Abstract
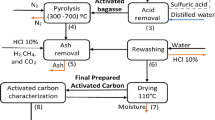
The waste biomass produced from sugar industry and Chinese medicine factory such as bagasse, reed root residue, pueraria residue and liquorice residue were selected as the raw material for the preparation of activated carbon with zinc chloride as activator. With the same activation time, the influence of temperature and impregnation ratio on the preparation of activated carbon was investigated and the reasonable preparation conditions of activated carbon were monitored and analyzed. The obtained activated carbon samples were characterized by scanning electron microscopy, Brunauer–Emmett–Teller, methylene blue adsorption, thermogravimetric analysis and nitrogen adsorption–desorption. Analysis from the experimental data, bagasse, reed root residue, pueraria residue are suitable for preparing activated carbon. For bagasse, the optimum preparation condition was 700 °C and the impregnation ratio was 1:1, the adsorption capacity of methylene blue reached 246.83 mg/g at the moment. For reed root residue, the optimum preparation condition was 600 °C and the impregnation ratio was 1:2, the adsorption capacity of methylene blue reached 268.07 mg/g at the moment. For Pueraria residue, the optimum preparation condition was 700 °C and the impregnation ratio was 1:2. The adsorption capacity of methylene blue reached 297.33 mg/g at the moment.








Similar content being viewed by others
Change history
27 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42768-021-00078-9
References
Chingombe P, Saha B, Wakeman RJ. Surface modification and characterisation of a coal-based activated carbon. Carbon. 2005;43:3132–43.
Wang T, Tan S, Liang C. Preparation and characterization of activated carbon from wood via microwave-induced ZnCl2 activation. Carbon N Y. 2009;47:1880–3.
Liu Z, Fang Z-H, Jin X, et al. Research on preparation of walnut shell-based activated carbon and its adsorption effect on methylene Blue. J Xi’an Univ (Natural Science Edition). 2019;22(2):81–5.
Ning P, Yang Y-H, Peng J-H, et al. Study on pyrolysis characteristics of macadamia shell during preparation of activated carbon. Chem Ind For Prod. 2006;26(4):61–4.
Zhang YD. Application of activated carbon adsorption in industrial wastewater treatment. Coal Chem Ind. 2011;34(6):74–6.
Jiang J, Sun K. Review on preparation technology of activated carbon and its application. Chem Ind For Prod. 2017;37(1):1–13.
Kazmierczak J, Nowicki P, Pietrzak R. Sorption properties of activated carbons obtained from corn cobs by chemical and physical activation. Adsorption. 2013;19:273–81.
Nakagawa Y, Molina-Sabio M, Rodríguez-Reinoso F. Modification of the porous structure along the preparation of activated carbon monoliths with H3PO4 and ZnCl2. Microporous Mesoporous Mater. 2007;103:29–34.
Nagano S, Tamon H, Adzumi T, Nakagawa K, Suzuki T. Activated carbon from municipal waste. Carbon. 2000;38:915–20.
Guo J, Lua AC. Textural and chemical characterizations of activated carbon prepared from oil-palm stone with H2SO4 and KOH impregnation. Microporous Mesoporous Mater. 2007;32:111–7.
Lillo-Ródenas MA, Marco-Lozar JP, Cazorla-Amoros D, Linares-Solano A. Activated carbons prepared by pyrolysis of mixtures of carbon precursor/alkaline hydroxide. J Anal Appl Pyrolysis. 2007;80:166–74.
Gercel O, Gercel HF. Adsorption of lead (II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia Rigida. Chem Eng J. 2007;132:289–97.
Carvalho AP, Gomes M, Mestre AS, Pires J, Carvalho BM. Activated carbons from cork waste by chemical activation with K2CO3, Application to adsorption of natural gas components. Carbon. 2004;42:672–4.
Angin D. Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel. 2014;115:804–11.
Namasivayam C, Kadirvelu K. Activated carbons prepared from coir pith by physical and chemical activation methods. Bioresour Technol. 1997;62:123–7.
Kaghazchi T, Asasian Kolur N, Soleimani M. Licorice residue and Pistachio-nut shell mixture: a promising precursor for activated carbon. J Ind Eng Chem. 2010;16(2010):368–74.
Ghaedi M, Danaei Ghazanfarkhani M, Khodadoust S, Sohrabi N, Oftade M. Acceleration of methylene blue adsorption onto activated carbon prepared from dross licorice by ultrasonic: equilibrium, kinetic and thermodynamic studies. J Ind Eng Chem. 2014;20(2014):2548–60.
Olivares-Marin M, Fernandez-Gonzalez C, Macias-Garcia A, Gomez-Serrano V. Preparation of activated carbon from cherry stones by chemical activation with ZnCl2. Appl Surf Sci. 2006;252:5967–71.
Saqer SM, Kondarides DI, Verykios XE. Catalytic oxidation of toluene over binary mixtures of copper, manganese and cerium oxides supported on γ-Al2O3. Appl Catal B Environ. 2011;103:275–86.
Test methods of wooden activated carbon—Determination of methylene blue adsorption (GB/T12496.10-1999) [S]. Beijing: China Standards Press; 2003. p 1–3.
Worasuwannarak N, Sonobe T, Tanthapanichakoon W. Pyrolysis behaviors of rice straw, rice husk, and corncob by TG-MS technique. J Anal Appl Pyrol. 2007;78(2):265–71.
Sayğılı H, Güzel F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. J Clean Prod. 2016;113:995–1004.
Köseoğlu E, Akmil-Başar C. Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv Powder Technol. 2015;26(3):811–8.
Brunauer S, Deming LS, Deming WE, Teller E. On a Theory of the van der Waals adsorption of gases. J Am Chem Soc. 1940;62(7):1723–32.
Hu L, Cheng W, Zhang W, Wu F, Peng S, Li J. Monolithic bamboo-based activated carbons for dynamic adsorption of toluene. J Porous Mater. 2016;24(2):1–9.
Kang S, Jiang JC, Lu XC. Preparation of activated carbon from pericarp and its application in glycerol decolorization and deactivation. For Chem Ind. 2009;v01.29(10):77–81.
Ma CY, Mao Y, Xu PF, et al. Preparation and characterization of high specific area activated carbon from bandoned capsicum straw by KOH/NaOH. J Funct Maer. 2013;44(3):3337–42.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Han, Q., Zong, Z. et al. Exploring on the optimal preparation conditions of activated carbon produced from solid waste produced from sugar industry and Chinese medicine factory. Waste Dispos. Sustain. Energy 2, 65–77 (2020). https://doi.org/10.1007/s42768-019-00032-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-019-00032-w