5221–5229. https://doi.org/10.1016/j.jece.2018.07.045 [online].
Norma Técnica: Controle de carga orgânica em efluentes líquidos industriais N. 2001 de 03 de novembro de 2003 (2003) Recife, Companhia Pernambucana Do Meio Ambiente (CPRH). Retrieved May 18, 2021 from http://www2.cprh.pe.gov.br/wp-content/uploads/2021/01/normas-cprh-2001.pdf
Olivares-Marín, M., Del Prete, V., Garcia-Moruno, E., Fernández-González, C., Macías-García, A., & Gómez-Serrano, V. (2009). The development of an activated carbon from cherry stones and its use in the removal of ochratoxin A from red wine. Food Control, 20(3), 298–303.
Patel, H., & Vashi, R. T. (2015). Chapter 4 - Batch adsorption treatment of textile wastewater. In H. Patel, & R. T. Vashi (Eds.), Characterization and treatment of textile wastewater (pp. 111–125). Elsevier
Pavan, F. A., Lima, E. C., Dias, S. L. P., & Mazzocato, A. C. (2008). Methylene blue biosorption from aqueous solutions by yellow passion fruit waste. Journal of Hazardous Materials, 150(3), 703–712.
Pereira, M. F. R., Soares, S. F., Órfão, J. J. M., & Figueiredo, J. L. (2003). Adsorption of dyes on activated carbons: Influence of surface chemical groups. Carbon, 41(4), 811–821.
Regalbuto, J. R., & Robles, J. O. (2004). The engineering of Pt/carbon catalyst preparation.
Sahu, A. K., Srivastava, V. C., Mall, I. D., & Lataye, D. H. (2008). Adsorption of furfural from aqueous solution onto activated carbon: Kinetic, equilibrium and thermodynamic study. Separation Science and Technology, 43(5), 1239–1259.
Silva, D. D. (2010). Avaliação das características dos efluentes segregados e tratabilidade biológica dos efluentes gerados por uma indústria têxtil do APL de confecção do Agreste de Pernambuco. Monograph - Centro Acadêmico do Agreste, Universidade Federal de Pernambuco, Caruaru
Tahir, H., Sultan, M., Akhtar, N., Hameed, U., & Abid, T. (2016). Application of natural and modified sugar cane bagasse for the removal of dye from aqueous solution. Journal of Saudi Chemical Society, 20, S115–S121.
Talaiekhozani, A., Mosayebi, M. R., Fulazzaky, M. A., Eskandari, Z., & Sanayee, R. (2020). Combination of TIO2 microreactor and electroflotation for organic pollutant removal from textile dyeing industry wastewater. Alexandria Engineering Journal, 59, 549–563.
Thabede, P. M., Shooto, N. D., & Naidoo, E. B. (2020). Removal of methylene blue dye and lead ions from aqueous solution using activated carbon from black cumin seeds. South African Journal of Chemical Engineering, 33, 39–50.
Thommes, M., Kaneko, K., Neimark, A. V., Olivier, J. P., Rodriguez-Reinoso, F., Rouquerol, J., & Sing, K. S. W. (2015). Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure and Applied Chemistry, 87(9–10), 1051–1069.
Tkaczyk, A., Mitrowska, K., & Posyniak, A. (2020). Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Science of the Total Environment, 717, 137222. https://doi.org/10.1016/j.scitotenv.2020.137222 [online].
Tounsadi, H., Metarfi, Y., Taleb, M., El Rhazi, K., & Rais, Z. (2020). Impact of chemical substances used in textile industry on the employee’s health: Epidemiological study. Ecotoxicology and Environmental Safety, 197, 110594.
Yakout, S. M. (2014). Removal of the hazardous, volatile, and organic compound benzene from aqueous solution using phosphoric acid activated carbon from rice husk. Chemistry Central Journal, 8(1), 1–7.
Zazou, H., Afanga, H., Akhouairi, S., Ouchtak, H., Addi, A. A., Akbour, R. A., Assabbane, A., Douch, J., Elmchaouri, A., Duplay, J., Jada, A., & Hamdani, M. (2019). Treatment of textile industry wastewater by electrocoagulation coupled with electrochemical advanced oxidation process. Journal of Water Process Engineering, 28, 214–221.
Zhang, Z., Lei, Y., Li, D., Zhao, J., Wang, Y., Zhou, G., Yan, C., & He, Q. (2020). Sudden heating of H3PO4-loaded coconut shell in CO2 flow to produce super activated carbon and its application for benzene adsorption. Renewable Energy, 153, 1091–1099. https://doi.org/10.1016/j.renene.2020.02.059 [online].
Acknowledgements
The authors acknowledge the funding agencies, FACEPE (PRONEX Proc. APQ-0603-3.07/14), Núcleo de Química Analítica Avançada de Pernambuco, NUQAAPE (FACEPE grants APQ-0346-1.06/14), and Instituto Nacional de Tecnologias Analíticas Avançadas, INCTAA (CNPq grant 465768/2014-8 and FAPESP grant 2014/50951-4), for supporting this work. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES), finance code 001. Research fellowships (CNPq, 307397/2018-1 and 304862/2018-5) are also gratefully acknowledged. We are also thankful to professor Fabrício Motteran from the Laboratory of Environmental Sanitation, Department of Civil Engineering, Federal University of Pernambuco, for his assistance with Langmuir and Freundlich models. The English version was revised by Sidney Pratt, Canadian, BA, MAT (The Johns Hopkins University), RSAdip (TEFL) (Cambridge University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
A low-cost bio-sorbent was fabricated from agro-industrial waste sugarcane bagasse
The experiments were carried out with a real effluent from a textile jeans laundry
A color reducing up to 93% was obtained under the optimum adsorption conditions
Rights and permissions
About this article
Cite this article
da Costa, W.K.O.C., Gavazza, S., Duarte, M.M.M.B. et al. Preparation of Activated Carbon from Sugarcane Bagasse and Removal of Color and Organic Matter from Real Textile Wastewater. Water Air Soil Pollut 232, 358 (2021). https://doi.org/10.1007/s11270-021-05306-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05306-w